Abstract
Background
Infants (<366 days of age) with acute lymphoblastic leukemia (ALL) have a poor prognosis. Most treatment failures occur within 6–9 months of diagnosis, primarily from relapse.
Procedure
The Children’s Oncology Group P9407 study was designed to test if early intensified treatment would improve outcome for infants with ALL. Due to a significant number of early deaths (< 90 days from enrollment), Induction therapy was amended three times. Cohorts 1 + 2 (n = 68), received identical Induction therapy except for reduced daunorubicin dose in Cohort 2. Cohort 3 (n = 141) received prednisone (40 mg/m2/day) instead of dexamethasone (10 mg/m2/day) and short infusion daunorubicin (30 minutes) instead of continuous infusion (48 hours), as well as additional supportive care measures throughout therapy.
Results
Early deaths occurred in 17/68 (25%) infants in Cohorts 1 + 2 and 8/141 (5.7%) infants in Cohort 3 (P < 0.0001). Among infants ≤90 days of age at diagnosis, early death occurred in 10/17 (58.8%) in Cohorts 1 + 2 and 4/27 (14.8%) in Cohort 3 (P = 0.006). Among infants >90 days of age at diagnosis, early death occurred in 7/51 (13.7%) in Cohorts 1 + 2 and 4/114 (3.5%) in Cohort 3 (P = 0.036). Bacterial, viral, and fungal infections were more common in Cohorts 1 + 2 versus Cohort 3.
Conclusions
Early morbidity and mortality for infants with ALL were reduced by substitution of prednisone (40 mg/m2/day) for dexamethasone (10 mg/m2/day), the delivery of daunorubicin over 30 minutes instead of a continuous infusion for 48 hours, and the provision of more specific supportive care measures.
Keywords: infant acute lymphoblastic leukemia, mortality
INTRODUCTION
Event-free survival (EFS) of children with acute lymphoblastic leukemia (ALL) now exceeds 80% [1–7]. However, infants less than or equal to 1 year of age with ALL continue to fare poorly with EFS rates of 23–51% [8–15]. Younger infants fare worse: <3 months EFS 0–29%, 3 to <6 months EFS 26–50%, and >6 months 49–71% [12,14–16]. In keeping with their poor prognosis, infants with ALL are more likely to have an initial white blood cell (WBC) count >100,000/μl (50–57%) [8,11,14,15], central nervous system (CNS) leukemia (7–24%) [8,11–15], lack of CD10 expression (59–76%) [8,11,13–15], mixed-lineage leukemia (MLL) gene rearrangements (69–79%) [13–15], and poor early response to therapy [11,12,15] when compared to older children. Treatment failures on studies conducted prior to 1995 were predominately due to relapse (22–62%), with fewer failures from infectious deaths (3–14%) [8–12,16,17]. Most relapses occurred within the first year of therapy [8,11,12,16,18], suggesting that alternative therapeutic approaches were needed for this aggressive disease.
The Children’s Oncology Group (COG) P9407 infant ALL study was designed to test the hypothesis that shortened, intensified therapy would improve outcome by decreasing early relapse. This report focuses on the unexpectedly high incidence and etiology of treatment related mortality during Induction and Induction Intensification. The overall outcome for patients treated on this study will be reported separately.
METHODS
Patients
From June 1996 until October 2006, 209 eligible children less than 366 days of age with previously untreated ALL or acute undifferentiated leukemia were enrolled on COG P9407. Institutional immunophenotype and MLL gene rearrangements were confirmed at COG central reference laboratories. Patients with CNS disease (WBC ≥ 5 with blasts present on cytospin examination) or testicular disease were eligible. Infants with (1) mature B-cell ALL, (2) receiving chronic steroids prior to diagnosis (after February 1997 amendment), or (3) congenital leukemia with a gestational age <36 weeks (after July 1998 amendment) were not eligible.
The study was approved by the National Cancer Institute and by Institutional Review Boards at the individual COG member institutions prior to patient enrollment. Informed consent was obtained from parents or guardians according to Department of Health and Human Services Guidelines.
Treatment
COG P9407 delivered intensified therapy to infants with ALL, with most drugs dosed per meter squared. The total duration of therapy was 46 weeks. Treatment was divided into four phases: (1) Induction (weeks 1–3) and Induction Intensification (weeks 4–6), (2) Re-induction (weeks 8–10), (3) Consolidation (weeks 11–17), and (4) Continuation (weeks 18–46). Prior to February 2004, bone marrow transplantation was permitted for selected infants with MLL gene rearrangements following completion of Consolidation therapy [19]. Toxicities were graded using the Toxicity and Complication Criteria utilized by the Pediatric Oncology Group during this time period, scaled as Grade 0 (normal) through Grade 5 (death). Toxicities during Induction + Induction Intensification resulted in three amendments revising Induction therapy (Table I). There were no statistically significant differences in toxicities or deaths between Cohorts 1 and 2, therefore the data were analyzed as two groups: Cohorts 1 + 2 and Cohort 3.
TABLE I.
