Abstract
Purpose
MLN8237, a selective small-molecule inhibitor of Aurora kinase A, has activity in a broad range of preclinical pediatric cancer models. We conducted a phase I trial in children with refractory/recurrent solid tumors to define the maximum-tolerated dose, toxicities, and pharmacokinetic properties of MLN8237.
Experimental Design
MLN8237 was administered orally either once daily or divided twice daily for seven days, every 21 days. Using a rolling-six design, four dose levels (45, 60, 80, and 100 mg/m2/day) were evaluated on the once-daily schedule, and two dose levels (60 and 80 mg/m2/d) on the twice-daily schedule. Pharmacokinetic studies were conducted with the initial dose and trough drug concentrations also measured at the steady state.
Results
Thirty-seven patients were enrolled. On the once-daily dosing schedule, myelosuppression was dose limiting in three of four patients at 100 mg/m2, and one of six patients had dose-limiting mood alteration at 80 mg/m2. At 45 mg/m2, one of six patients experienced dose-limiting mucositis. Mucositis and myelosuppression were dose limiting at 80 mg/m2 on the twice-daily schedule, and one of five patients at 60 mg/m2 on the twice-daily schedule experienced a dose-limiting alkaline phosphatase. Five of 11 patients experienced hand–foot–skin syndrome with twice-daily dosing versus one of 21 after once-daily dosing. There was one partial response and six with prolonged stable disease among 33 evaluable subjects.
Conclusion
The twice-daily dose regimen is well tolerated in adults; however, children experienced a greater frequency of myelosuppression and hand–foot–skin syndrome on this schedule. Children tolerated a higher dose and the recommended pediatric phase II dose is 80 mg/m2/d once daily for seven days.
Introduction
The Aurora family of serine/threonine protein kinases plays a critical role in the regulation of chromosomal segregation and cytokinesis during mitotic progression. Aurora A and B are expressed in all actively dividing cells, whereas Aurora C expression is largely restricted to dividing germ cells (1). Aurora A is critical for mitotic spindle assembly and stability as well as regulation of centrosomal and kinetochore formation (2, 3). The Aurora A kinase gene is amplified or overexpressed in many tumors, including colon, ovarian (4), pancreatic (5), and bladder cancers (6, 7). Aurora A overexpression is associated with aneuploidy and centrosome amplification, and overexpression of Aurora A kinase results in the transformation of normal cells (8), supporting the hypothesis that Aurora A is an oncogene (9). Reports have shown that in cultured cells, reduction of Aurora A using RNA interference (RNAi) reduces Aurora A protein content and results in mitotic spindle defects, mitotic delay, and apoptosis (10).
MLN8237 is a selective small-molecule inhibitor of Aurora A kinase being developed for treatment of advanced malignancies. The safety experience in adults on a 7-day schedule in cycles repeated every 21 days supports a recommended phase II dose of 50 mg orally twice daily (11, 12). The most commonly observed adverse events are generally reversible and include neutropenia, thrombocytopenia, mucositis, alopecia, and asthenia; other adverse events include transient schedule-dependent somnolence and confusion, consistent with the benzodiazepine-like effects of MLN8237 (11, 12).
MLN8237 has shown activity against a broad range of both in vitro and in vivo preclinical models (13). Because of promising results from the Pediatric Preclinical Testing Program (PPTP) showing substantial preclinical activity in neuroblastoma and leukemia (13), MLN8237 was prioritized for rapid clinical development in pediatrics.
Patients and Methods
Institutional Review Boards at participating institutions approved the study. Informed consent was obtained from patients, ages 18 years or older, or from parents/legal guardians of children aged less than 18 years, with child assent when appropriate, according to individual institutional policies.
Patients and eligibility
Eligible patients were younger than 22 years with a recurrent or refractory solid tumor, excluding central nervous system (CNS) tumors or known CNS metastatic disease. Patients were required to have a Karnofsky or Lansky performance score of at least 50 for those older or younger than 16 years, respectively. Adequate bone marrow function (absolute neutrophil count ≥1,000/μL, platelet count ≥100,000/μL, and hemoglobin ≥8 gm/dL), renal (normal serum creatinine for age), and hepatic [total bilirubin ≤1.5× normal and alanine aminotransferase (ALT) ≤5.0× upper limit of normal, for this study, the upper limit of normal was 45 U/L] function was required. Patients were required to have recovered from the acute toxic effects of all prior treatment and should not have received myelosuppressive chemotherapy within 3 weeks of study entry; palliative radiation within 2 weeks or radiation to more than 50% of the pelvis or craniospinal axis within 6 months; or biologic therapy or growth factors within 7 days; or stem cell transplantation within 3 months. Patients were required to be able to swallow capsules. Exclusion criteria included uncontrolled infection, pregnancy or lactation, concurrent administration of certain P-glycoprotein substrates (digoxin, cyclosporine, tacrolimus, or sirolimus), and use of daily benzodiazepines because of the potential benzodiazepine-like effects of MLN8237.
