Abstract
Objectives
Among patients with initially unresectable colorectal cancer liver metastases (CLM), a subset are rendered resectable following the administration of systemic chemotherapy. This study reports the results achieved in liver resections performed at a single hepatobiliary referral centre after downsizing chemotherapy in patients with initially unresectable CLM.
Methods
All liver resections for CLM performed over a 10-year period at the Toronto General Hospital were considered. Data on initially non-resectable patients who received systemic therapy and later underwent surgery were included for analysis.
Results
Between January 2002 and July 2012, 754 liver resections for CLM were performed. A total of 24 patients were found to meet the study inclusion criteria. Bilobar CLM were present in 23 of these 24 patients. The median number of tumours was seven (range: 2–15) and median tumour size was 7.0 cm (range: 1.0–12.8 cm) before systemic therapy. All patients received oxaliplatin-or irinotecan-based chemotherapy. Fourteen patients received combined treatment with bevacizumab. Negative margin (R0) resection was accomplished in 21 of 24 patients. There was no perioperative mortality. Ten patients suffered perioperative morbidity. Eighteen patients suffered recurrence of disease within 9 months. Rates of disease-free survival at 1, 2 and 3 years were 47.6% [95% confidence interval (CI) 30.4–74.6%], 23.8% (95% CI 11.1–51.2%) and 19.0% (95% CI 7.9–46.0%), respectively. Overall survival at 1, 2 and 3 years was 91.5% (95% CI 80.8–100%), 65.3% (95% CI 48.5–88.0%) and 55.2% (95% CI 37.7–80.7%), respectively.
Conclusions
Liver resection in initially unresectable CLM can be performed with low rates of morbidity and mortality in patients who respond to systemic chemotherapy, although these patients do experience a high frequency of disease recurrence.
Introduction
The rationale for the use of local therapies in the management of advanced colorectal cancer derives from the recognition that up to 30% of patients with metastatic colorectal cancer have liver-limited disease.1 Effective local therapy should therefore lead to improved clinical outcomes in at least a subset of these patients. Advances in systemic therapy, with the now routine use of a variety of cytotoxic and biologic therapies, have led to definite improvements in the survival of patients with advanced colorectal cancer.2 However, equivalent benefit has also been attributed to the increased use of hepatic resections for metastatic disease.3 In fact, despite the additional options of systemic therapy now available, the chance of longterm survival for patients with metastatic colorectal cancer remains low, with reported 5-year survival rates of <10% with chemotherapy alone.4 Consequently, in patients with liver-limited stage IV disease, hepatic resection of colorectal cancer liver metastases (CLM) remains the only potentially curative option. Contemporary reports demonstrate that patients with resectable disease demonstrate median overall survival (OS) of 24–42 months, and 3-and 5-year survival rates of 50% and 35%, respectively, following resection of CLM.5,6
Unfortunately, fewer than 25% of patients with CLM are considered to be candidates for surgery at the time of diagnosis as a result of the presence of diffuse bilobar disease, critical vascular involvement and/or the inability to preserve two contiguous anatomic segments at the time of curative liver resection. Patients with CLM who are initially considered non-resectable are candidates for systemic chemotherapy. The goals for these patients refer to the palliation of symptoms and the prolonging of survival, although the chance of longterm survival on systemic therapy alone remains low.4 Nonetheless, the improved tumour control rates realized with these newer regimens of systemic therapy have led to the development of a novel paradigm of neoadjuvant treatment for the purpose of tumour downsizing to the point of surgical cure.
In an 11-year experience reported by Adam et al., 138 of 1104 non-resectable patients underwent operative management for CLM with curative intent,7 having demonstrated an adequate response to chemotherapy. This and other experiences have shown that modern chemotherapy regimens may in fact downsize tumours in up to 12.5% of patients with initially unresectable CLM, rendering them candidates for surgery. These patients demonstrate 5-year OS rates of 33–40%,7–10 but the likelihood of tumour recurrence remains high. The introduction of biologic agents, such as the vascular endothelial growth factor (VEGF) monoclonal antibody, bevacizumab, to oxaliplatin-or irinotecan-based chemotherapy has increased progression-free survival in patients with CLM.11,12 Currently, bevacizumab is used selectively and many patients with initially unresectable CLM are offered first-line treatment with irinotecan-or oxaliplatin-based chemotherapy in combination with bevacizumab.
