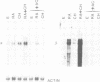
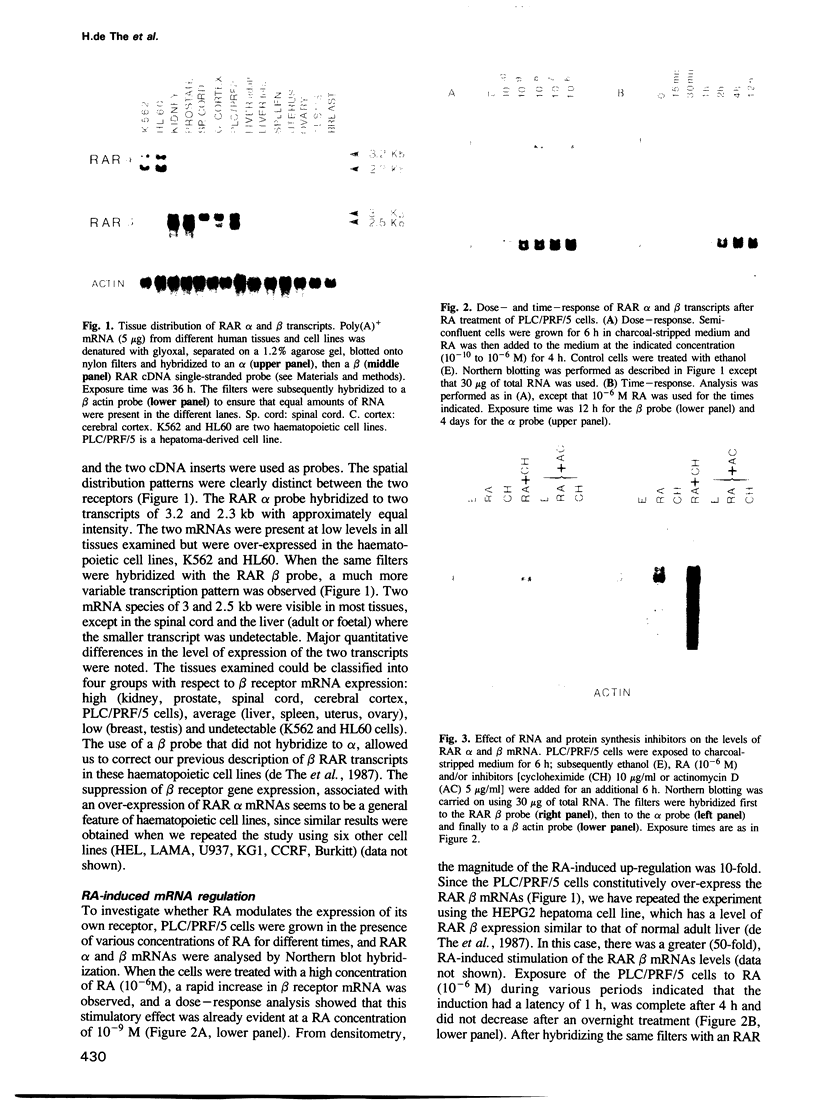
Abstract
Retinoic acid (RA) is a vitamin A derivative that exhibits major effects on biological processes such as cell differentiation and embryo pattern formation. Two human retinoic acid receptors (RAR alpha and beta) have been recently characterized. These receptors are encoded by two genes and their affinities for RA differ, suggesting that these two nuclear receptors may have distinct roles in mediating the varied biological effects of RA. Here we show that RAR alpha and beta differ in the regulation of expression of their mRNAs. Different levels of RAR alpha and beta transcripts were found in the various human tissues analysed. In addition, treatment of human hepatoma cells with RA leads to a rapid 10- to 50-fold increase in RAR beta mRNA levels, whereas RAR alpha mRNA expression is not affected. The induction of RAR beta transcription does not require de novo protein synthesis but is completely abolished by inhibitors of RNA synthesis. Nuclear transcript elongation assays indicate that the mechanism of RAR beta mRNA induction lies at the transcriptional level. These data demonstrate that the RAR beta gene is a primary target for RA. The differences in regulation of RAR gene expression might be a fundamental aspect of retinoid physiology and may prove especially important in the analysis of the morphogenic properties of RA.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brand N., Petkovich M., Krust A., Chambon P., de Thé H., Marchio A., Tiollais P., Dejean A. Identification of a second human retinoic acid receptor. Nature. 1988 Apr 28;332(6167):850–853. doi: 10.1038/332850a0. [DOI] [PubMed] [Google Scholar]
- Breitman T. R., Selonick S. E., Collins S. J. Induction of differentiation of the human promyelocytic leukemia cell line (HL-60) by retinoic acid. Proc Natl Acad Sci U S A. 1980 May;77(5):2936–2940. doi: 10.1073/pnas.77.5.2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies P. J., Murtaugh M. P., Moore W. T., Jr, Johnson G. S., Lucas D. Retinoic acid-induced expression of tissue transglutaminase in human promyelocytic leukemia (HL-60) cells. J Biol Chem. 1985 Apr 25;260(8):5166–5174. [PubMed] [Google Scholar]
- Dejean A., Bougueleret L., Grzeschik K. H., Tiollais P. Hepatitis B virus DNA integration in a sequence homologous to v-erb-A and steroid receptor genes in a hepatocellular carcinoma. Nature. 1986 Jul 3;322(6074):70–72. doi: 10.1038/322070a0. [DOI] [PubMed] [Google Scholar]
- Evans R. M. The steroid and thyroid hormone receptor superfamily. Science. 1988 May 13;240(4854):889–895. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giguere V., Ong E. S., Segui P., Evans R. M. Identification of a receptor for the morphogen retinoic acid. Nature. 1987 Dec 17;330(6149):624–629. doi: 10.1038/330624a0. [DOI] [PubMed] [Google Scholar]