Study design
This dose-escalation study used a rolling-six design (14). Briefly, up to 6 patients were enrolled concurrently at the starting dose. Enrollment to subsequent dose levels was determined by the number of enrolled patients, the number with dose-limiting toxicity (DLT), and the number at risk for DLT. Toxicity was graded according to Common Terminology Criteria for Adverse Events v3.0. Hematologic DLT was defined as MLN8237-related grade 4 neutropenia for greater than 7 days, grade 4 thrombocytopenia on 2 separate days, or requiring platelet transfusion on 2 separate days, within a 7-day period, or myelosuppression that caused a delay of 14 days or more between treatment cycles. Nonhematologic DLT was defined as any grade 3 or 4 nonhematologic toxicity possibly, probably, or definitely attributable to MLN8237 with the exception of the following grade 3 toxicities: nausea or vomiting that resolved within 3 days, fever, infection, or serum mineral or electrolyte disturbances that resolved with oral supplementation. Any toxicity that resulted in a treatment delay of greater than 14 days was considered dose limiting. The maximum tolerated dose (MTD) was exceeded if 2 or more subjects in a cohort of 2 to 6 subjects experienced a DLT during cycle 1.
Dose escalation, drug formulation, and administration
MLN8237 (Millennium Pharmaceuticals, Inc.) was administered orally once daily (part A1) or twice daily (part A2) for 7 days, every 21 days. The starting dose was 45 mg/m2 per os once daily, with dose escalation to 60, 80, and 100 mg/m2. Following completion of enrollment to part A1 (once-daily dosing), patients enrolled on part A2 (twice-daily dosing) and received 40 mg/m2 per os twice daily (80 mg/m2 total daily dose) or 30 mg/m2 per os twice daily (60 mg/m2 total daily dose). A dosing nomogram based on body surface area was used to determine the daily dose in each patient using MNL8237 capsules (2.5, 5, and 25 mg). Capsules were swallowed intact by patients who had been fasting for 1-hour before and 1-hour after dose.
Response
Subjects were evaluable for response if they received 1 complete cycle of MLN8237. Disease evaluations were conducted at baseline and before cycle 2 and then every other treatment cycle. Response Evaluation Criteria in Solid Tumors (RECIST) criteria were used for response assessment (15). The overall response rate was based on best response. Responses were required to be sustained for a minimum of 2 consecutive imaging evaluations at least 6 weeks apart. All institutional reports of response or prolonged stable disease (≥6 cycles) were confirmed by central radiographic review.
Pharmacokinetic studies
Serial blood samples for pharmacokinetic studies of MLN8237 were obtained on day 1, cycle 1 in consenting patients. Whole blood (3 mL) was collected in K2EDTA before, and 0.5, 1, 2, 4, 6 to 8, and 24 hours after the first dose. For patients receiving twice-daily dosing (n = 7), the second dose of MLN8237 on day 1 was not administered to permit sampling at 24 hours and estimation of the terminal half-life. Blood samples were stored refrigerated on wet ice for up to 48 hours and then centrifuged. Plasma was stored at –80°C.
The MLN8237 concentration in plasma was quantified using a validated liquid chromatography/tandem mass spectrometry method with a lower limit of quantification of 10 nmol/L and interday reproducibility less than 5% (16). The MLN8237 plasma concentration–time data were analyzed using noncompartmental methods. The peak concentration (Cmax) and time to peak concentration (Tmax) were determined from a concentration–time curve for each patient. Area under the concentration curve to the last measured time point (AUC0–last) was calculated using the linear trapezoidal method and extrapolated to infinity (AUC0–∞) by adding the final measured plasma concentration divided by the terminal rate constant, which was derived from the slope of the natural log-transformed concentrations and times on the terminal elimination phase of the decay curve. The half-life was determined by dividing 0.693 by the terminal rate constant. Apparent clearance (CL/F) was calculated by dividing the actual dose administered to the patient by the AUC0–∞.