The aim of this study is to report the results achieved in liver resection following downsizing chemotherapy in patients with initially unresectable CLM, performed at a single major hepatobiliary referral centre.
Materials and methods
All liver resections for CLM performed at Toronto General Hospital (TGH) over a 10-year period from January 2002 to July 2012 were considered. Patients eligible for inclusion in this retrospective analysis comprised those undergoing liver surgery for CLM, who were initially considered to be non-resectable and were given systemic therapy.
For the purposes of the present study, cross-sectional imaging at the time of CLM diagnosis, including computed tomography (CT) and magnetic resonance imaging (MRI) scans, were retrospectively reviewed by two hepatopancreatobiliary (HPB) surgeons unaffiliated with the original case. Criteria for resectability were based on an intent to preserve two contiguous segments of the liver and to ensure an adequate functional liver remnant (FLR) at the time of surgery.
Patient demographics, perioperative details and clinicopathologic factors were collected retrospectively by a trained postgraduate hepatobiliary surgical fellow from paper office charts, hospital electronic records and from a prospective HPB surgical database. Death clearances were performed by the Ontario Cancer Registry to confirm vital status.
This study was approved by the Research Ethics Board, University Health Network, Toronto, Ontario, Canada.
Statistical analysis
Outcomes of interest in this study included perioperative results, disease-free survival (DFS) and OS. Disease-free survival was defined as the time from liver resection to the date of radiographic disease recurrence among patients in whom a negative margin (R0) resection was achieved. Overall survival was defined as the time from hepatic resection to the date of death or last follow-up. Subgroup analyses referred to DFS and OS in patients who received bevacizumab along with oxaliplatin-or irinotecan-based chemotherapy, compared with patients who received chemotherapy only.
Descriptive statistics and frequency tables were used to summarize perioperative results. The Kaplan–Meier method was used to estimate DFS and OS. Outcomes in patients who received bevacizumab along with oxaliplatin-or irinotecan-based chemotherapy were compared with those in patients who received chemotherapy only using the log-rank test and Cox proportional hazard regressions.
Results
From January 2002 to July 2012, a total of 754 liver resections were performed at TGH for the treatment of CLM. All patients underwent resection of the colorectal primary malignancy prior to the resection of liver metastases. In this group, 31 patients (4.1%) were initially considered to be non-resectable either as a result of their tumour burden or for reasons of vascular involvement, but became candidates for surgery after treatment with combination systemic therapy.
An independent review of baseline imaging demonstrated that seven of these 31 patients were likely to have been resectable at presentation; data for these patients were therefore excluded from the analysis. These patients had begun chemotherapy, usually before surgical referral, had been classified as non-resectable by the attending surgeon and had been entered as such in the HPB database.
Patient demographics
The cohort for analysis included 24 patients with a median age of 59.5 years (range: 28–87 years), 20 of whom presented with synchronous disease. A total of 23 patients had diffuse bilobar disease. One patient with unilobar disease was initially considered to be non-resectable as a result of the encasement of the inferior vena cava. The median number of liver metastatic lesions was seven (range: 2–15); the median size of metastatic lesions was 7.0 cm (range: 1.0–12.8 cm). Median serum carcinoembryonic antigen (CEA) was 20.4 μg/l (range: 1.8–690.2 μg/l). Only two patients had extrahepatic metastatic disease at the time of diagnosis. Both of these had pulmonary metastases, which were also considered resectable with curative intent (Table 1).
Table 1.