COG P9407 Induction and Induction Intensification Therapy and Modifications
| Cohort 1 (1996–1997)
|
Cohort 2 (1997–2000)
|
Cohort 3 (2001–2006)
|
|
|---|---|---|---|
| June 1996 | November 1997 | July 1998 | Jan 2001 |
| TITa days 1, 8, 15, 22, 29 | |||
| VCR 0.05 mg/kg, days 1, 15; VCR 0.03 mg/kg, day 8 | |||
| Dex 10 mg/m2/day div TID, days 1–21 | PDN 40 mg/m2/day div TID, days 1–21 | ||
| Daun days 1, 2 | Daun days 1, 2 | Daun days 1, 2 | Daun days 1, 2 |
| All ages—120 mg/m2 CI over 48 hours | ≤90 days 2 mg/kg/day IV over 30 minutes | ||
| <6 months at diagnosis 2 mg/kg/day CI | >90 days to <6 months 2 mg/kg/day CI | <6 months 2 mg/kg/day IV over 30 minutes | |
| 6 to <9 months 2.5 mg/kg/day CI | 6 to <9 months 2.5 mg/kg/day CI | 6 to <9 months 2.5 mg/kg/day IV over 30 minutes | |
| ≥9 months 3 mg/kg/day CI | ≥9 months 3 mg/kg/day CI | ≥9 months 3 mg/kg/day IV over 30 minutes | |
| Cy 250 mg/m2 every 12 hours × 4 doses, days 3, 4 (with MESNA) | |||
| Asp 6,000 U/m2 IM days 4, 6, 8, 10, 12, 15, 17, 19 | |||
| HD MTX 4 g/m2 over 24 hours days 22, 29 (followed by leucovorin rescue) | |||
| VP-I6 100 mg/m2/day days 36–40 | |||
| Cy 300 mg/m2/day days 36–40 (with MESNA) | |||
TIT to <365 days of age—intrathecal methotrexate 7.5 mg, hydrocortisone 7.5 mg, cytosine arabinoside 15 mg; >365 days of age—intrathecal methotrexate 8 mg, hydrocortisone 8 mg, cytosine arabinoside 16 mg; VCR—vincristine; Dex—dexamethasone; Daun—daunorubicin; Cy—cyclophosphamide; Asp—native L-asparaginase; HD MTX—high dose methotrexate; VP-16—etoposide; PDN—prednisone; TID—three times daily; CI—continuous infusion; Leucovorin rescue beginning 42 hours after start of HD MTX infusion—10 mg/m2 every 6 hours for five doses.
Cohort 1 (n = 16) included infants enrolled prior to any changes to protocol therapy (June 1996–July 1997). Daunorubicin was administered during Induction and Re-induction as a 120 mg/m2 continuous infusion (CI) over 48 hours. Due to excessive mucositis, skin breakdown, infection, and death (primarily among infants ≤6 months of age at diagnosis) the study was temporarily closed to accrual in July 1997.
Cohort 2 (n = 52) included infants enrolled following two amendments (November 1997–June 2000). Beginning in November 1997, the daunorubicin dosage during Induction was based on age at diagnosis. Infants <6 months, 6 to <9 months, and ≥9 months at diagnosis received 2, 2.5, and 3 mg/kg/day CI for 2 days, respectively. In July 1998, the daunorubicin dosage during Induction was amended to 2 mg/kg/day over 30 minutes for 2 days, versus a 48 hour continuous infusion, for infants ≤90 days of age at diagnosis. Additionally, infants with congenital leukemia and a gestational age <36 weeks were no longer eligible. Excessive toxic deaths continued to be observed, and the study was again temporarily closed to accrual in June 2000.
Cohort 3 (n = 141) included infants enrolled following a January 2001 amendment (January 2001–October 2006). This amendment replaced dexamethasone (10 mg/m2/day) during Induction, Re-induction, and Continuation with prednisone (40 mg/m2/day), and replaced continuous infusion (48 hours) daunorubicin during Induction and Re-induction with short infusion (30 minutes) daunorubicin for all infants.
Supportive Care
General supportive care guidelines initially included: (1) broad spectrum intravenous antibiotics for fever (≥37.9°C) and neutropenia (absolute neutrophil count (ANC) < 1,000) with no specific anti-bacterial coverage recommendations, (2) amphotericin B if no etiology was determined and the fever continued for >7 days, (3) prophylaxis against Pneumocystis jiroveci with trimethoprim/sulfamethoxazole (first line) or pentamidine (second line), with no other specific recommendations for prophylactic anti-bacterial or anti-fungal therapy, (4) intravenous immunoglobulin (IVIG) therapy for infants with IgG levels <500 mg/dl, and (5) close monitoring of nutritional status with the use of enteral or parenteral feedings as necessary. No specific recommendations were made for length of hospital admission.
Additional supportive care recommendations with the January 2001 amendment included: (1) double intravenous antibiotic coverage for fever (≥38.1°C) and neutropenia (ANC ≤ 1,000), (2) anti-viral therapy when serious viral infection/pneumonia was suspected, (3) anti-fungal prophylaxis for all patients, with fluconazole as the preferred choice, (4) holding amphotericin therapy during and for 24 hours after completion of high dose methotrexate (HD MTX) infusion, (5) use of a Foley catheter during daunorubicin and HD MTX administration to reduce perineal skin breakdown, (6) holding daunorubicin and HD MTX infusions until grade 3–4 perineal breakdown and/or mucositis began to heal, (7) caution when using enteric feedings after extensive mucositis/perineal breakdown, and (8) monitoring infants in the hospital during Induction therapy for a minimum of 14 days.
Statistical Considerations
Data frozen as of 01 February 2010 are included in this report. Two hundred nine eligible patients (68 patients in Cohorts 1 + 2; 141 patients in Cohort 3) were included in the analyses. Fisher’s Exact test was used to test differences in rates of Grade 3/4 toxicities experienced during Induction and Induction Intensification, between Cohorts 1 + 2 and Cohort 3 patients. The Wilcoxon Rank-Sum test (one-sided) was used to determine if the median number of days (overall and by age at diagnosis) to an early death in Cohorts 1 + 2 patients was greater than that for Cohort 3 patients. Statistical significance was defined as P-value less than 0.05. All analyses were performed using SAS® software.