Results
Thirty-seven patients were enrolled onto the study from October 2008 to September 2009 (Table 1). Five patients were not fully assessable for toxicity: 2 patients were deemed ineligible (1 patient each did not meet minimum recovery period after receiving radiotherapy or a biologic agent) and 3 were deemed inevaluable for toxicity assessment before completion of the first cycle.
Table 1.
Patient characteristics (eligible patients, n = 35)
| Characteristic | Number (%) |
|---|---|
| Age (y) | |
| Median | 12.3 |
| Range | 3.2–21.6 |
| Sex | |
| Male | 18 (51.4) |
| Female | 17 (48.6) |
| Race | |
| White | 24 (68.6) |
| Asian | 2 (5.7) |
| Black or African American | 5 (14.3) |
| Unknown | 4 (11.4) |
| Ethnicity | |
| Non-Hispanic | 30 (85.7) |
| Hispanic | 4 (11.4) |
| Unknown | 1 (2.9) |
| Diagnosis | |
| Ewings sarcoma | 5 (14.3) |
| Hepatoblastoma | 2 (5.7) |
| Osteosarcoma | 6 (17.2) |
| Wilms tumor | 2 (5.7) |
| Neuroblastoma | 11 (31.4) |
| Renal cell carcinoma | 1 (2.9) |
| Rhabdomyosarcoma | 1 (2.9) |
| Soft tissue sarcomas | 7 (20.1) |
| Prior therapy | |
| Chemotherapy regimens | |
| Median | 3 |
| Range | 1–10 |
| Radiotherapy | 23 |
Toxicity
Toxicity attributable to MLN8237 is presented in Table 2. In part A1 (once-daily dosing), 21 patients were fully evaluable to toxicity. At 45 mg/m2, 1 of 6 patients had dose-limiting (grade 3) mucositis/stomatitis; no DLTs were observed at 60 mg/m2; at 80 mg/m2, 1 of 6 patients experienced a DLT (grade 4 mood alteration/depression). Hematologic DLTs were not observed until dose level 4 at 100 mg/m2, with 3 of 4 subjects experiencing 5 total DLTs, including grade 4 neutropenia, grade 4 platelets, and a delay in platelet recovery to more than 100,000/μL resulting in a delay of 14 days or more between treatment cycles. Therefore, the MTD on part A1 was 80 mg/m2 per os once daily for 7 days, every 21 days. Table 3 lists the nondose–limiting hematologic and nonhematologic toxicities observed in all patients during cycle 1. In patients treated at the MTD, non-DLTs during cycle 1 included hematologic toxicity and mood alteration.
Table 2.
Dose-limiting toxicities attributable to MLN8237
| Part and schedule | Dose level (mg/m2/dose) | Number entered | Number eligible | Number evaluable | Number with DLT | Type of DLT (n) |
|---|---|---|---|---|---|---|
| A1 (daily) | 45 | 7 | 7 | 6 | 1 | Mucositis/stomatitis (1) |
| 60 | 6 | 6 | 5 | 0 | ||
| 80 | 6 | 6 | 6 | 1 | Mood alteration/depression (1) | |
| 100 | 5 | 4 | 4 | 3 | Neutropenia > 7 days (2) | |
| Platelets (3)a | ||||||
| A2 (twice daily) | 30 | 6 | 5 | 5 | 1 | Alkaline phosphatase (1) |
| 40 | 7 | 7 | 6 | 2 | ANC (2) | |
| Mucositis/stomatitis (1) |
Abbreviation: ANC, absolute neutrophil count.
Two patients requiring transfusions on 2 separate days within a 7-day period, and 1 did not recover to baseline within 14 days.
Table 3.
