Abstract
Objectives
The aim of this study was to identify factors that predict the failure of a ‘no drain’ policy in laparoscopic hepatectomy.
Methods
Surgical outcomes in 342 consecutive patients undergoing laparoscopic hepatectomy were reviewed. Drains were placed only for the following predefined criteria: (i) intraoperative bile leak; (ii) bilioenteric anastomosis, and (iii) increased risk for postoperative bleeding (‘no drain’ policy). Factors leading to need for postoperative drainage or reoperation were evaluated.
Results
Drains were placed in 44 patients (drainage group). Postoperatively, additional procedures were required in five (11.4%) patients in the drainage group and in 18 (6.0%) patients in the no-drainage group. Multivariate analysis suggested that blood loss of >400 ml [odds ratio (OR) 4.50, 95% confidence interval (CI) 1.41–14.2; P = 0.010] and preoperative chemotherapy (OR = 2.24, 95% CI 0.82–6.48; P = 0.120) may increase the risk for need for postoperative procedures when intraoperative prophylactic drainage is not used.
Conclusions
Prophylactic drainage during liver resection should be considered not only in the presence of uncontrollable bile leak or concern for postoperative bleeding risk, but also in patients who have undergone neoadjuvant chemotherapy and those in whom intraoperative blood loss is >400 ml. Otherwise, a ‘no drain’ policy is safe and would enhance the advantages of minimally invasive liver surgery.
Introduction
The use of prophylactic abdominal drainage in liver resection is a controversial. Traditionally, prophylactic drainage has been advocated for the prevention of postoperative fluid collections, the detection and drainage of bile leak, and the identification of bleeding,1 especially in complicated liver resection.2,3 In addition, in the largest Asian series of liver resection without mortality, routine abdominal drainage was employed.4–6 However, the extent to which the placement of prophylactic drainage contributes to satisfactory surgical outcomes remains unclear.
By contrast, since the late 1990s most Western series have advised against the routine placement of drains in elective liver surgery.7–12 Negative effects of routine abdominal drainage on the incidence of postoperative infection10 and development of ascites7,10 have been demonstrated in open liver resection. Recently, a pooled meta-analysis comparing routine abdominal drainage (n = 234) with no abdominal drainage (n = 231) in elective liver surgery found no difference between the two groups with respect to mortality, intra-abdominal collections requiring reoperation, infected intra-abdominal collections, wound infection, ascitic leak or hospital stay.13 To the present authors' knowledge, however, there have been no reports focusing on the role of prophylactic abdominal drainage in the setting of minimally invasive liver surgery, in which surgeons may be more reluctant to use prophylactic drains than in open hepatectomy. The present study evaluated surgical outcomes of laparoscopic hepatectomy (LH) carried out using a restrictive drain placement strategy in 342 consecutive patients with the aim of identifying clinical factors that predict failure of the ‘no drain’ policy.
Materials and methods
Patients
Subjects included 342 consecutive patients who underwent laparoscopic hepatic resection at the Institute Mutualiste Montsouris, Paris, France between 1995 and 2010. During the study period, 136 patients underwent open hepatic surgery and were excluded from this analysis. The laparoscopic approach was offered to patients without contraindications specific to pneumoperitoneum. Predefined exclusion criteria included closed-angle glaucoma, intracranial hypertension, diffuse bullous emphysema, and contraindications specific to surgical techniques including a need for complex vascular and/or biliary reconstruction. Previous open abdominal surgery was considered to be a relative contraindication for LH because of the complexity of dissection.
Preoperative evaluation and care
Resectability was assessed preoperatively by ultrasonography, computed tomography and/or magnetic resonance imaging. Portal vein embolization or ligation was indicated if the estimated remnant liver volume was <25–30% of total liver volume in patients without cirrhosis or <40% in patients with cirrhosis. Neoadjuvant chemotherapy [infusional fluorouracil, leucovorin and oxaliplatin (FOLFOX) and/or infusional fluorouracil, leucovorin and irinotecan (FOLFIRI)] was administered in patients with colorectal liver metastasis if they had synchronous metastasis, more than three resectable metastases or initially unresectable metastases. In patients with synchronous metastases, hepatic and colorectal resection was considered during the same operation.