RESULTS
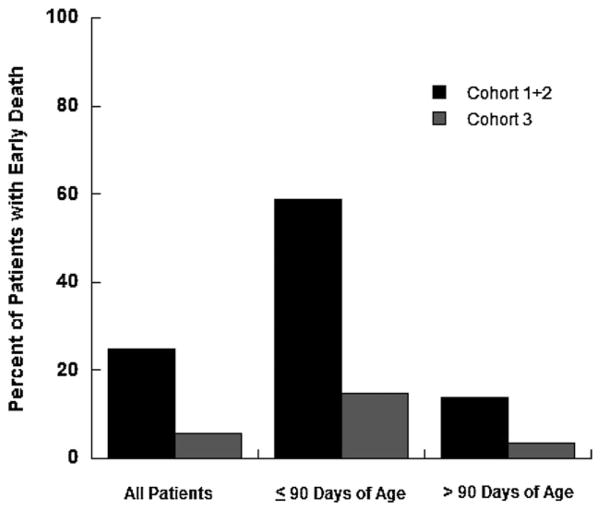
Early death (death within 90 days of enrollment) occurred in 25 patients (Fig. 1), 17/68 (25%) in Cohorts 1 + 2 versus 8/141 (5.7%) in Cohort 3 [odds ratio (OR) = 5.5; 95% CI [2.3, 13.6]; P< 0.0001]. Among patients ≤90 days of age at diagnosis, 10/17 (58.8%) in Cohorts 1 + 2 versus 4/27 (14.8%) in Cohort 3 experienced an early death (OR = 8.2; 95% CI [2,34.5]; P = 0.006). Among patients >90 days of age at diagnosis, 7/51 (13.7%) in Cohorts 1 + 2 versus 4/114 (3.5%) in Cohort 3 experienced an early death (OR = 4.4; 95% CI [1.2, 15.7]; P = 0.036). The last course of therapy received for infants experiencing early death was Induction (n = 21) and Induction Intensification (n = 4).
Fig. 1.
Early death rate (death within 90 days of enrollment) in Cohorts 1 + 2 and Cohort 3 for all patients, those ≤90 days of age at diagnosis, and those >90 days of age at diagnosis.
The median time to death was 21 days (range 0–44) in Cohorts 1 + 2 versus 24 days (range 5–53) in Cohort 3 (P = 0.154). For infants ≤90 days of age at diagnosis, the median time to death was 15 days (range 0–26) in Cohorts 1 + 2 versus 34.5 days (range 5–53) in Cohort 3 (P = 0.132); and for infants >90 days of age at diagnosis, the median time to death was 23 days (range 15–44) in Cohorts 1 + 2 versus 24 days (range 14–44) in Cohort 3 (P = 0.373).
Grades 3 and 4 toxicities are summarized in Table II. Bacterial, viral, and fungal infections were statistically more common in Cohorts 1 + 2 versus Cohort 3. Within the bacterial and fungal groups, bacterial sepsis and fungal abscess were more common in Cohorts 1 + 2 compared to Cohort 3 (P = 0.003 vs. 0.040, respectively). Incidence rates of other infectious toxicities were similar between cohorts.
TABLE II.
Grade 3/4 Toxicity During Induction/Induction Intensification
| Grade 3/4 toxicity | Cohorts 1 + 2 (n = 68), N (%) | Cohort 3 (n = 141), N (%) | P |
|---|---|---|---|
| Infectious toxicitya | |||
| Bacterial | 45 (66.2) | 60 (42.6) | 0.002 |
| Sepsis | 42 (61.8) | 56 (39.7) | |
| Pneumonia | 2 (2.9) | 1 (0.7) | |
| Other | 7 (10.3) | 6 (4.3) | |
| Viral | 13 (19.1) | 9 (6.4) | 0.008 |
| Generalized | 4 (5.9) | 2 (1.4) | |
| Pneumonia | 9 (13.2) | 7 (5.0) | |
| Varicella-Zoster | 1 (1.5) | 0 | |
| Fungal | 9 (13.2) | 3 (2.1) | 0.002 |
| Sepsis | 3 (4.4) | 1 (0.7) | |
| Abscess | 4 (5.9) | 1 (0.7) | |
| Pneumocystis jirovecii | 2 (2.9) | 1 (0.7) | |
| Other | |||
| Infection not otherwise specified | 1 (1.5) | 10 (7.1) | 0.108 |
| Non-hematologic toxicity | |||
| Hemorrhage | 3 (4.4) | 6 (4.3) | 1.000 |
| Thrombosis | 1 (1.5) | 1 (0.7) | 0.546 |
| Cortical (seizure) | 8 (11.8) | 10 (7.1) | 0.296 |
| Diarrhea | 10 (14.7) | 11 (7.8) | 0.142 |
| Local skin | 13 (19.1) | 16 (11.3) | 0.139 |
| Stomatitis | 14 (20.6) | 43 (30.5) | 0.140 |
| Hematologic toxicity | |||
| Absolute neutrophil count | 62 (91.2) | 117 (83.0) | 0.142 |
| Hemoglobin | 58 (85.3) | 111 (78.7) | 0.348 |
| Platelets | 61 (89.7) | 114 (80.9) | 0.114 |
Infectious sub-categories do not total to the categories of bacterial, viral, and fungal as N = number affected and not total number of episodes.