Nondose Limiting MLN8237-related toxicity more than grade 1 during cycle 1
| Dose level (mg/m2/dose) |
||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 45 qd (n = 6) Grade |
60 qd (n = 5) Grade |
60 divided bid (n = 5) Grade |
80 qd (n = 6) Grade |
80 divided bid (n = 6) Grade |
100 qd (n = 4) Grade |
|||||||||||||
| Toxicity type | 2 | 3 | 4 | 2 | 3 | 4 | 2 | 3 | 4 | 2 | 3 | 4 | 2 | 3 | 4 | 2 | 3 | 4 |
| Hematologic | ||||||||||||||||||
| Anemia | 2 | 1 | 3 | 1 | 2 | 1 | 1 | 3 | 1 | |||||||||
| Leukopenia | 3 | 1 | 1 | 4 | 2 | 3 | 4 | 2 | 1 | 1 | ||||||||
| Lymphopenia | 2 | 1 | 1 | 3 | 1 | 1 | 2 | 1 | 3 | 2 | ||||||||
| Neutropenia | 2 | 1 | 1 | 1 | 1 | 1 | 3 | 1 | 1 | 3 | 1 | 3 | 2 | |||||
| Thrombocytopenia | 1 | 1 | 1 | 2 | 1 | |||||||||||||
| Constitutional | ||||||||||||||||||
| Fatigue | 1 | 1 | ||||||||||||||||
| Fever | 1 | |||||||||||||||||
| Dermatology | ||||||||||||||||||
| Alopecia | 1 | 2 | 3 | 1 | ||||||||||||||
| Rash/desquamation | 1 | 2 | 1 | |||||||||||||||
| Hand–foot–skin reaction | 1 | 1 | ||||||||||||||||
| Gastrointestinal | ||||||||||||||||||
| Anorexia | 1 | 1 | ||||||||||||||||
| Diarrhea | 1 | |||||||||||||||||
| Mucositis/stomatitis | 1 | 1 | 2 | 3 | 1 | |||||||||||||
| Nausea | 1 | |||||||||||||||||
| Vomiting | 1 | |||||||||||||||||
| Infection | ||||||||||||||||||
| Febrile neutropenia | 1 | 1 | 2 | 1 | ||||||||||||||
| Infection | 1 | |||||||||||||||||
| Metabolic/lab | ||||||||||||||||||
| Alkaline phosphatase | 1 | |||||||||||||||||
| ALT | 1 | 1 | ||||||||||||||||
| AST | 1 | 1 | ||||||||||||||||
| Hyperbilirubinemia | 1 | |||||||||||||||||
| Hypocalcemia | 1 | 1 | ||||||||||||||||
| Gamma glutamyl transpeptidase | 1 | |||||||||||||||||
| Hypophosphatemia | 2 | 1 | ||||||||||||||||
| Hypokalemia | 1 | |||||||||||||||||
| Hyponatremia | 1 | |||||||||||||||||
| Other metabolic | 1 | |||||||||||||||||
| Neurology | ||||||||||||||||||
| Memory impairment | 1 | |||||||||||||||||
| Mood alteration agitation | 1 | |||||||||||||||||
| Euphoria | 1 | |||||||||||||||||
| Somnolence | 2 | 1 | 1 | 1 | ||||||||||||||
| Ocular | ||||||||||||||||||
| Flashing lights/floaters | 1 | |||||||||||||||||
| Photophobia | 1 | |||||||||||||||||
| Pain | ||||||||||||||||||
| Pain abdomen NOS | 1 | |||||||||||||||||
| Pain extremity | 1 | |||||||||||||||||
| Pain eye | 1 | |||||||||||||||||
| Pain joint | 1 | |||||||||||||||||
| Pain oral cavity | 1 | |||||||||||||||||
Abbreviations: AST, aspartate aminotransferase; NOS, nitric oxide synthase.
The study was amended to allow for twice-daily dosing to evaluate whether this schedule could decrease neurotoxicity by lowering the maximum plasma drug concentration (Cmax). In part A2, 11 patients were fully evaluable for toxicity. At the 80 mg/m2 dose level (40 mg/m2/dose twice daily), 2 of 6 patients experienced hematologic toxicity (grade 4 neutrophils/granulocytes), and one of those patients also experienced a nonhematologic DLT (grade 3 mucositis). At the next lowest dose level 60 mg/m2/dose (30 mg/m2/dose twice daily), 1 of 5 patients developed transient grade 4 alkaline phosphatase elevation. In addition, 5 of 11 patients experienced nondose–limiting hand–foot–skin syndrome, manifested as erythema and peeling, on the twice-daily dosing regimen.
Thirteen of 21 patients on part A1 and 5 of 11 patients on part A2 experienced transient, fully reversible neurotoxicity (grade 1–4) during cycle 1 (Table 4). All neurotoxicity was transient and reversible. In addition, a greater proportion of patients receiving twice-daily dosing experienced hematologic toxicity or hand–foot–skin syndrome. One patient on the once-daily dosing developed grade 2 hand–foot–skin syndrome. Because of DLT and nondose–limiting toxicity profile in patients receiving 30 mg/m2 twice daily, enrollment to part A2 was discontinued. The toxicity profile did not change for patients receiving up to 13 cycles in part A1 and 5 cycles in part A2. Neurologic and hematologic toxicity did not seem to be cumulative.