Patient demographics and tumour characteristics in patients resected for initially unresectable colorectal liver metastases downsized by systemic chemotherapy
| Patients, n | 24 |
| Age, years, median (range) | 59.5 (28–87) |
| Sex, male, n | 12 |
| Primary tumour, n | |
| Colon | 19 |
| Rectum | 5 |
| Number of metastases, median (range) | 7 (2–15) |
| Size of metastases, cm, median (range) | 7.0 (1.0–12.8) |
| Bilobar disease, n | 23 |
| Carcinoembryonic antigen, ng/ml, median (range) | 20.4 (1.8–690.2) |
| Extrahepatic disease, n | |
| Lung | 2 |
Systemic therapy
Details of systemic therapy were available for 21 of the 24 patients (Table 2). All of these patients received chemotherapy based on 5-fluorouracil which included either irinotecan (n = 13) or oxaliplatin (n = 6), or both (n = 2). Of the two patients who received both oxaliplatin and irinotecan, one required two lines of sequential therapy and the other was treated with a combination that included both drugs (IROX). In 14 patients, chemotherapy was combined with bevacizumab, whereas eight patients received cytotoxic therapy alone. Two patients were treated on a placebo-controlled clinical trial which included an anti-angiogenic.
Table 2.
Details of systemic therapy in patients resected for initially unresectable colorectal liver metastases downsized by systemic chemotherapy (n = 24)
| Type of therapy | Patients, n |
|---|---|
| Cytotoxic | |
| 5-fluorouracil | 24 |
| Oxaliplatin | 7 |
| Irinotecan | 13 |
| Oxaliplatin and irinotecan | 2 |
| Unknown | 2 |
| Anti-angiogenic | |
| Bevacizumab | 14 |
| None | 8 |
| Unknown | 2 |
| Treatment on clinical trial | 2 |
| Lines of systemic therapy, n | |
| 1 | 23 |
| 2 | 1 |
Resectability in these 24 patients was rendered after verification of a response to chemotherapy based on tumour downsizing, allowing critical inflow and outflow vascular sparing and the preservation of two contiguous liver anatomic segments in a single-stage or a two-stage approach.
Surgical outcomes
A total of 27 liver resections were performed. These included 21 major hepatectomies (four or more Couinaud segments) and six minor resections (fewer than three Couinaud segments). The median estimated blood loss was 650 ml (range: 200–6800 ml). A curative intent resection was accomplished in 21 patients, including five who required concomitant radiofrequency ablation (RFA).
Preoperative portal vein embolization was required in three patients. A two-stage procedure was planned for five patients; however, two did not complete the second resection as a result of tumour progression in the interval following the first-stage procedure (Table 3). The third patient in whom an R0 resection was not achieved was scheduled for RFA of a remaining lesion after hepatectomy, but experienced tumour progression before RFA was completed.
Table 3.
Surgical outcomes in patients resected for initially unresectable colorectal liver metastases downsized by systemic chemotherapy (n = 24)
| Operative procedure, n | |
| Major hepatic resection (≥4 segments) | 21 |
| Minor hepatic resection (<4 segments) | 6 |
| Total | 27 |
| Curative resection (R0), n | 21/24 |
| Preoperative portal vein embolization, n | 3/24 |
| Two-stage surgery, n | 5/24 |
| Combined radiofrequency ablation, n | 5/24 |
| Estimated blood loss, ml, median (range) | 650 (200–6800) |
| Hospital stay, days, median (range) | 7 (4–53) |
The median hospital stay was 7 days (range: 4–53 days). Perioperative morbidity within 60 days occurred in 10 patients. Complications included three bile leaks (managed with percutaneous drainage), one subcapsular bleed and one pleural effusion (Table 4). There were no reoperations for perioperative morbidity. Perioperative mortality at 60 days was zero.
Table 4.
Postoperative complications in patients resected for initially unresectable colorectal liver metastases downsized by systemic chemotherapy (n = 24)
| Patients, n | |
|---|---|
| Complication grade (Dindo–Clavien) | |
| I | 2 |
| II | 5 |
| IIIa | 3 |
| IIIb | 0 |
| IV | 0 |
| V | 0 |
| Complication type | |
| Bile leak | 3 |
| Subcapsular bleed | 1 |
| Pleural effusion | 1 |
| Pneumonia | 1 |
| Wound infection | 1 |
| Urinary tract infection | 2 |
| Abdominal pain | 1 |
| Total, n | 10/24 |
Survival analysis
Median follow-up was 18.8 months (range: 4–102 months); no patients were lost to follow-up. Median OS was 56.8 months.