There were no differences in the incidence of Grades 3–4 non-hematologic and hematologic toxicities between cohorts. Cortical toxicity (seizure) occurred in 18 (8.6%) patients. Local skin toxicity and stomatitis occurred in 29 (13.9%) and 57 (27.3%) patients, respectively. Grade 3–4 ANC, hemoglobin, and platelet count occurred in 179 (85.6%), 169 (80.9%), and 175 (83.7%) of patients, respectively.
The etiologies of early deaths are presented in Table III. The majority of early deaths, 14/17 (82.4%) in Cohorts 1 + 2 and 6/8 (75%) in Cohort 3, were infection-related. Identified infectious etiologies included both Gram negative and positive bacteria, fungal and viral causes. Infectious complications occurred early, with the last course of therapy reported as Induction for all but three of the deaths due to infectious causes (one in Cohorts 1 + 2 and two in Cohort 3), with 8/14 (57.1%) deaths due to infectious causes in Cohorts 1 + 2 and 2/6 (33.3%) in Cohort 3 occurring within the first 3 weeks of therapy.
TABLE III.
Early Deaths
| Age at diagnosis (days) | Time to death (days) | Cause of death |
|---|---|---|
| Cohort 1 | ||
| 5 | 16 | Aspergillus spp. |
| 55 | 15 | Enterobacter spp. |
| 57 | 24 | Progressive disease |
| Cohort 2 | ||
| 4 | 26 | Aspergillus spp. |
| 34 | 0 | Leukostasis—pulmonary hemorrhage |
| 46 | 9 | Sepsis—no etiology identified |
| 50 | 15 | Staphylococcus spp. |
| 54 | 5 | Enterococcus spp. and Serratia spp. |
| 67 | 14 | Enterobacter spp. |
| 81 | 26 | Pneumocystis jiroveci |
| 137 | 44 | Respiratory syncytial virus |
| 140 | 23 | Respiratory syncytial virus |
| 141 | 15 | Respiratory syncytial virus |
| 147 | 21 | Pseudomonas spp. |
| 160 | 38 | Sepsis—no etiology identified |
| 166 | 40 | Respiratory failure—no etiology identified |
| 179 | 23 | Sepsis—no etiology identified |
| Cohort 3 | ||
| 1 | 5 | Tumor lysis syndrome |
| 2 | 51 | Candida spp. |
| 62 | 53 | Staphylococcus spp. |
| 63 | 18 | Pseudomonas spp. |
| 200 | 14 | Hemorrhage |
| 260 | 27 | Pseudomonas spp. |
| 264 | 44 | Sepsis—no etiology identified |
| 335 | 21 | Adenovirus |
The 5-year EFS was 47 ± 6.2% for Cohorts 1 + 2 versus 42.3 ± 6% in Cohort 3 (P = 0.96), with a 5-year EFS of 44 ± 4.3% for the three cohorts combined. The 5-year OS was 53% for Cohorts 1 + 2 and 53% for Cohort 3 patients (P = 0.48). Relapses occurred in 12 patients in Cohorts 1 + 2 versus 53 patients in Cohort 3 (P = 0.004).
DISCUSSION
Dexamethasone has been associated with an improvement in EFS and has enhanced CNS penetration when compared to prednisone [20–24], and anthracyclines delivered by continuous infusion have been associated with a decrease in cardiac toxicity in adult trials [25,26]. Given the increased risk of CNS disease and cardiac toxicity in infants, these approaches to therapy were incorporated in COG P9407. Unexpected toxicity and unacceptable mortality however necessitated modifications to Induction therapy. Changes to the daunorubicin dose and infusion rate for those ≤ 90 days of age were implemented for Cohort 2 but early deaths continued. In Cohort 3, dexamethasone 10 mg/m2/day was replaced by prednisone 40 mg/m2/day, continuous infusion daunorubicin (48 hours) was changed to short infusion (30 minutes × 2 days) for all patients, and expanded supportive care recommendations were provided. Although the latter changes resulted in a 77% reduction in early deaths, the early death rate of infants in Cohort 3 (5.7%) remained in excess of the induction death rate of older children with ALL (0.4–1.4%) [5,6,27–29].
Administration of dexamethasone during induction chemotherapy has been associated with improved long-term outcomes compared to prednisone [20–23]. However, the addition of an anthracycline to dexamethasone during induction has been associated with increased mortality in some [22,30] but not all studies [21]. Induction therapy on the Dana-Farber Cancer Institute (DFCI) 91–01P which included dexamethasone (6 mg/m2/day × 28 days) and doxorubicin was closed as a result of induction deaths in 4/38 (10.5%) patients [30]. Modifications, the substitution of prednisone (40 mg/m2/day × 28 days) for dexamethasone and elimination of asparaginase, resulted in a decrease in induction deaths to 2/377 (0.5%) patients on the subsequent DFCI 91–01 [31]. Induction therapy for higher risk precursor B-ALL in children 1–21 years old on Pediatric Oncology Group study 9906 included dexamethasone (6 mg/m2/day × 28 days) and daunorubicin. Induction deaths occurred in 2/34(5.9%) patients, systemic Candida spp. (n = 1) and Bacillus spp. (n = 1). The amended study substituted prednisone (40 mg/m2/day × 28 days) for dexamethasone with a decrease in induction deaths to 18/1035 (1.74%) patients (unpublished data). Induction therapy on the Associazione Italiana Ematologia Oncologia Pediatrica-Berlin-Frankfürt-Münster (AIEOP-BFM) ALL 2000 randomized patients to dexamethasone (10 mg/m2/day × 21 days) versus prednisone (60 mg/m2/day × 21 days) on a regimen that included daunorubicin. The study was amended to halt randomization to dexamethasone for patients >10 years of age because of an increase in induction deaths in patients randomized to dexamethasone, 2.0% versus 0.9% (P = 0.003) [22]. The combined myelotoxic effects of an anthracycline, mucosal breakdown associated with an anthracycline, and lympholytic effects of dexamethasone may cause greater myelosuppression, immune suppression, and severity of infectious complications with resultant increased mortality.