Table 4.
Neurologic toxicity regardless of attribution while on MLN8237
| Part and schedule | Dose level (mg/m2/dose) | Pt. no. | Description and grade |
|---|---|---|---|
| A1 (daily) | 45 | 4 | Somnolence, Gr 2 |
| 5 | Anxiety, Gr 1; depression, Gr 1; somnolence, Gr 2 | ||
| 6 | Depression, Gr 2 | ||
| 7 | Agitation, Gr 1 | ||
| 60 | 13 | Somnolence, Gr 2; memory impairment, Gr 2 | |
| 80 | 15 | Euphoria, Gr 2 | |
| 16 | Depression, Gr 4a | ||
| 17 | Agitation; Gr 2 | ||
| 19 | Depression, Gr 1 | ||
| 100 | 20 | Agitation, Gr 1 | |
| 21 | Somnolence, Gr 2; dizziness, Gr 1 | ||
| 23 | Agitation, Gr 1 | ||
| 24 | Dizziness, Gr 1 | ||
| A2 (twice daily) | 40 | 27 | Personality/behavioral, Gr 1 |
| 29 | Somnolence, Gr 2 | ||
| 31 | Depression, Gr 1; dysphoria, Gr 1 | ||
| 30 | 32 | Agitation, Gr 1 | |
| 36 | Personality/behavioral, Gr 1 |
Grade 1 depression at baseline.
The MTD and recommended dose of MLN8237 in children with refractory or relapse solid tumors is 80 mg/m2 per os once daily for 7 days followed by 2 weeks rest (21-day treatment cycle).
Pharmacokinetic studies
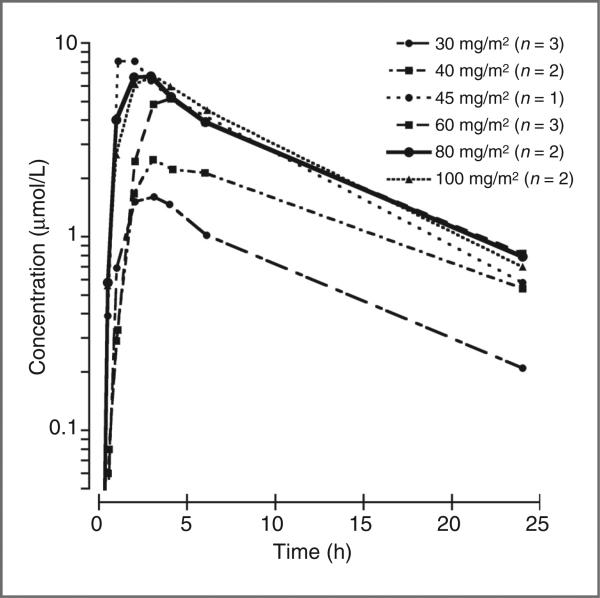
The average plasma concentration–time profiles of MLN8237 at each dose level are presented in Fig. 1. MLN8237 was rapidly absorbed after an oral dose, with a median (range) Tmax of 2.3 (1.1–3.1) hours. MLN8237 plasma pharmacokinetic parameters for the 13 subjects with complete serial pharmacokinetic sampling are summarized in Table 5. The AUC0–∞ increased in proportion to the dose over the dosage range from 30 to 100 mg/m2. Wide interpatient variability in drug disposition was observed with a median (range) apparent clearance (CL/F) of 34 (16–59) mL/min/m2.
Figure 1.
Average concentration × time curves for each dose level (mg/m2/dose). Plasma samples were obtained on cycle 1, day 1 for patients receiving once-daily dosing (45, 60, 80, and 100 mg/m2/dose) and twice-daily dosing (30 or 40 mg/m2/dose, twice daily). For patients receiving twice-daily dosing the second dose of MLN8237 on day 1 was not administered for accurate determination of pharmacokinetic parameters.
Table 5.
