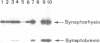Abstract
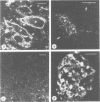
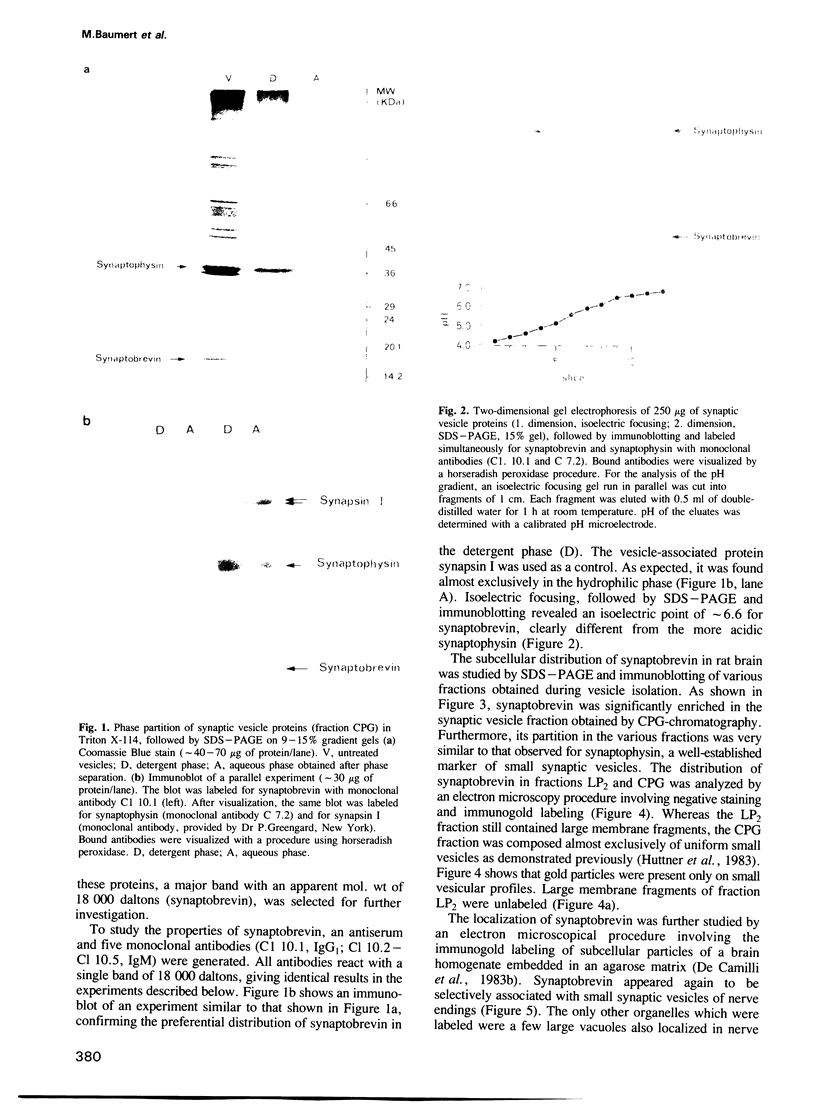
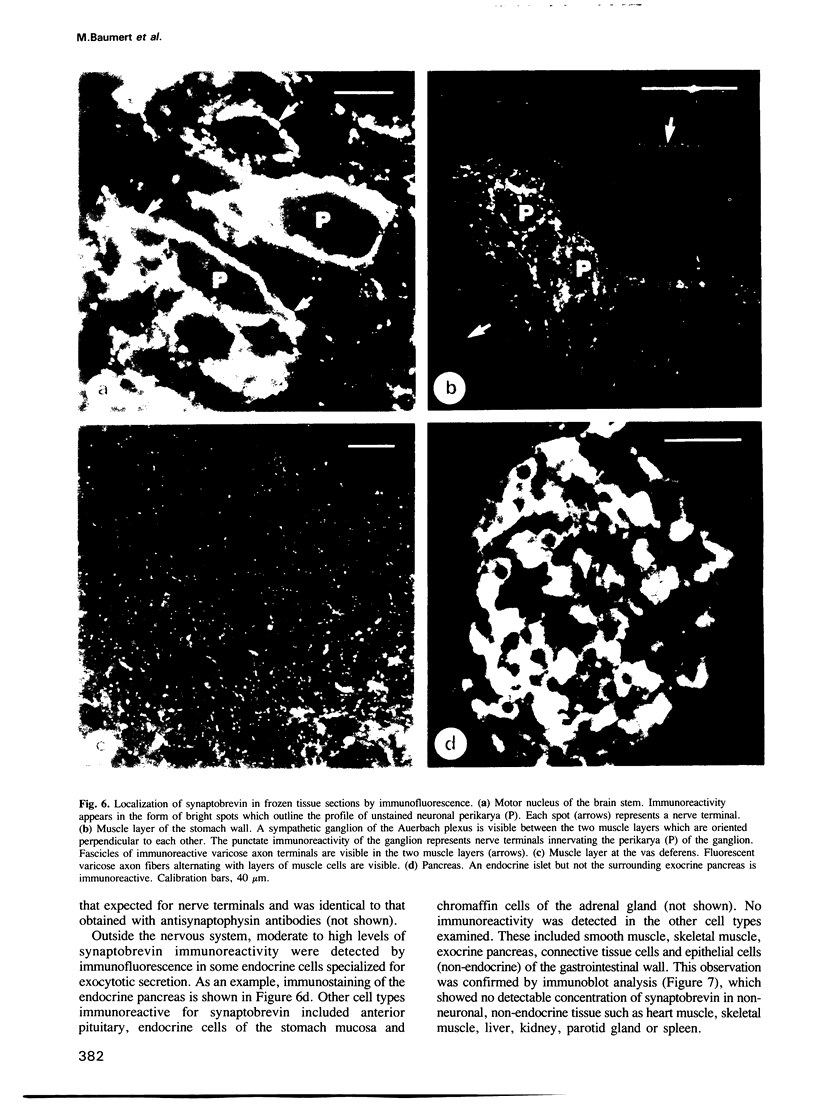
A protein with an apparent mol. wt of 18,000 daltons (synaptobrevin) was identified in synaptic vesicles from rat brain. Some of its properties were studied using monoclonal and polyclonal antibodies. Synaptobrevin is an integral membrane protein with an isoelectric point of approximately 6.6. During subcellular fractionation, synaptobrevin followed the distribution of small synaptic vesicles, with the highest enrichment in the purified vesicle fraction. Immunogold electron microscopy of subcellular particles revealed that synaptobrevin is localized in nerve endings where it is concentrated in the membranes of virtually all small synaptic vesicles. No significant labeling was observed on the membranes of peptide-containing large dense core vesicles. In agreement with these results, synaptobrevin immunoreactivity has a widespread distribution in nerve terminal-containing regions of the central and peripheral nervous system as shown by light microscopy immunocytochemistry. Outside the nervous system, synaptobrevin immunoreactivity was found in endocrine cells and cell lines (endocrine pancreas, adrenal medulla, PC12 cells, insulinoma cells) but not in other cell types, for example smooth muscle, skeletal muscle and exocrine pancreas. Thus, the distribution of synaptobrevin is similar to that of synaptophysin, a well-characterized membrane protein of small vesicles in neurons and endocrine cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bordier C. Phase separation of integral membrane proteins in Triton X-114 solution. J Biol Chem. 1981 Feb 25;256(4):1604–1607. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Browning M. D., Huang C. K., Greengard P. Similarities between protein IIIa and protein IIIb, two prominent synaptic vesicle-associated phosphoproteins. J Neurosci. 1987 Mar;7(3):847–853. doi: 10.1523/JNEUROSCI.07-03-00847.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley K. M., Floor E., Kelly R. B. Cloning and sequence analysis of cDNA encoding p38, a major synaptic vesicle protein. J Cell Biol. 1987 Dec;105(6 Pt 1):2447–2456. doi: 10.1083/jcb.105.6.2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley K., Kelly R. B. Identification of a transmembrane glycoprotein specific for secretory vesicles of neural and endocrine cells. J Cell Biol. 1985 Apr;100(4):1284–1294. doi: 10.1083/jcb.100.4.1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceccarelli B., Hurlbut W. P. Vesicle hypothesis of the release of quanta of acetylcholine. Physiol Rev. 1980 Apr;60(2):396–441. doi: 10.1152/physrev.1980.60.2.396. [DOI] [PubMed] [Google Scholar]