Dexamethasone, when given in combination with the highly myelosuppresive induction regimen of COG P9407, was not tolerated. Two critical changes were made in Cohort 3. Dexamethasone 10 mg/m2/day was replaced with prednisone 40 mg/m2/day; this is both a change in the corticosteroid and a relative dose reduction of corticosteroids. The mode of administration of anthracycline was changed from a 48 hours continuous infusion to a 30 minutes infusion daily. Continuous infusion anthracycline has been associated with more mucositis than when given by short intravenous infusion [32,33]. In contrast, induction therapy on the Interfant 99 study utilized a prednisone prophase (60 mg/m2/day × 7 days) followed by multi-agent chemotherapy including dexamethasone (6 mg/m2/day × 21 days) and daunorubicin (30 mg/m2 infusion over 60 minutes, days 8, 9). There were 18/474 (3.8%) induction deaths [15], similar to that of Cohort 3. It is possible that the most deleterious effects of dexamethasone occur during the initial week of induction or that early clearance of blasts during the prednisone prophase alters neutrophil recovery. More likely, the lower dexamethasone dose utilized during induction on Interfant 99 decreased toxicities or the inclusion of cyclophosphamide during induction therapy on COG P9407 further exacerbated the toxicities associated with an anthracycline and dexamethasone.
Although dexamethasone has been associated with improved outcomes when used during induction chemotherapy, sufficiently high dosages of prednisone have been associated with equivalent outcomes without increased toxicities. When studied in randomized clinical trials, administration of prednisone and dexamethasone at a dosing ratio ≤6.67, demonstrated superior outcomes for the dexamethasone regimens [21,22,34–36]. In contrast, a recent systematic review and meta-analysis concluded that when prednisone was administered during induction therapy at a dosing ratio >6.67 as compared to dexamethasone, equivalent outcomes were seen [36]. The prednisone to dexamethasone ratio on COG P9407 was ≤6.67. However, dexamethasone utilized during Induction on COG P9407 was not tolerated, and thus a higher prednisone dosage on this regimen may result in increased toxicity/mortality. Similarly, though anthracycline by continuous infusion has been associated with a decrease in cardiac toxicity in adults, pediatric studies do not demonstrate a protective effect [37,38].
Although the combination of dexamethasone and an anthracycline has been associated with increased mortality during induction therapy on some studies, this does not appear to be the case during post-induction therapy. Children’s Cancer Group (CCG) 1953 for infants included dexamethasone during Induction/Intensification and Re-induction therapy. Deaths due to infectious causes during Induction/Intensification occurred in 19/115 (16.5%) versus 1/92 (1.1%) during Re-induction [39]. The DFCI 00–01 trial randomized patients to dexamethasone versus prednisone during post-induction chemotherapy. Dexamethasone was associated with improved 5 year EFS (90 ± 2% vs. 81 ± 3%, respectively) [35]. Therefore it may be important to administer dexamethasone during post-induction period, especially on regimens where dexamethasone cannot be tolerated during induction.
The January 2001 amendment included three anti-microbial recommendations: (1) double antibiotic coverage during fever and neutropenia; (2) anti-fungal prophylaxis, fluconazole preferred; and (3) anti-viral therapy for serious viral infections, including noting respiratory syncytial virus (RSV) as a significant pathogen. Following this amendment, there was no clear change in the pattern of bacterial or fungal isolates that resulted in early death. Moreover, the pattern of deaths due to fungal infections did not appear to be influenced by fluconazole prophylaxis, with two Aspergillus spp. associated deaths in Cohorts 1 + 2 and one Candida spp. associated death in Cohort 3, as fluconazole would not be active against Aspergillus spp. Additionally, three of five deaths due to fungal infections (two Aspergillus spp. and one mucormycosis) on the concurrently conducted CCG 1953 [39] would not have been sensitive to fluconazole. Taken together, at least 5/8 (62.5%) fungal isolates resulting in early death on these studies would not be prevented by fluconazole prophylaxis. Future studies are needed to assess the utility of anti-fungal prophylaxis in this age group.
The pattern of deaths due to viral infections may have changed, with three RSV associated deaths in Cohorts 1 + 2 and one adenovirus associated death in Cohort 3. An additional four RSV associated induction deaths occurred on the CCG 1953 [39]. Combining this data, RSV infection resulted in the early death of 7/183 patients enrolled on the concurrently conducted studies (Cohorts 1 + 2 and CCG 1953) versus 0/141 enrolled on Cohort 3. These data suggest that the recommendation to provide specific anti-viral therapy and heightened awareness of RSV infections may have reduced the number of deaths related to RSV. On the current COG infant ALL protocol, AALL0631, palivizumabprophylaxis is recommended during the RSV season.
Little is known about the pharmacokinetics (PK) of chemotherapeutic agents in infants <1 month of age. However, drug exposure could be affected by lower plasma protein levels, decreased capacity of the liver to metabolize drugs, and decreased renal function [40,41]. For example, full term newborns have a glomerular filtration rate of 2–40 ml/min/m2 increasing to approximately 70 ml/min/m2 in the first 2 weeks of life [42–44]. The average body surface area (BSA) of the newborn using the Haycock BSA method [45] is 0.22 m2, providing the average newborn enrolled on COG P9407 with 34 mg/m2 of methotrexate intrathecally on day 1 of therapy. Since methotrexate levels were not followed and leucovorin rescue was not administered after day 1 intrathecal therapy, it is possible that systemic exposure to methotrexate [20,46] and/or, decreased excretion of methotrexate may have led to increased toxicity in the youngest patients. These considerations might also apply to somewhat older infants (1–6 months) as well.