Of the 21 patients in whom an R0 resection was achieved, 18 suffered disease recurrence, of which eight died. The median time to recurrence in these R0 patients was 8.9 months (range: 2.0–55.3 months).
Sites of recurrence were liver only (n = 6), lung only (n = 5), liver and lung (n = 5), the peritoneal cavity (peritoneal carcinomatosis) (n = 1) and the mediastinum (n = 1).
Median DFS at 1, 2 and 3 years was 47.6% [95% confidence interval (CI) 30.4–74.6%], 23.8% (95% CI 11.1–51.2%) and 19.0% (95% CI 7.9–46.0%), respectively. Median OS at 1, 2 and 3 years was 91.5% (95% CI 80.8–100%), 65.3% (95% CI 48.5–88.0%), and 55.2% (95% CI 37.7–80.7%), respectively (Figs 1 and 2).
Figure 1.
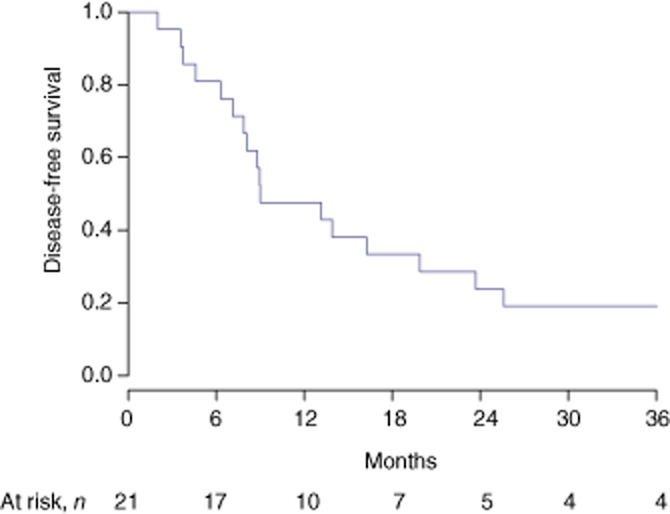
Disease-free survival in patients resected for initially unresectable colorectal liver metastases downsized by systemic chemotherapy
Figure 2.
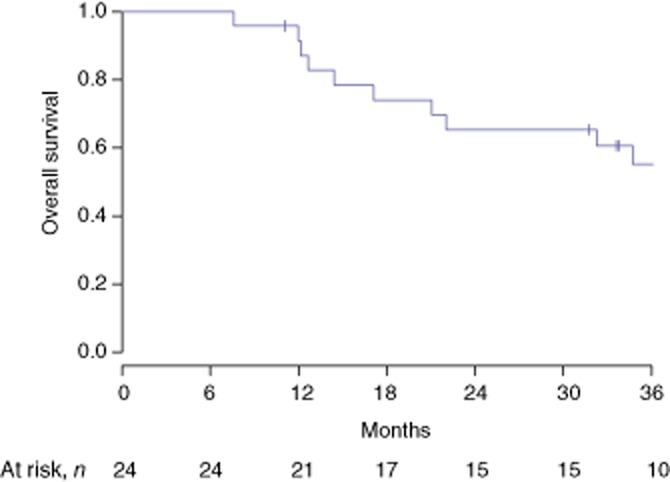
Overall survival in patients resected for initially unresectable colorectal liver metastases downsized by systemic chemotherapy
Subgroup analysis demonstrated differences in outcome between patients treated with a regimen containing bevacizumab and those treated with cytotoxic agents alone. Median DFS in patients treated with bevacizumab was 8.9 months, whereas in those who did not receive the anti-angiogenic agent was 25.6 months [log-rank test, P = 0.025; hazard ratio (HR) 3.6, 95% CI 1.1–12.0; P = 0.033]. There was no significant difference in OS (log-rank test, P = 0.583; HR 1.4, 95% CI 0.4–5.2; P = 0.585).
Discussion
Surgery for CLM now represents the standard of care, providing clinical benefit, longterm survival and a cure in 10–25% of patients who present with resectable disease.13,14 By contrast, the management of patients with unresectable CLM is largely restricted to systemic therapy.