- Czernik A. J., Pang D. T., Greengard P. Amino acid sequences surrounding the cAMP-dependent and calcium/calmodulin-dependent phosphorylation sites in rat and bovine synapsin I. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7518–7522. doi: 10.1073/pnas.84.21.7518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Camilli P., Cameron R., Greengard P. Synapsin I (protein I), a nerve terminal-specific phosphoprotein. I. Its general distribution in synapses of the central and peripheral nervous system demonstrated by immunofluorescence in frozen and plastic sections. J Cell Biol. 1983 May;96(5):1337–1354. doi: 10.1083/jcb.96.5.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Camilli P., Greengard P. Synapsin I: a synaptic vesicle-associated neuronal phosphoprotein. Biochem Pharmacol. 1986 Dec 15;35(24):4349–4357. doi: 10.1016/0006-2952(86)90747-1. [DOI] [PubMed] [Google Scholar]
- De Camilli P., Harris S. M., Jr, Huttner W. B., Greengard P. Synapsin I (Protein I), a nerve terminal-specific phosphoprotein. II. Its specific association with synaptic vesicles demonstrated by immunocytochemistry in agarose-embedded synaptosomes. J Cell Biol. 1983 May;96(5):1355–1373. doi: 10.1083/jcb.96.5.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fykse E. M., Fonnum F. Uptake of gamma-aminobutyric acid by a synaptic vesicle fraction isolated from rat brain. J Neurochem. 1988 Apr;50(4):1237–1242. doi: 10.1111/j.1471-4159.1988.tb10599.x. [DOI] [PubMed] [Google Scholar]
- Hager D. A., Burgess R. R. Elution of proteins from sodium dodecyl sulfate-polyacrylamide gels, removal of sodium dodecyl sulfate, and renaturation of enzymatic activity: results with sigma subunit of Escherichia coli RNA polymerase, wheat germ DNA topoisomerase, and other enzymes. Anal Biochem. 1980 Nov 15;109(1):76–86. doi: 10.1016/0003-2697(80)90013-5. [DOI] [PubMed] [Google Scholar]
- Hell J. W., Maycox P. R., Stadler H., Jahn R. Uptake of GABA by rat brain synaptic vesicles isolated by a new procedure. EMBO J. 1988 Oct;7(10):3023–3029. doi: 10.1002/j.1460-2075.1988.tb03166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper J. E., Kelley R. B. Calcium-dependent calmodulin binding to cholinergic synaptic vesicles. J Biol Chem. 1984 Jan 10;259(1):141–147. [PubMed] [Google Scholar]