The dose modification of daunorubicin in the November 1997 amendment, resulted in dose reductions of 47.8%, 39.7%, 17.2%, and −3.6% in those patients who were 0 days, 90 days, 6 months, and 9 months of age at diagnosis, respectively, when converted from BSA to per kilogram dosing [45]. Despite larger reduction in daunorubicin dosage for the youngest age groups, the early death rate did not decrease. The PK properties of daunorubicin were analyzed in 21 infants, 5 of whom were <6 months of age, enrolled on the Interfant 99 study [47]. The daunorubicin dosage on this study was reduced to 3/4 for infants 6 months to 1 year of age and 2/3 for infants <6 months of age, from standard BSA dosing. In this small cohort, no significant PK parameter differences were found according to the age of the patient. Despite the dose reduction for younger infants on the Interfant 99, infection during induction was higher in infants <6 months of age compared to older infants, while other toxicities were similar [47]. These data suggest that factors other than daunorubicin metabolism are responsible for the increased infectious complications of the younger infants: decreased clearance of other drugs, increased tissue susceptibility to damage, or decreased host immune defenses or repair mechanisms.
The substitution of prednisone (40 mg/m2/day) for dexamethasone (10 mg/m2/day) and shortened daunorubicin during Induction therapy, as well as additional supportive care recommendations, resulted in a decrease in the early death rate from 25% to 5.7% on COG P9407. Despite a decrease in early death rate, the 5-year EFS and OS of Cohorts 1 + 2 and Cohort 3 were not statistically different, suggesting the changes in Induction therapy may have resulted in an increased relapse rate. However, the use of dexamethasone with different multi-agent induction, such as Interfant 99, as well as its use during post-induction therapy may be well tolerated and improve outcomes.
Acknowledgments
Supported by grants from the National Institutes of Health (grants CA30969, CA29139, CA13539, CA98543, and CA98413). A complete listing of grant support for research conducted by Pediatric Oncology Group (POG) and Children’s Cancer Group (CCG) before initiation of the COG grant in 2003 is available online at: http://www.childrensoncologygroup.org/admin/grantinfo.htm.
Footnotes
Conflict of interest: Nothing to declare.
The opinions and assertions contained herein are the private views of the author(s) and are not to be construed as the official policy or position of the U.S. Government, the Department of Defense, the Department of the Air Force, or the US Food and Drug Administration.
References
- 1.Hunger S, Devidas M, Camitta B, et al. Improved survival for children with acute lymphoblastic leukemia (ALL) from 1990–2005: A report from the Children’s Oncology Group (COG) Berlin, Germany: Wiley-Liss, Inc; Oct 3–6, 2008. p. 31. [Google Scholar]
- 2.Schrappe M, Nachman J, Hunger S, et al. Educational symposium on long-term results of large prospective clinical trials for childhood acute lymphoblastic leukemia (1985–2000) Leukemia. 2010;24:253–254. doi: 10.1038/leu.2009.276. [DOI] [PubMed] [Google Scholar]
- 3.Moricke A, Zimmermann M, Reiter A, et al. Long-term results of five consecutive trials in childhood acute lymphoblastic leukemia performed by the ALL-BFM study group from 1981 to 2000. Leukemia. 2009;24:265–284. doi: 10.1038/leu.2009.257. [DOI] [PubMed] [Google Scholar]
- 4.Silverman LB, Stevenson KE, O’Brien JE, et al. Long-term results of Dana-Farber Cancer Institute ALL Consortium protocols for children with newly diagnosed acute lymphoblastic leukemia (1985–2000) Leukemia. 2009;24:320–334. doi: 10.1038/leu.2009.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pui CH, Campana D, Pei D, et al. Treating childhood acute lymphoblastic leukemia without cranial irradiation. N Engl J Med. 2009;360:2730–2741. doi: 10.1056/NEJMoa0900386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seibel NL, Steinherz PG, Sather HN, et al. Early postinduction intensification therapy improves survival for children and adolescents with high-risk acute lymphoblastic leukemia: A report from the Children’s Oncology Group. Blood. 2008;111:2548–2555. doi: 10.1182/blood-2007-02-070342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matloub Y, Bostrom BC, Hunger SP, et al. Escalating intravenous methotrexate improves event-free survival in children with standard-risk acute lymphoblastic leukemia: A report from the Children’s Oncology Group. Blood. 2011;118:243–251. doi: 10.1182/blood-2010-12-322909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reaman G, Zeltzer P, Bleyer WA, et al. Acute lymphoblastic leukemia in infants less than one year of age: A cumulative experience of the Children’s Cancer Study Group. J Clin Oncol. 1985;3:1513–1521. doi: 10.1200/JCO.1985.3.11.1513. [DOI] [PubMed] [Google Scholar]