The current study adds to an increasing body of literature supporting the use of ‘rescue surgery’, defined as definitive surgery in patients originally deemed to be non-resectable prior to treatment with chemotherapy. It reports a 10-year clinical experience at a single high-volume centre and demonstrates a median OS of 56.8 months following conversion surgery, with 2-and 3-year survival rates of 65.3% and 55.2%, respectively. These results are consistent with prior reports in similar patient populations using equivalent neoadjuvant strategies.5,6
One clear limitation of this study refers to its lack of an appropriate control group in the form of a cohort of patients with good tumour control, treated with systemic therapy, who did not proceed to definitive surgery. It is therefore difficult to attribute the perceived improvement in survival solely to the ability to complete surgical resection. Historical controls suggest that patients with advanced disease managed with chemotherapy alone have a 3-year OS of <10% and that there are no 5-year survivors.15–18 It is therefore reasonable to extrapolate that, in this and other series, clinical benefit is a partial consequence of the surgical procedure itself and is not solely attributable to a tumour response to systemic therapy that renders a patient operable. In this context, however, any comparison with historical controls should carry the caveat that evolutions in clinical practice with the incorporation of novel (and targeted) therapies in the management of advanced disease and improved supportive care have also contributed to improved outcomes in contemporary patient cohorts.
Despite good rates of OS, DFS was still relatively low (23.8% at 2 years and 19.0% at 3 years), but was, again, comparable with DFS rates established in prior reports, notably by Adam et al., who reported a tumour recurrence rate of 80% and 5-year DFS of 17%.7
The current data parallel previously published outcomes in patients with initially resectable CLM who proceeded straight to surgery. Nanji et al. reported a total of 320 patients with initially resectable CLM who were treated with upfront surgery and displayed median DFS and OS of 19.0 months and 35.1 months, respectively.19 A retrospective study by Nordlinger et al.20 demonstrated recurrent disease in over two-thirds of patients who underwent surgery for resectable CLM. Therefore, these findings suggest that the majority of patients who are submitted to surgery following conversion therapy in whom an R0 resection is achieved are not usually ‘cured’ of disease despite the survival benefit conferred by resection. This supports the notion that CLM should be treated as a ‘chronic’ disease in some cases. Furthermore, data also suggest that patients with unresectable CLM who are rendered candidates for surgery by downsizing chemotherapy can expect a prognosis comparable with that of patients who present with initially resectable CLM, as originally shown by Adam et al.7
The vast majority of patients in the present study in whom R0 resections were achieved experienced disease recurrence, with a median DFS of 8.9 months. These data suggest that even with current approaches and despite the achievement of R0 resections, residual microscopic disease remains and ultimately leads to treatment failure. Further improvement in clinical outcomes will depend on improvements in imaging that facilitate the detection of even smaller metastases and the incorporation of more efficacious systemic therapies. There is ongoing work evaluating the addition of novel targeted agents to chemotherapy in the perioperative management of CLM. Van Cutsem et al. demonstrated the feasibility of this strategy using a combination of FOLFIRI [folinic acid (leucovorin), fluorouracil (5-FU), irinotecan hydrochloride] and the epidermal growth factor receptor (EGFR) monoclonal antibody, cetuximab, and noted improved rates of metastatic resection, but stable clinical outcomes.21 Again, given the heterogeneity of tumour biology and clinical outcomes, prospective studies are required to definitively address these issues.
In the present study, no patients were treated with regimens containing cetuximab or panitumumab as part of downsizing therapy. This is pertinent as both EGFR-targeting antibodies are approved for use in metastatic disease. However, because current government funding restrictions in Ontario, Canada, limit the use of EGFR-directed therapies to the third-line setting after progression on both irinotecan-and oxaliplatin-based regimens, or within the confines of an approved clinical trial, this was not a component of the systemic therapy of patients included in this analysis.
Approximately half of the patients were treated with perioperative bevacizumab, which allowed for the examination of the potential impact of this anti-angiogenic on acute complication rates and DFS. During the study period, bevacizumab was used selectively with irinotecan-based chemotherapy. Although some statistically significant differences were noted, this analysis is limited by power and selection bias. The trend towards inferior clinical outcomes in the bevacizumab-treated cohort may be explained by the omission of anti-angiogenic therapy in patients who were considered to be more likely to be rendered resectable after a short course of systemic therapy and included among patients who were either considered for palliative care only or to have a low likelihood of becoming resectable. Patients who may have been considered less likely to demonstrate downsizing in response to systemic therapy were treated with an optimal regimen of palliative chemotherapy for advanced disease with bevacizumab.