- Huttner W. B., Schiebler W., Greengard P., De Camilli P. Synapsin I (protein I), a nerve terminal-specific phosphoprotein. III. Its association with synaptic vesicles studied in a highly purified synaptic vesicle preparation. J Cell Biol. 1983 May;96(5):1374–1388. doi: 10.1083/jcb.96.5.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn R., Schiebler W., Ouimet C., Greengard P. A 38,000-dalton membrane protein (p38) present in synaptic vesicles. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4137–4141. doi: 10.1073/pnas.82.12.4137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanner B. I., Schuldiner S. Mechanism of transport and storage of neurotransmitters. CRC Crit Rev Biochem. 1987;22(1):1–38. doi: 10.3109/10409238709082546. [DOI] [PubMed] [Google Scholar]
- Kelly R. B. The cell biology of the nerve terminal. Neuron. 1988 Aug;1(6):431–438. doi: 10.1016/0896-6273(88)90174-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leube R. E., Kaiser P., Seiter A., Zimbelmann R., Franke W. W., Rehm H., Knaus P., Prior P., Betz H., Reinke H. Synaptophysin: molecular organization and mRNA expression as determined from cloned cDNA. EMBO J. 1987 Nov;6(11):3261–3268. doi: 10.1002/j.1460-2075.1987.tb02644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthew W. D., Tsavaler L., Reichardt L. F. Identification of a synaptic vesicle-specific membrane protein with a wide distribution in neuronal and neurosecretory tissue. J Cell Biol. 1981 Oct;91(1):257–269. doi: 10.1083/jcb.91.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maycox P. R., Deckwerth T., Hell J. W., Jahn R. Glutamate uptake by brain synaptic vesicles. Energy dependence of transport and functional reconstitution in proteoliposomes. J Biol Chem. 1988 Oct 25;263(30):15423–15428. [PubMed] [Google Scholar]
- McCaffery C. A., DeGennaro L. J. Determination and analysis of the primary structure of the nerve terminal specific phosphoprotein, synapsin I. EMBO J. 1986 Dec 1;5(12):3167–3173. doi: 10.1002/j.1460-2075.1986.tb04625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy A., Baker R. R., Morris S. J., Whittaker V. P. The preparation and characterization of synaptic vesicles of high purity. Brain Res. 1976 Jun 11;109(2):285–309. doi: 10.1016/0006-8993(76)90531-x. [DOI] [PubMed] [Google Scholar]
- Navone F., Jahn R., Di Gioia G., Stukenbrok H., Greengard P., De Camilli P. Protein p38: an integral membrane protein specific for small vesicles of neurons and neuroendocrine cells. J Cell Biol. 1986 Dec;103(6 Pt 1):2511–2527. doi: 10.1083/jcb.103.6.2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelles L. P., Bamburg J. R. Rapid visualization of protein--dodecyl sulfate complexes in polyacrylamide gels. Anal Biochem. 1976 Jun;73(2):522–531. doi: 10.1016/0003-2697(76)90202-5. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Obata K., Kojima N., Nishiye H., Inoue H., Shirao T., Fujita S. C., Uchizono K. Four synaptic vesicle-specific proteins: identification by monoclonal antibodies and distribution in the nervous tissue and the adrenal medulla. Brain Res. 1987 Feb 24;404(1-2):169–179. doi: 10.1016/0006-8993(87)91368-0. [DOI] [PubMed] [Google Scholar]