Surgical techniques
The specific technical details of LH have been described previously.14–16 Briefly, patients were placed in the low lithotomy position, with the legs spread apart and bent at the knees (French position). Usually, five or six trocars were placed in the right upper quadrant of the abdomen, using one 12-mm port for the laparoscope and another for intraoperative ultrasonography. For LH of Couinaud segments VII and VIII, two trocars were placed through the diaphragm in addition to three trocars placed in the right upper quadrant of the abdomen (the lateral approach).16 Intra-abdominal pressure was maintained at 10–12 mmHg.14
Prior to parenchymal dissection, an umbilical tape was passed around the hepatoduodenal ligament for inflow occlusion, when needed. Liver anatomy and dissection planes were mapped using intraoperative ultrasonography with a flexible laparoscopic probe (BK Medical APS, Herlev, Denmark). The liver parenchyma was then dissected using bipolar forceps (MicroFrance CEV134; Medtronic, Inc., Minneapolis, MN, USA) and ultrasonic shears, usually a SonoSurg (Olympus Co., Tokyo, Japan) or Harmonic Scalpel (Ethicon Endo-Surgery, Inc., Cincinnati, OH, USA). During parenchymal dissection, central venous pressure was kept low and monitored visually by confirming inferior vena cava fluctuation and heartbeat and ventilator data.
‘No drain’ policy
After parenchymal dissection was completed, any areas of obvious bile leakage were addressed with absorbable sutures or clips. Bile leak testing was performed after major hepatectomy by injecting air through a transcystic catheter and evaluating visually for the presence of bubbles. Radiographic cholangiography was not routinely used for this purpose. Criteria indicating the need to deviate from the ‘no drain’ policy were predefined. Closed suction drains were used in the following circumstances: (i) intraoperative bile leak from liver parenchyma or bile ducts; (ii) bilioenteric anastomosis, and (iii) increased risk for postoperative bleeding. Fibrin glue or a carrier-bound fibrin sealant (Tachosil™; Nycomed Austria GmbH, Linz, Austria) was applied on the raw surface for bleeding control in complex hepatic resections.
Postoperatively, patients were followed clinically and liver function tests were assessed. Ultrasonography and other examinations were ordered as indicated. Drains were removed usually on postoperative day (PoD) 3 if the amount and visual inspection of the drainage was not concerning. Fluid collections in the abdominal cavity were drained percutaneously under ultrasound guidance or by reoperation when the fluid collection was symptomatic (abdominal pain, fever of >38 °C and/or white blood cell count of >100 000/μl and rising).
Statistical analysis
Patients' background characteristics and surgery-related factors were analysed retrospectively to identify possible risk factors contributing to the occurrence of postoperative complications that required additional procedures. Categorical data and continuous data [expressed as median (range)] were compared using Fisher's exact test and the Wilcoxon rank sum test, respectively. Differences of statistical significance were indicated by a P-value of <0.05. In a multiple logistic regression analysis, a P-value of <0.20 was set as the cut-off value for elimination. The results were expressed as adjusted odds ratios (ORs) with 95% confidence intervals (CIs), and P-values were calculated using the likelihood ratio test. Calculations were performed using jmp Version 9.0.0 (SAS Institute, Inc., Cary, NC, USA).
Results
Demographic data
The study population included 204 men and 138 women, with a median age of 61 years (range: 18–88 years). Laparoscopic hepatectomy was indicated for malignancy in 273 patients (secondary liver malignancy, n = 228; primary liver malignancy, n = 36; hilar bile duct cancer, n = 8; gallbladder cancer, n = 1) and for benign lesions in the remaining 69 patients (liver cell adenoma, n = 16; focal nodular hyperplasia, n = 15; liver cysts, n = 9; hepatic hamartoma, n = 6; other, n = 23). A total of 24 (7.0%) patients had chronic hepatitis or liver cirrhosis and, of the 147 patients who had undergone neoadjuvant chemotherapy, 38 (25.8%) had steatohepatitis and/or hepatic sinusoidal obstruction based on pathological diagnosis.