- 9.Chessells JM, Eden OB, Bailey CC, et al. Acute lymphoblastic leukaemia in infancy: Experience in MRC UKALL trials. Report from the Medical Research Council Working Party on Childhood Leukaemia Leukemia. 1994;8:1275–1279. [PubMed] [Google Scholar]
- 10.Frankel LS, Ochs J, Shuster JJ, et al. Therapeutic trial for infant acute lymphoblastic leukemia: The Pediatric Oncology Group experience (POG 8493) J Pediatr Hematol Oncol. 1997;19:35–42. doi: 10.1097/00043426-199701000-00005. [DOI] [PubMed] [Google Scholar]
- 11.Dordelmann M, Reiter A, Borkhardt A, et al. Prednisone response is the strongest predictor of treatment outcome in infant acute lymphoblastic leukemia. Blood. 1999;94:1209–1217. [PubMed] [Google Scholar]
- 12.Reaman GH, Sposto R, Sensel MG, et al. Treatment outcome and prognostic factors for infants with acute lymphoblastic leukemia treated on two consecutive trials of the Children’s Cancer Group. J Clin Oncol. 1999;17:445–455. doi: 10.1200/JCO.1999.17.2.445. [DOI] [PubMed] [Google Scholar]
- 13.Hilden JM, Dinndorf PA, Meerbaum SO, et al. Analysis of prognostic factors of acute lymphoblastic leukemia in infants: Report on CCG 1953 from the Children’s Oncology Group. Blood. 2006;108:441–451. doi: 10.1182/blood-2005-07-3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tomizawa D, Koh K, Sato T, et al. Outcome of risk-based therapy for infant acute lymphoblastic leukemia with or without an MLL gene rearrangement, with emphasis on late effects: A final report of two consecutive studies, MLL96 and MLL98, of the Japan Infant Leukemia Study Group. Leukemia. 2007;21:2258–2263. doi: 10.1038/sj.leu.2404903. [DOI] [PubMed] [Google Scholar]
- 15.Pieters R, Schrappe M, De Lorenzo P, et al. A treatment protocol for infants younger than 1 year with acute lymphoblastic leukaemia (Interfant-99): An observational study and a multicentre randomised trial. Lancet. 2007;370:240–250. doi: 10.1016/S0140-6736(07)61126-X. [DOI] [PubMed] [Google Scholar]
- 16.Silverman LB, McLean TW, Gelber RD, et al. Intensified therapy for infants with acute lymphoblastic leukemia: Results from the Dana-Farber Cancer Institute Consortium. Cancer. 1997;80:2285–2295. [PubMed] [Google Scholar]
- 17.Ferster A, Bertrand Y, Benoit Y, et al. Improved survival for acute lymphoblastic leukaemia in infancy: The experience of EORTC-Childhood Leukaemia Cooperative Group. Br J Haematol. 1994;86:284–290. doi: 10.1111/j.1365-2141.1994.tb04727.x. [DOI] [PubMed] [Google Scholar]
- 18.Igarashi S, Manabe A, Ohara A, et al. No advantage of dexamethasone over prednisolone for the outcome of standard- and intermediate-risk childhood acute lymphoblastic leukemia in the Tokyo Children’s Cancer Study Grou L95–14 Protocol. J Clin Oncol. 2005;23:6489–6498. doi: 10.1200/JCO.2005.01.982. [DOI] [PubMed] [Google Scholar]
- 19.Dreyer ZE, Dinndorf PA, Camitta B, et al. Analysis of the role of hematopoietic stem-cell transplantation in infants with acute lymphoblastic leukemia in first remission and MLL gene rearrangements: A report from the Children’s Oncology Group. J Clin Oncol. 2011;29:214–222. doi: 10.1200/JCO.2009.26.8938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bostrom BC, Erdmann GR, Kamen BA. Systemic methotrexate exposure is greater after intrathecal than after oral administration. J Pediatr Hematol Oncol. 2003;25:114–117. doi: 10.1097/00043426-200302000-00006. [DOI] [PubMed] [Google Scholar]
- 21.Mitchell CD, Richards SM, Kinsey SE, et al. Benefit of dexamethasone compared with prednisolone for childhood acute lymphoblastic leukaemia: Results of the UK Medical Research Council ALL97 randomized trial. Br J Haematol. 2005;129:734–745. doi: 10.1111/j.1365-2141.2005.05509.x. [DOI] [PubMed] [Google Scholar]
- 22.Schrappe M, Zimmermann M, Moricke A, et al. Dexamethasone in induction can eliminate one third of all relapses in childhood acute lymphoblastic leukemia (ALL): Results of an international randomized trial in 3655 patients (Trial AIEOP-BFM ALL 2000) ASH Annu Meet Abstr. 2008;112:7. [Google Scholar]
- 23.Winick NJ, Salzer WL, Devidas M, et al. Dexamethasone (DEX) versus prednisone (PRED) during induction for children with high risk acute lymphoblastic leukemia (HR-ALL): A report from the Children’s oncology group study AALL 0232. ASCO Meet Abstr; p. 9504. [Google Scholar]
- 24.Balis FM, Lester CM, Chrousos GP, et al. Differences in cerebrospinal fluid penetration of corticosteroids: Possible relationship to the prevention of meningeal leukemia. J Clin Oncol. 1987;5:202–207. doi: 10.1200/JCO.1987.5.2.202. [DOI] [PubMed] [Google Scholar]
- 25.Hortobagyi GN, Frye D, Buzdar AU, et al. Decreased cardiac toxicity of doxorubicin administered by continuous intravenous infusion in combination chemotherapy for metastatic breast carcinoma. Cancer. 1989;63:37–45. doi: 10.1002/1097-0142(19890101)63:1<37::aid-cncr2820630106>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 26.Legha SS, Benjamin RS, Mackay B, et al. Reduction of doxorubicin cardiotoxicity by prolonged continuous intravenous infusion. Ann Intern Med. 1982;96:133–139. doi: 10.7326/0003-4819-96-2-133. [DOI] [PubMed] [Google Scholar]