The focus of analysis in this study concerned outcomes in initially non-resectable patients who were converted to resectable status by systemic chemotherapy. Hence, this is a highly selected patient population and therefore it is not possible to comment on the proportion of initially non-resectable patients rendered resectable, or the efficacy of specific chemotherapy regimens in achieving conversion. In addition, the potential contributions of postoperative chemotherapy and systemic therapy for recurrent disease were considered to lie beyond the scope of this study. Nonetheless, these factors are certainly crucial to better characterizing DFS and OS in this patient cohort and remain the focus of ongoing work.
Morbidity rates in this study were acceptable and consistent with those reported previously. There were no perioperative mortalities at 60 days despite the high proportion of major hepatectomies. Furthermore, the majority of perioperative morbidities in this cohort were minor and none required an intensive care unit admission or repeat surgery.
In summary, the results of this study demonstrate that surgical resection following chemotherapy for initially unresectable CLM confers good OS compared with the historical survival of patients treated with chemotherapy alone; however, this does not lead to cure in the majority of patients. Although the present study is limited by the inherent biases of a retrospective design, its findings suggest that initially unresectable CLM treated with systemic chemotherapy followed by surgery with curative intent is feasible and is associated with acceptable rates of perioperative morbidity and mortality. The selection of the most effective chemotherapeutic regimen and potentially suitable candidates for surgery remain challenging and justify the call for future prospective randomized trials, and germline and somatic genetic cataloguing to resolve these key issues.
Conflicts of interest
None declared.
References
- 1.Weiss L, Grundmann E, Torhorst J, Hartveit F, Moberg I, Eder M, et al. Haematogenous metastatic patterns in colonic carcinoma: an analysis of 1541 necropsies. J Pathol. 1986;150:195–203. doi: 10.1002/path.1711500308. [DOI] [PubMed] [Google Scholar]
- 2.Giacchetti S, Itzhaki M, Gruia G, Adam R, Zidani R, Kunstlinger F, et al. Longterm survival of patients with unresectable colorectal cancer liver metastases following infusional chemotherapy with 5-fluorouracil, leucovorin, oxaliplatin and surgery. Ann Oncol. 1999;10:663–669. doi: 10.1023/a:1008347829017. [DOI] [PubMed] [Google Scholar]
- 3.Kopetz S, Chang GJ, Overman MJ, Eng C, Sargent DJ, Larson DW, et al. Improved survival in metastatic colorectal cancer is associated with adoption of hepatic resection and improved chemotherapy. J Clin Oncol. 2009;27:3677–3683. doi: 10.1200/JCO.2008.20.5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferrarotto R, Pathak P, Maru D, Agarwal A, Overman M, Hoff PM, et al. Durable complete responses in metastatic colorectal cancer treated with chemotherapy alone. Clin Colorectal Cancer. 2011;10:178–182. doi: 10.1016/j.clcc.2011.03.023. [DOI] [PubMed] [Google Scholar]
- 5.Fong Y. Surgical therapy of hepatic colorectal metastasis. CA Cancer J Clin. 1999;49:231–255. doi: 10.3322/canjclin.49.4.231. [DOI] [PubMed] [Google Scholar]