MLN8237 pharmacokinetic parameters for QD dosing
| Dose (mg/m2/dose) | Age (y) | BSA (m2) | Cmax (μmol/L) | Tmax (h) | AUC0–24 (μmol/L·h) | T1/2 (h) | CL/F (mL/min/m2) |
|---|---|---|---|---|---|---|---|
| 30 | 7 | 0.87 | 1.1 | 2.1 | 11.4 | 9 | 67.7 |
| 20 | 1.54 | 2.1 | 3.3 | 22.0 | 6.8 | 32.6 | |
| 6 | 0.84 | 2.4 | 2.0 | 9.2 | 3.1 | 68.9 | |
| Mean | 1.8 | 3 | 14.2 | 6.3 | 56.4 | ||
| SD | 0.7 | 2.5 | 6.9 | 3 | 20.6 | ||
| 40 | 11 | 1.4 | 2.7 | 3.1 | 32.7 | 9 | 30.8 |
| 9 | 1.1 | 2.3 | 3.0 | 33.2 | 8.3 | 31.3 | |
| Mean | 2.5 | 3.1 | 32.9 | 8.6 | 31 | ||
| SD | 0.3 | 0.1 | 0.4 | 0.5 | 0.3 | ||
| 45 | 11 | 1.15 | 8.1 | 1.1 | 80.5 | 6.1 | 16.3 |
| 60 | 15 | 1.52 | 4.2 | 3.1 | 36.5 | 5.5 | 52.3 |
| 14 | 1.62 | 2.2 | 3.1 | 23.9 | 7.1 | 74.5 | |
| 19 | 1.97 | 9.6 | 4.1 | 118 | 8.2 | 14.8 | |
| Mean | 5.3 | 3.4 | 49.5 | 6.9 | 47.2 | ||
| SD | 3.8 | 0.6 | 51.2 | 1.3 | 30.2 | ||
| 80 | 12 | 1.25 | 7.5 | 1.0 | 65.7 | 8.8 | 35.5 |
| 15 | 2.1 | 7.6 | 3.0 | 84.7 | 7.8 | 24.2 | |
| Mean | 7.5 | 2.0 | 75.2 | 8.3 | 29.9 | ||
| SD | 0.1 | 1.4 | 13.5 | 0.7 | 8.0 | ||
| 100 | 9 | 1.5 | 8.2 | 2.0 | 65.3 | 8.1 | 44.4 |
| 13 | 1.2 | 7.8 | 3.2 | 95.2 | 6.4 | 28.6 | |
| Mean | 8.0 | 2.6 | 80.3 | 7.2 | 36.5 | ||
| SD | 0.3 | 0.8 | 21.2 | 1.3 | 11.2 |
Best response
In the 33 response-evaluable subjects, (23 measurable disease, 10 evaluable disease only) a median of 1 cycle (range 1–35) was administered. In those with measurable disease, 1 patient with hepatoblastoma had a partial response (PR) and 6 patients had stable disease (2 neuroblastoma and 4 sarcoma for 10, 35, 7, 13, 5, and 5 cycles, respectively). Two patients with evaluable disease, both with neuroblastoma, had stable disease for 6 and 9 cycles. There was not a significant difference in the number of prior therapies in the subjects with PR/SD compared with the subjects who had progressive disease. There was also no difference in response between subjects who received once-daily dosing versus those who received twice-daily dosing.
Discussion
MLN8237 is a small-molecule reversible inhibitor of AURKA that acts via competition with ATP binding. A recent review on mitotic kinase inhibitors (17) focused on the relatively limited antitumor activity seen with these agents in adult trials, raising concern that the mitotic proteins (Aurora A, B, and C), may not be a productive drug development pathway to pursue. On the basis of preclinical data and the results of the current trial, these concerns may not necessarily translate over into the pediatric realm.
The MTD of MLN8237 in children with solid tumors, 80 mg/m2/d administered orally once daily for 7 days, is approximately 1.5-fold greater than the adult MTD of 50 mg twice daily for 7 days. Overall, toxicities were similar to those observed in adults, with hematologic toxicities being the most common DLT observed in children. Although the twice-daily dosing regimen seemed to be well tolerated in adult studies, the schedule did not prove to be better tolerated in children who developed more frequent myelosuppression causing delays and hand–foot–skin syndrome.
This is the first phase I trial in children to use the rolling-six design, which allows enrollment of up to 6 patients at a dose level versus the standard 3, in an attempt to shorten overall study duration by eliminating the observation period when a cohort is expanded from 3 to 6. Although the overall performance of this design cannot be assessed from a single trial, we note that it did not result in an apparent increased risk in toxicity. A comprehensive analysis of this trial design will be possible after the completion of more trials that use the design.