The median operating time required for hepatectomy was 195 min (range: 15–515 min). Median blood loss amounted to 150 ml (range: 5–1500 ml). Red blood cell transfusions were required in 26 patients. Conversion to open surgery was required in 18 patients as a result of haemorrhage (n = 8), severe adhesions (n = 4), localization of the tumour (n = 3), necessity for complex bile duct reconstruction (n = 2), and opening of the thoracic cavity (n = 1).
The presence or absence of abdominal drainage and postoperative course
Figure 1 summarizes the postoperative course in the 342 patients with respect to the placement of abdominal drainage at the time of surgery. Abdominal drainage was used in 44 patients (drainage group). The remaining 298 patients did not undergo drain placement at the time of surgery and thus were considered as the ‘no-drainage group’ in this study. The proportions of patients submitted to major hepatectomy (hemi-hepatectomy or extended hepatectomy) were 77.3% (n = 34) in the drainage group and 27.8% (n = 83) in the no-drainage group.
Figure 1.
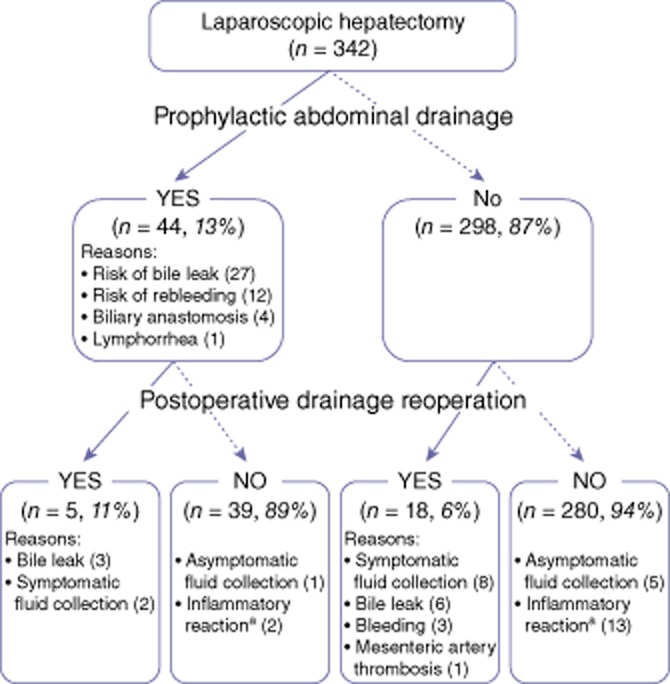
Postoperative course in 342 consecutive patients submitted to laparoscopic hepatectomy who did (n = 44, drainage group) and did not (n = 298, no-drainage group) receive prophylactic drainage. Postoperatively, additional procedures (percutaneous drainage and/or haemostasis/lavage by reoperation) were required in five patients in the drainage group and 18 patients in the no-drainage group. aInflammatory reaction (fever of >38 °C and white blood cell count of >100 000/μl and rising) not associated with abdominal fluid collection
Postoperatively, additional procedures (percutaneous drainage and/or haemostasis/lavage by reoperation) were required in five (11.4%) patients in the drainage group and in 18 (6.0%) patients in the no-drainage group. Overall, 280 of 342 subjects did not require either prophylactic drainage or postoperative procedures. The incidence of postoperative bile leak was 15.9% (n = 7) in the drainage group and 2.0% (n = 6) in the no-drainage group.