- 6.Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230:309–318. doi: 10.1097/00000658-199909000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adam R, Delvart V, Pascal G, Valeanu A, Castaing D, Azoulay D, et al. Rescue surgery for unresectable colorectal liver metastases downstaged by chemotherapy: a model to predict longterm survival. Ann Surg. 2004;240:644–657. doi: 10.1097/01.sla.0000141198.92114.f6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bismuth H, Adam R, Levi F, Farabos C, Waechter F, Castaing D, et al. Resection of non-resectable liver metastases from colorectal cancer after neoadjuvant chemotherapy. Ann Surg. 224:509–520. doi: 10.1097/00000658-199610000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rivoire M, De Cian F, Meeus P, Negrier S, Sebban H, Kaemmerlen P. Combination of neoadjuvant chemotherapy with cryotherapy and surgical resection for the treatment of unresectable liver metastases from colorectal carcinoma. Cancer. 2002;95:2283–2292. doi: 10.1002/cncr.10973. [DOI] [PubMed] [Google Scholar]
- 10.Alberts SR, Horvath WL, Sternfeld WC, Goldberg RM, Mahoney MR, Dakhil SR, et al. Oxaliplatin, fluorouracil, and leucovorin for patients with unresectable liver-only metastases from colorectal cancer: a North Central Cancer Treatment Group phase II study. J Clin Oncol. 2005;23:9243–9249. doi: 10.1200/JCO.2005.07.740. [DOI] [PubMed] [Google Scholar]
- 11.Saltz LB, Clarke S, Diaz-Rubio E, Scheithauer W, Figer A, Wong R, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol. 2008;26:2013–0027. doi: 10.1200/JCO.2007.14.9930. [DOI] [PubMed] [Google Scholar]
- 12.Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 13.Adson MA. Resection of liver metastases – when is it worthwhile? World J Surg. 1987;11:511–520. doi: 10.1007/BF01655817. [DOI] [PubMed] [Google Scholar]
- 14.Doci R, Gennari L, Bignami P, Montalto F, Morabito A, Bozzetti F. One hundred patients with hepatic metastases from colorectal cancer treated by resection: analysis of prognostic determinants. Br J Surg. 1991;78:797–801. doi: 10.1002/bjs.1800780711. [DOI] [PubMed] [Google Scholar]
- 15.Sanoff HK, Sargent DJ, Campbell ME, Morton RF, Fuchs CS, Ramanathan RK, et al. Five-year data and prognostic factor analysis of oxaliplatin and irinotecan combinations for advanced colorectal cancer: N9741. J Clin Oncol. 2008;26:5721–5727. doi: 10.1200/JCO.2008.17.7147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wong R, Cunningham D, Barbachano Y, Saffery C, Valle J, Hickish T, et al. A multicentre study of capecitabine, oxaliplatin plus bevacizumab as perioperative treatment of patients with poor-risk colorectal liver-only metastases not selected for upfront resection. Ann Oncol. 2011;22:2042–2048. doi: 10.1093/annonc/mdq714. [DOI] [PubMed] [Google Scholar]
- 17.Maughan TS, Adams RA, Smith CG, Meade AM, Seymour MT, Wilson RH, et al. Addition of cetuximab to oxaliplatin-based first-line combination chemotherapy for treatment of advanced colorectal cancer: results of the randomized phase 3 MRC COIN trial. Lancet. 2011;377:2103–2114. doi: 10.1016/S0140-6736(11)60613-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Folprecht G, Gruenberger T, Bechstein WO, Raab HR, Lordick F, Hartmann JT, et al. Tumour response and secondary resectability of colorectal liver metastases following neoadjuvant chemotherapy with cetuximab: the CELIM randomized phase 2 trial. Lancet Oncol. 2010;11:38–47. doi: 10.1016/S1470-2045(09)70330-4. [DOI] [PubMed] [Google Scholar]
- 19.Nanji S, Cleary S, Ryan P, Guindi M, Selvarajah S, Al-Ali H, et al. Erratum to: up-front hepatic resection for metastatic colorectal cancer results in favorable long-term survival. Ann Surg Oncol. 2013;20:295–304. doi: 10.1245/s10434-012-2424-1. [DOI] [PubMed] [Google Scholar]
- 20.Nordlinger B, Guiguet M, Vaillant JC, Balladur P, Boudjema K, Bachellier P, et al. Surgical resection of colorectal carcinoma metastases to the liver. A prognostic scoring system to improve case selection, based on 1568 patients. Association Française de Chirurgie. Cancer. 1996;77:1254–1262. [PubMed] [Google Scholar]
- 21.Van Cutsem E, Kohne CH, Hitre E, Zaluski J, Chang Chien CR, Makhson A, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360:1408–1417. doi: 10.1056/NEJMoa0805019. [DOI] [PubMed] [Google Scholar]