Despite the wide interpatient variability in drug disposition, the systemic MLN8237 exposure (AUC) seemed to increase in proportion to dose. Correlation of plasma pharmacokinetic parameters to toxicity may have been limited by the small sample size on this study, although we did observe that patients with any grade of neurotoxicity during cycle 1 had a higher median Cmax (5.7 vs. 3.6 μmol/L, P < 0.05) and AUC (69.7 vs. 47.8 μmol/L•h, P < 0.05) compared with those without neurotoxicity.
MLN8237 showed broad and potent activity against both acute lymphoblastic leukemia (ALL) and neuroblastoma panels in preclinical models. The dose and schedule used in PPTP experiments (20 mg/kg twice daily for 5 days for 6 weeks consecutively in solid tumor xenografts and for 3 weeks consecutively for ALL xenografts) was predicted to prove tolerable in humans. However, the adult phase I experience proved these to be overestimates. Despite limited clinical activity observed on the current trial, the number of patients studied at the MTD is too small to provide for a good assessment for efficacy. The preclinical data, coupled with the observations that children tolerate 1.5-fold greater doses than adult patients do, support moving forward with the ongoing phase II program in pediatrics. Important considerations for future development of this agent will include the availability of a liquid formulation for younger children and definition of the mechanism by which Aurora kinase A provides a potential oncogenic vulnerability to a subset of pediatric cancer cells, as genomic amplification does not seem to be a driving event but stabilization of myelocytomatosis viral related oncogene, neuroblastoma-derived may be important (18).
Translational Relevance.
MLN8237 is a small-molecule reversible inhibitor of Aurora kinase A via competition with ATP-binding that is being developed for the treatment of cancer. The activity of this agent was evaluated within the Pediatric Preclinical Testing Program against preclinical models of pediatric cancer and found to have significant activity against both solid tumor (notably neuroblastoma) and leukemia xenografts. These data provided the preclinical rationale for rapid development of MLN8237 in childhood cancer. We report the toxicity, pharmacokinetic properties, and response in the pediatric phase I trial of MLN8237 in children with relapsed/refractory cancers and show that the maximum-tolerated dose and schedule in children differs from adults. To our knowledge, this is the first pediatric phase I trial to use the rolling-six design, and one of the first studies to report the experience with MLN8237 in humans.
Acknowledgments
The authors thank Elizabeth O'Connor and Biljana Georgievska of the COG Phase I/Pilot Consortium Coordinating Center for outstanding administrative support throughout the development and conduct of this trial.
Footnotes
Authors’ Contributions
Conception and design: Y.P. Mossé, J.M. Maris, P.C. Adamson, S.M. Blaney
Development of methodology: Y.P. Mossé, E. Lipsitz, P.C. Adamson
Acquisition of data (provided animals, acquired and managed patients, provided facilities, etc.): Y.P. Mossé, E. Lipsitz, E. Fox, J.M. Maris, B. Weigel, M.A. Ingle
Analysis and interpretation of data (e.g., statistical analysis, biostatistics, computational analysis): Y.P. Mossé, E. Lipsitz, E. Fox, D.T. Teachey, J.M. Maris, P.C. Adamson, M.A. Ingle, C.H. Ahern, S.M. Blaney
Writing, review, and/or revision of the manuscript: Y.P. Mossé, E. Lipsitz, E. Fox, D.T. Teachey, J.M. Maris, B. Weigel, P.C. Adamson, M.A. Ingle, C.H. Ahern, S.M. Blaney
Administrative, technical, or material support (i.e., reporting or organizing data, constructing databases): Y.P. Mossé, D.T. Teachey, M.A. Ingle, S.M. Blaney
Study supervision: Y.P. Mossé, B. Weigel, M.A. Ingle, S.M. Blaney
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Nigg EA. Mitotic kinases as regulators of cell division and its checkpoints. Nat Rev Mol Cell Biol. 2001;2:21–32. doi: 10.1038/35048096. [DOI] [PubMed] [Google Scholar]