Major postoperative complications (grade III or higher according to Clavien–Dindo classification17) occurred in 11 (25.0%) patients in the drainage group and in 24 (8.1%) patients in the no-drainage group (P = 0.002). These included symptomatic fluid collection with or without evidence of bacterial infection (drainage group, n = 1; no-drainage group, n = 9), bile leak (drainage group, n = 7; no-drainage group, n = 6), bleeding (drainage group, n = 0; no-drainage group, n = 3), liver insufficiency (drainage group, n = 1; no-drainage group, n = 2), sepsis (drainage group, n = 1; no-drainage group, n = 1), cerebral infarction (drainage group, n = 1; no-drainage group, n = 1), stenosis of the bile duct (drainage group, n = 0; no-drainage group, n = 1), and mesenteric artery embolism (drainage group, n = 0; no-drainage group, n = 1). Of the 342 subjects, three (0.9%) patients in the no-drainage group died. One of these exsanguinated from the stump of the portal vein as a result of the migration of vascular clips on PoD 6, after hospital discharge. The other patients experienced, respectively, cerebral infarction on PoD 9, and hepatic and renal insufficiency on PoD 43 following severe late-onset cholestasis without intrahepatic biliary dilatation or abnormal hepatic blood flow, probably as a result of chemotherapy-associated steatohepatitis diagnosed by pathological examination. The median postoperative hospital stay was 7 days (range: 2–68 days).
Risk factors for additional postoperative procedures
Background characteristics and surgery-related factors were compared among 298 patients in the no-drainage group, including the 18 patients in whom additional postoperative procedures were required, and the remaining 280 patients who did not require intervention (Table 1). Five potential risk factors for which univariate analysis showed P-values of <0.20 [i.e. preoperative chemotherapy, location and extent of resection, amount of blood loss (>400 ml or <400 ml), operation time (>300 min or <300 min)], were included in multivariate analysis. Blood loss of >400 ml (n = 47; OR = 4.50, 95% CI 1.41–14.2; P = 0.010) and preoperative chemotherapy (n = 118; OR = 2.24, 95% CI 0.82–6.48; P = 0.120) were estimated to increase the risk for need for additional procedures postoperatively when prophylactic abdominal drainage was not used at the time of surgery (Table 2).
Table 1.
Risk factors for postoperative drainage procedures to treat complications associated with liver resection in 298 patients submitted to laparoscopic hepatectomy without prophylactic abdominal drain placement (no-drainage group)
| Postoperative interventions | P-value | ||
|---|---|---|---|
| Yes | No | ||
| Age, years, median (range) | 63 (32–79) | 62 (18–89) | 0.571 |
| Hepatitis or cirrhosis, present/absent, n | 1/17 | 19/261 | >0.999 |
| Preoperative chemotherapy, yes/no, n | 11/7 | 107/173 | 0.080 |
| History of previous hepatectomy, yes/no, n | 1/17 | 40/240 | 0.484 |
| Simultaneous colorectal resection, yes/no, n | 3/15 | 28/252 | 0.415 |
| Extent of hepatectomy, hemiliver or extended/lessa, n | 8/10 | 75/205 | 0.112 |
| Number of hepatectomy, multiple/single, n | 2/16 | 49/231 | 0.748 |
| Location of resection, superior or dorsal regionb/other, n | 13/5 | 123/157 | 0.027 |
| Distribution of resection, bilobar/unilobar, n | 4/14 | 35/245 | 0.271 |
| Operation time, min, median (range) | 210 (120–420) | 180 (15–515) | 0.037 |
| Amount of blood loss, ml, median (range) | 400 (100–1300) | 100 (5–1500) | 0.001 |
Including central bisectorectomy (segments IV, V and VIII, n = 1), anatomic bisegmentectomy (n = 14), segmentectomy (n = 35), subsegmentectomy (n = 10), left lobectomy (segments II and III, n = 24), and non-anatomic partial resection (n = 121).
Liver resections associated with segments I, VII, VIII and/or the superior region of segment IV (subsegment IVa).
Table 2.