- Olmsted J. B. Analysis of cytoskeletal structures using blot-purified monospecific antibodies. Methods Enzymol. 1986;134:467–472. doi: 10.1016/0076-6879(86)34112-0. [DOI] [PubMed] [Google Scholar]
- Parsons S. M., Bahr B. A., Gracz L. M., Kaufman R., Kornreich W. D., Nilsson L., Rogers G. A. Acetylcholine transport: fundamental properties and effects of pharmacologic agents. Ann N Y Acad Sci. 1987;493:220–233. doi: 10.1111/j.1749-6632.1987.tb27203.x. [DOI] [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Rahamimoff R., DeRiemer S. A., Sakmann B., Stadler H., Yakir N. Ion channels in synaptic vesicles from Torpedo electric organ. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5310–5314. doi: 10.1073/pnas.85.14.5310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehm H., Wiedenmann B., Betz H. Molecular characterization of synaptophysin, a major calcium-binding protein of the synaptic vesicle membrane. EMBO J. 1986 Mar;5(3):535–541. doi: 10.1002/j.1460-2075.1986.tb04243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Südhof T. C., Lottspeich F., Greengard P., Mehl E., Jahn R. A synaptic vesicle protein with a novel cytoplasmic domain and four transmembrane regions. Science. 1987 Nov 20;238(4830):1142–1144. doi: 10.1126/science.3120313. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimble W. S., Cowan D. M., Scheller R. H. VAMP-1: a synaptic vesicle-associated integral membrane protein. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4538–4542. doi: 10.1073/pnas.85.12.4538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittaker V. P. The structure and function of cholinergic synaptic vesicles. The Third Thudichum Lecture. Biochem Soc Trans. 1984 Aug;12(4):561–576. doi: 10.1042/bst0120561. [DOI] [PubMed] [Google Scholar]
- Wiedenmann B., Franke W. W. Identification and localization of synaptophysin, an integral membrane glycoprotein of Mr 38,000 characteristic of presynaptic vesicles. Cell. 1985 Jul;41(3):1017–1028. doi: 10.1016/s0092-8674(85)80082-9. [DOI] [PubMed] [Google Scholar]
- de Camilli P., Navone F. Regulated secretory pathways of neurons and their relation to the regulated secretory pathway of endocrine cells. Ann N Y Acad Sci. 1987;493:461–479. doi: 10.1111/j.1749-6632.1987.tb27231.x. [DOI] [PubMed] [Google Scholar]