- 2.Macurek L, Lindqvist A, Lim D, Lampson MA, Klompmaker R, Freire R, et al. Polo-like kinase-1 is activated by aurora A to promote checkpoint recovery. Nature. 2008;455:119–23. doi: 10.1038/nature07185. [DOI] [PubMed] [Google Scholar]
- 3.Sasai K, Parant JM, Brandt ME, Carter J, Adams HP, Stass SA, et al. Targeted disruption of Aurora A causes abnormal mitotic spindle assembly, chromosome misalignment and embryonic lethality. Oncogene. 2008;27:4122–7. doi: 10.1038/onc.2008.47. [DOI] [PubMed] [Google Scholar]
- 4.Yang G, Chang B, Yang F, Guo X, Cai KQ, Xiao XS, et al. Aurora kinase A promotes ovarian tumorigenesis through dysregulation of the cell cycle and suppression of BRCA2. Clin Cancer Res. 2010;16:3171–81. doi: 10.1158/1078-0432.CCR-09-3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Macarulla T, Ramos FJ, Tabernero J. Aurora kinase family: a new target for anticancer drug. Recent Pat Anticancer Drug Discov. 2008;3:114–22. doi: 10.2174/157489208784638785. [DOI] [PubMed] [Google Scholar]
- 6.Sen S, Zhou H, Zhang RD, Yoon DS, Vakar-Lopez F, Ito S, et al. Amplification/overexpression of a mitotic kinase gene in human bladder cancer. J Natl Cancer Inst. 2002;94:1320–9. doi: 10.1093/jnci/94.17.1320. [DOI] [PubMed] [Google Scholar]
- 7.Bischoff JR, Anderson L, Zhu Y, Mossie K, Ng L, Souza B, et al. A homologue of Drosophila aurora kinase is oncogenic and amplified in human colorectal cancers. EMBO J. 1998;17:3052–65. doi: 10.1093/emboj/17.11.3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Katayama H, Brinkley WR, Sen S. The Aurora kinases: role in cell transformation and tumorigenesis. Cancer Metastasis Rev. 2003;22:451–64. doi: 10.1023/a:1023789416385. [DOI] [PubMed] [Google Scholar]
- 9.Gautschi O, Heighway J, Mack PC, Purnell PR, Lara PN, Jr, Gandara DR. Aurora kinases as anticancer drug targets. Clin Cancer Res. 2008;14:1639–48. doi: 10.1158/1078-0432.CCR-07-2179. [DOI] [PubMed] [Google Scholar]
- 10.Hirota T, Kunitoku N, Sasayama T, Marumoto T, Zhang D, Nitta M, et al. Aurora-A and an interacting activator, the LIM protein Ajuba, are required for mitotic commitment in human cells. Cell. 2003;114:585–98. doi: 10.1016/s0092-8674(03)00642-1. [DOI] [PubMed] [Google Scholar]
- 11.Dees EC, Infante JR, Burris HA, Astsaturov IA, Stinchcombe TE, Liu H, editors. Phase I study of the investigational drug MLN8237, an Aurora A kinase (AAK) inhibitor, in patients (pts) with solid tumors. J Clin Oncol. 2010;28:15s. (suppl; abstr 3010) [Google Scholar]
- 12.Sharma S, Kurzrock R, Gouw L, Hong DS, Jones K, Zhou X, editors. Phase I dose-escalation study of the investigational Aurora A kinase (AAK) inhibitor MLN8237 as an enteric-coated tablet (ECT) formulation in patients with nonhematologic malignancies. J Clin Oncol. 2011;29 (suppl; abstr 3094) [Google Scholar]
- 13.Maris JM, Morton CL, Gorlick R, Kolb EA, Lock R, Carol H, et al. Initial testing of the aurora kinase a inhibitor MLN8237 by the Pediatric Preclinical Testing Program (PPTP). Pediatr Blood Cancer. 2010;55:26–34. doi: 10.1002/pbc.22430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Skolnik JM, Barrett JS, Jayaraman B, Patel D, Adamson PC. Shortening the timeline of pediatric phase I trials: the rolling six design. J Clin Oncol. 2008;26:190–5. doi: 10.1200/JCO.2007.12.7712. [DOI] [PubMed] [Google Scholar]
- 15.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 16.Lipsitz E, Moorthy G, Mosse Y, Fox E, Adamson PC. A sensitive and selective liquid chromatography/tandem mass spectrometry method for determination of MLN8237 in human plasma. J Chromatogr B: Anal Technol Biomed Life Sci. 878:2369–73. doi: 10.1016/j.jchromb.2010.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bates SE, Amiri-Kordestani L, Giaccone G. Drug development: portals of discovery. Clin Cancer Res. 2012;18:23–32. doi: 10.1158/1078-0432.CCR-11-1001. [DOI] [PubMed] [Google Scholar]
- 18.Otto T, Horn S, Brockmann M, Eilers U, Schuttrumpf L, Popov N, et al. Stabilization of N-Myc is a critical function of Aurora A in human neuroblastoma. Cancer Cell. 2009;15:67–78. doi: 10.1016/j.ccr.2008.12.005. [DOI] [PubMed] [Google Scholar]