Multivariate analysis to identify risk factors for postoperative intervention in patients submitted to laparoscopic hepatectomy
| Variable | OR | 95% CI | P-value |
|---|---|---|---|
| Amount of blood loss, >400 ml | 4.50 | 1.41–14.2 | 0.010 |
| Preoperative chemotherapy, yes | 2.24 | 0.82–6.48 | 0.120 |
| Location of resection, superior or dorsal regiona | 2.05 | 0.51–8.10 | 0.295 |
| Operation time, >300 min | 1.38 | 0.36–4.81 | 0.626 |
| Extent of hepatectomy, hemi-hepatectomy or more | 1.33 | 0.35–4.90 | 0.667 |
Liver resections associated with Couinaud segments I, VII, VIII and/or the superior region of segment IV (subsegment IVa).
OR, odds ratio; 95% CI, 95% confidence interval.
In patients with intraoperative blood loss of >400 ml (n = 7), the leading causes of additional postoperative procedures were bleeding (n = 3) and symptomatic fluid collection (n = 3), whereas symptomatic fluid collection (n = 6) was the most prevalent indication for postoperative procedures among patients submitted to preoperative chemotherapy (n = 11).
Discussion
In this series of LH utilizing a ‘no drain’ policy, 87.1% of all treated patients did not undergo prophylactic drain placement (the no-drainage group); the remaining 12.9% of patients were drained during surgery because of bile leakage (7.9%), concern for the adequacy of haemostasis (3.5%), biliary anastomosis (1.2%) and lymphorrhea (0.3%). Overall, 94.0% of patients in the no-drainage group did not require postoperative drainage procedures (percutaneous drainage and/or reoperation) to treat complications specific to the liver resection. The percentages of patients who required postoperative procedures did not differ significantly between the drainage group (11.4%) and the no-drainage group (6.0%). Surgical outcomes and the proportion of patients who required postoperative procedures in this series are comparable with those established in previous randomized controlled trials of drain placement in open surgery,7,8,10–12 as well as in a large case series1 describing the routine use of prophylactic drains (Table 3). Although this is a retrospective cohort study, the current results support the validity of a ‘no drain’ policy [i.e. the restrictive use of prophylactic drainage only if there is a perceived clinically significant risk for postoperative bile leak (intraoperative bile leakage or biliary anastomosis/suture) or concern for the adequacy of haemostasis prior to abdominal closure].
Table 3.
Surgical outcomes after liver resection in patients with prophylactic drain placement and in those without intraoperative drainage
| Arm (n) | Bile leak, % | Percutaneous drainage, % | Reoperation, % | Mortality, % | Exclusion criteria for no-drainage group | HCC, % | Hemi-or extended hepatectomy, % | |
|---|---|---|---|---|---|---|---|---|
| Belghiti et al.7a | Drain (42) | 4.8% | 35.7% | 2.4% | 2.4% | Biliary enteric anastomosis, gastrointestinal procedure, total hepatic vascular exclusion, ex situ perfused liver, devitalized hepatic stump, bile duct injury | 26% | 30% |
| No drain (39) | 5.1% | 15.8% | 2.5% | 2.6% | ||||
| Fong et al.8a | Drain (60) | 5.0% | 8.3% | 1.6% | 3.3% | Biliary enteric anastomosis, thoracoabdominal incision, preoperative biliary stent | 14% | 73% |
| No drain (60) | 5.0% | 18.3% | 0% | 3.3% | ||||
| Fuster et al.11a | Drain (20) | 0% | 0% | 0% | 0% | Major liver resection or tumour ≥ 5 cm | 100% | 0% |
| No drain (20) | 0% | 10.0% | 5.0% | 0% | ||||
| Liu et al.10a | Drain (52) | 3.8% | 3.8% | 5.8% | 5.8% | Biliary enteric anastomosis | 96% | 60% |
| No drain (52) | 0% | 0% | 1.9% | 1.9% | ||||
| Sun et al.12a | Drain (60) | 0% | 5.0% | 2.0% | 0% | Biliary anastomosis, operation on other organ, infection | 72% | 41% |
| No drain (60) | 0% | 2.0% | 0% | 2.0% | ||||
| Burt et al.9b | Drain (184) | – | 26.1% | – | 7.1% | Thoracotomy, uncontrollable bile leak, biliary anastomosis, abdominal infection | 9% | 58% |
| No drain (981) | – | 10.5% | – | 2.0% | ||||
| Kyoden et al.1c | Drain (1269) | 8.7% | 2.0% | 1.6% | 0.07% | (Routine drainage) | 71% | 13% |
| Present studyd | Drain (44) | 16.0% | 9.1% | 4.6% | 0% | Uncontrollable bile leak, biliary anastomosis, concern for postoperative bleeding risk | 10% | 34% |
| No drain (298) | 2.0% | 5.4% | 2.4% | 1.0% |
Randomized controlled trial.