- Greenberg M. E., Hermanowski A. L., Ziff E. B. Effect of protein synthesis inhibitors on growth factor activation of c-fos, c-myc, and actin gene transcription. Mol Cell Biol. 1986 Apr;6(4):1050–1057. doi: 10.1128/mcb.6.4.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattei M. G., Petkovich M., Mattei J. F., Brand N., Chambon P. Mapping of the human retinoic acid receptor to the q21 band of chromosome 17. Hum Genet. 1988 Oct;80(2):186–188. doi: 10.1007/BF00702866. [DOI] [PubMed] [Google Scholar]
- Mattei M. G., de Thé H., Mattei J. F., Marchio A., Tiollais P., Dejean A. Assignment of the human hap retinoic acid receptor RAR beta gene to the p24 band of chromosome 3. Hum Genet. 1988 Oct;80(2):189–190. doi: 10.1007/BF00702867. [DOI] [PubMed] [Google Scholar]
- McDonnell D. P., Mangelsdorf D. J., Pike J. W., Haussler M. R., O'Malley B. W. Molecular cloning of complementary DNA encoding the avian receptor for vitamin D. Science. 1987 Mar 6;235(4793):1214–1217. doi: 10.1126/science.3029866. [DOI] [PubMed] [Google Scholar]
- Mezger V., Bensaude O., Morange M. Deficient activation of heat shock gene transcription in embryonal carcinoma cells. Dev Biol. 1987 Dec;124(2):544–550. doi: 10.1016/0012-1606(87)90507-0. [DOI] [PubMed] [Google Scholar]
- Okret S., Poellinger L., Dong Y., Gustafsson J. A. Down-regulation of glucocorticoid receptor mRNA by glucocorticoid hormones and recognition by the receptor of a specific binding sequence within a receptor cDNA clone. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5899–5903. doi: 10.1073/pnas.83.16.5899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petkovich M., Brand N. J., Krust A., Chambon P. A human retinoic acid receptor which belongs to the family of nuclear receptors. Nature. 1987 Dec 3;330(6147):444–450. doi: 10.1038/330444a0. [DOI] [PubMed] [Google Scholar]
- Robertson M. Retinoic acid receptor. Towards a biochemistry of morphogenesis. Nature. 1987 Dec 3;330(6147):420–421. doi: 10.1038/330420a0. [DOI] [PubMed] [Google Scholar]
- Shaw G., Kamen R. A conserved AU sequence from the 3' untranslated region of GM-CSF mRNA mediates selective mRNA degradation. Cell. 1986 Aug 29;46(5):659–667. doi: 10.1016/0092-8674(86)90341-7. [DOI] [PubMed] [Google Scholar]
- Slack J. M. Embryology: we have a morphogen! Nature. 1987 Jun 18;327(6123):553–554. doi: 10.1038/327553a0. [DOI] [PubMed] [Google Scholar]
- Strickland S., Mahdavi V. The induction of differentiation in teratocarcinoma stem cells by retinoic acid. Cell. 1978 Oct;15(2):393–403. doi: 10.1016/0092-8674(78)90008-9. [DOI] [PubMed] [Google Scholar]
- Thaller C., Eichele G. Identification and spatial distribution of retinoids in the developing chick limb bud. Nature. 1987 Jun 18;327(6123):625–628. doi: 10.1038/327625a0. [DOI] [PubMed] [Google Scholar]
- de Thé H., Marchio A., Tiollais P., Dejean A. A novel steroid thyroid hormone receptor-related gene inappropriately expressed in human hepatocellular carcinoma. Nature. 1987 Dec 17;330(6149):667–670. doi: 10.1038/330667a0. [DOI] [PubMed] [Google Scholar]