Retrospective case series of open liver resection with ‘no drain’ policy.
Retrospective case series of open liver resection with routine prophylactic drainage.
Retrospective case series of laparoscopic liver resection with ‘no drain’ policy.
HCC, hepatocellular carcinoma.
Although a recent systematic review indicates a paucity of evidence to support routine drainage after uncomplicated liver resection,13 the definition of ‘uncomplicated liver resection’ remains vague. In previous studies, biliary anastomosis has been regarded as the major clinical factor indicating the need for prophylactic drainage (Table 3).7–12 In the present study, prophylactic drains were placed in patients with intraoperative bile leak or biliary anastomosis or suturing. As a result, the incidence of postoperative bile leakage was higher in the drainage group (15.9%) than in the no-drainage group (2.0%), which suggests that the clinical stratification of patients according to risk for postoperative bile leak is effective. By contrast, 6.0% of patients in the no-drainage group required postoperative drainage procedures, indicating that additional criteria for placing prophylactic drains may be required in order to capture all patients who should receive a drain at the time of surgery.
In this study, multivariate analysis revealed that operative blood loss of >400 ml independently increased the risk for requiring postoperative additional procedures. Excessive intraoperative blood loss is a strong indicator of postoperative bleeding risk, and may also trigger postoperative ascitic fluid accumulation through water and sodium retention following a decrease in effective arterial blood volume18 and blood transfusion. This is demonstrated herein, as well as in a previous study.19 Based on confidence intervals in the multivariate analysis, preoperative chemotherapy was also estimated to be a risk factor for need for postoperative procedures. In patients who underwent preoperative chemotherapy, the leading cause of need for postoperative intervention was fluid collection with or without evidence of bacterial infection. This may be related to chemotherapy-associated liver injury.20 Although the mechanism by which this arises remains unclear, previous studies suggest an association with sinusoidal obstruction syndrome21 and steatosis or steatohepatitis22–24 as a result of preoperative chemotherapy, leading to an increased incidence of postoperative complications including bile leak and ascitic fluid retention, which may require drainage.
In the present series, all of the three postoperative deaths occurred in the no-drainage group. However, two of these patients died of cerebral infarction and postoperative liver insufficiency, respectively, which were not directly related to the presence or absence of prophylactic drains. The remaining patient died of exsanguination related to the failure of vascular clips at the stump of the right portal vein branch, just after hospital discharge on PoD 6. As abdominal drains are typically removed when effluent is minimal and does not indicate concern for bile leak or bleeding, normally on PoD 2 or 3, this postoperative death could not have been avoided even if a prophylactic drain had been placed.
In line with the available literature on restricted drain placement,7–12 the current study suggests that indications for the placement of prophylactic drains in liver resection may be similar in both laparoscopic and open surgery (Table 3). Nevertheless, the present study is limited by the fact that only a small proportion of the study population had chronic hepatitis or cirrhosis (7.0%) because the incidence of postoperative complications is potentially higher in these patients than in those with normal liver function.6 With regard to liver cirrhosis, the use of prophylactic drains with appropriate fluid and diuretic therapy may be an effective strategy by which to avoid the occurrence of massive postoperative ascites, which may lead to liver failure.19
In conclusion, the present data support the claim that there is no need for routine drainage in hepatic resection unless there is a clinical risk for bile leak (indicated by bile leakage at the completion of the operation or requirement for a biliary anastomosis or suturing) or concern for the adequacy of haemostasis. More specifically, the data suggest that the ‘no drain’ policy can be safely applied in LH and will enhance the advantages of minimally invasive surgery, unless intraoperative blood loss is >400 ml and the patient has undergone neoadjuvant chemotherapy.
Conflicts of interest
None declared.
Funding
This work was supported by grants from the Takeda Science Foundation (to TI), the Kanae Foundation for the Promotion of Medical Science (to TI), the Phillipe Foundation (to NBZ), the Canon Foundation in Europe, and the Ministry of Education, Culture, Sports, Science and Technology of Japan (to TI, no. 23689060).
References
- 1.Kyoden Y, Imamura H, Sano K, Beck Y, Sugawara Y, Kokudo N, et al. Value of prophylactic abdominal drainage in 1269 consecutive cases of elective liver resection. J Hepatobiliary Pancreat Sci. 2010;17:186–192. doi: 10.1007/s00534-009-0161-z. [DOI] [PubMed] [Google Scholar]
- 2.Bismuth H, Castaing D, Garden OJ. Major hepatic resection under total vascular exclusion. Ann Surg. 1989;210:13–19. doi: 10.1097/00000658-198907000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bona S, Gavelli A, Huguet C. The role of abdominal drainage after major hepatic resection. Am J Surg. 1994;167:593–595. doi: 10.1016/0002-9610(94)90104-x. [DOI] [PubMed] [Google Scholar]
- 4.Fan ST, Lo CM, Liu CL, Lam CM, Yuen WK, Yeung C, et al. Hepatectomy for hepatocellular carcinoma: toward zero hospital deaths. Ann Surg. 1999;229:322–330. doi: 10.1097/00000658-199903000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu CC, Yeh DC, Lin MC, Liu TJ, Peng FK. Improving operative safety for cirrhotic liver resection. Br J Surg. 2001;88:210–215. doi: 10.1046/j.1365-2168.2001.01653.x. [DOI] [PubMed] [Google Scholar]
- 6.Imamura H, Seyama Y, Kokudo N, Maema A, Sugawara Y, Sano K, et al. One thousand forty-six hepatectomies without mortality in 8 years. Arch Surg. 2003;138:1198–1206. doi: 10.1001/archsurg.138.11.1198. [DOI] [PubMed] [Google Scholar]
- 7.Belghiti J, Kabbej M, Sauvanet A, Vilgrain V, Panis Y, Fekete F. Drainage after elective hepatic resection. A randomized trial. Ann Surg. 218:748–753. doi: 10.1097/00000658-199312000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fong Y, Brennan MF, Brown K, Heffernan N, Blumgart LH. Drainage is unnecessary after elective liver resection. Am J Surg. 1996;171:158–162. doi: 10.1016/s0002-9610(99)80092-0. [DOI] [PubMed] [Google Scholar]
- 9.Burt BM, Brown K, Jarnagin W, DeMatteo R, Blumgart LH, Fong Y. An audit of results of a no-drainage practice policy after hepatectomy. Am J Surg. 2002;184:441–445. doi: 10.1016/s0002-9610(02)00998-4. [DOI] [PubMed] [Google Scholar]
- 10.Liu CL, Fan ST, Lo CM, Wong Y, Ng IO, Lam CM. Abdominal drainage after hepatic resection is contraindicated in patients with chronic liver diseases. Ann Surg. 2004;239:194–201. doi: 10.1097/01.sla.0000109153.71725.8c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fuster J, Llovet JM, Garcia-Valdecasas JC, Grande L, Fondevila C, Vilana R, et al. Abdominal drainage after liver resection for hepatocellular carcinoma in cirrhotic patients: a randomized controlled study. Hepatogastroenterology. 2004;51:536–540. [PubMed] [Google Scholar]
- 12.Sun HC, Qin LX, Lu L, Wang L, Ye QH, Ren N. Randomized clinical trial of the effects of abdominal drainage after elective hepatectomy using the crushing clamp method. Br J Surg. 2006;93:422–426. doi: 10.1002/bjs.5260. [DOI] [PubMed] [Google Scholar]
- 13.Gurusamy KS, Samraj K, Davidson BR. Routine abdominal drainage for uncomplicated liver resection. Cochrane Database Syst Rev. 2007;(18) doi: 10.1002/14651858.CD006232.pub2. CD006232. [DOI] [PubMed] [Google Scholar]
- 14.Vibert E, Perniceni T, Levard H, Denet C, Shahri NK, Gayet B. Laparoscopic liver resection. Br J Surg. 2006;93:67–72. doi: 10.1002/bjs.5150. [DOI] [PubMed] [Google Scholar]
- 15.Gayet B, Cavaliere D, Vibert E, Perniceni T, Levard H, Denet C, et al. Totally laparoscopic right hepatectomy. Am J Surg. 2007;194:685–689. doi: 10.1016/j.amjsurg.2006.11.044. [DOI] [PubMed] [Google Scholar]
- 16.Ishizawa T, Gumbs AA, Kokudo N, Gayet B. Laparoscopic segmentectomy of the liver: from segment I to VIII. Ann Surg. 2012;256:959–964. doi: 10.1097/SLA.0b013e31825ffed3. [DOI] [PubMed] [Google Scholar]
- 17.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ginès P, Cardenas A, Arroyo V, Rodés J. Management of cirrhosis and ascites. N Engl J Med. 2004;350:1646–1654. doi: 10.1056/NEJMra035021. [DOI] [PubMed] [Google Scholar]
- 19.Ishizawa T, Hasegawa K, Kokudo N, Sano K, Imamura H, Beck Y, et al. Risk factors and management of ascites after liver resection to treat hepatocellular carcinoma. Arch Surg. 2009;144:46–51. doi: 10.1001/archsurg.2008.511. [DOI] [PubMed] [Google Scholar]
- 20.Nordlinger B, Benoist S. Benefits and risks of neoadjuvant therapy for liver metastases. J Clin Oncol. 2006;24:4954–4955. doi: 10.1200/JCO.2006.07.9244. [DOI] [PubMed] [Google Scholar]
- 21.Karoui M, Penna C, Amin-Hashem M, Mitry E, Benoist S, Franc B, et al. Influence of preoperative chemotherapy on the risk of major hepatectomy for colorectal liver metastases. Ann Surg. 2006;243:1–7. doi: 10.1097/01.sla.0000193603.26265.c3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vauthey JN, Pawlik TM, Ribero D, Wu TT, Zorzi D, Hoff PM, et al. Chemotherapy regimen predicts steatohepatitis and an increase in 90-day mortality after surgery for hepatic colorectal metastases. J Clin Oncol. 2006;24:2065–2072. doi: 10.1200/JCO.2005.05.3074. [DOI] [PubMed] [Google Scholar]
- 23.Gomez D, Malik HZ, Bonney GK, Wong V, Toogood GJ, Lodge JP, et al. Steatosis predicts postoperative morbidity following hepatic resection for colorectal metastasis. Br J Surg. 2007;94:1395–1402. doi: 10.1002/bjs.5820. [DOI] [PubMed] [Google Scholar]
- 24.McCormack L, Petrowsky H, Jochum W, Furrer K, Clavien PA. Hepatic steatosis is a risk factor for postoperative complications after major hepatectomy: a matched case–control study. Ann Surg. 2007;245:923–930. doi: 10.1097/01.sla.0000251747.80025.b7. [DOI] [PMC free article] [PubMed] [Google Scholar]