- 27.Chauvenet AR, Martin PL, Devidas M, et al. Antimetabolite therapy for lesser-risk B-lineage acute lymphoblastic leukemia of childhood: A report from Children’s Oncology Group Study P9201. Blood. 2007;110:1105–1111. doi: 10.1182/blood-2006-12-061689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moghrabi A, Levy DE, Asselin B, et al. Results of the Dana-Farber Cancer Institute ALL Consortium Protocol 95–01 for children with acute lymphoblastic leukemia. Blood. 2007;109:896–904. doi: 10.1182/blood-2006-06-027714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Borowitz MJ, Devidas M, Hunger SP, et al. Clinical significance of minimal residual disease in childhood acute lymphoblastic leukemia and its relationship to other prognostic factors: A Children’s Oncology Group study. Blood. 2008;111:5477–5485. doi: 10.1182/blood-2008-01-132837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hurwitz CA, Silverman LB, Schorin MA, et al. Substituting dexamethasone for prednisone complicates remission induction in children with acute lymphoblastic leukemia. Cancer. 2000;88:1964–1969. [PubMed] [Google Scholar]
- 31.Silverman LB, Gelber RD, Dalton VK, et al. Improved outcome for children with acute lymphoblastic leukemia: Results of Dana-Farber Consortium Protocol 91–01. Blood. 2001;97:1211–1218. doi: 10.1182/blood.v97.5.1211. [DOI] [PubMed] [Google Scholar]
- 32.Ackland SP, Ratain MJ, Vogelzang NJ, et al. Pharmacokinetics and pharmacodynamics of long-term continuous-infusion doxorubicin. Clin Pharmacol Ther. 1989;45:340–347. doi: 10.1038/clpt.1989.39. [DOI] [PubMed] [Google Scholar]
- 33.Bielack SS, Erttmann R, Winkler K, et al. Doxorubicin: Effect of different schedules on toxicity and anti-tumor efficacy. Eur J Cancer Clin Oncol. 1989;25:873–882. doi: 10.1016/0277-5379(89)90135-1. [DOI] [PubMed] [Google Scholar]
- 34.Bostrom BC, Sensel MR, Sather HN, et al. Dexamethasone versus prednisone and daily oral versus weekly intravenous mercaptopurine for patients with standard-risk acute lymphoblastic leukemia: A report from the Children’s Cancer Group. Blood. 2003;101:3809–3817. doi: 10.1182/blood-2002-08-2454. [DOI] [PubMed] [Google Scholar]
- 35.Vrooman LM, Neuberg DS, Stevenson KE, et al. Dexamethasone and individualized asparaginase dosing are each associated with superior event-free survival in childhood acute lymphoblastic leukemia: Results from DFCI-ALL Consortium Protocol 00–01. ASH Annu Meet Abstr. 2009;114:321. [Google Scholar]
- 36.Teuffel O, Kuster SP, Hunger SP, et al. Dexamethasone versus prednisone for induction therapy in childhood acute lymphoblastic leukemia: A systematic review and meta-analysis. Leukemia. 2011 doi: 10.1038/leu.2011.84. [DOI] [PubMed] [Google Scholar]
- 37.Lipshultz SE, Giantris AL, Lipsitz SR, et al. Doxorubicin administration by continuous infusion is not cardioprotective: The Dana-Farber 91–01 Acute Lymphoblastic Leukemia protocol. J Clin Oncol. 2002;20:1677–1682. doi: 10.1200/JCO.2002.20.6.1677. [DOI] [PubMed] [Google Scholar]
- 38.Levitt GA, Dorup I, Sorensen K, et al. Does anthracycline administration by infusion in children affect late cardiotoxicity? Br J Haematol. 2004;124:463–468. doi: 10.1111/j.1365-2141.2004.04803.x. [DOI] [PubMed] [Google Scholar]
- 39.Salzer W, Dinndorf P, Dreyer Z, et al. Analysis of infectious complications in infants with acute lymphoblastic leukemia treated on the Children’s Cancer Group Protocol 1953 a report from the Children’s Oncology Group. J Pediatr Hematol Oncol. 2009;31:398–405. doi: 10.1097/MPH.0b013e3181a6dec0. [DOI] [PubMed] [Google Scholar]
- 40.Morselli PL. Clinical pharmacology of the perinatal period and early infancy. Clin Pharmacokinet. 1989;17:13–28. doi: 10.2165/00003088-198900171-00004. [DOI] [PubMed] [Google Scholar]
- 41.Kearns GL, Reed MD. Clinical pharmacokinetics in infants and children. A reappraisal. Clin Pharmacokinet. 1989;17:29–67. doi: 10.2165/00003088-198900171-00005. [DOI] [PubMed] [Google Scholar]
- 42.Guignard JP, Torrado A, Da Cunha O, et al. Glomerular filtration rate in the first three weeks of life. J Pediatr. 1975;87:268–272. doi: 10.1016/s0022-3476(75)80600-7. [DOI] [PubMed] [Google Scholar]
- 43.Bartelink IH, Rademaker CM, Schobben AF, et al. Guidelines on paediatric dosing on the basis of developmental physiology and pharmacokinetic considerations. Clin Pharmacokinet. 2006;45:1077–1097. doi: 10.2165/00003088-200645110-00003. [DOI] [PubMed] [Google Scholar]
- 44.Anderson GD, Lynn AM. Optimizing pediatric dosing: A developmental pharmacologic approach. Pharmacotherapy. 2009;29:680–690. doi: 10.1592/phco.29.6.680. [DOI] [PubMed] [Google Scholar]
- 45.Haycock GB, Schwartz GJ, Wisotsky DH. Geometric method for measuring body surface area: A height-weight formula validated in infants, children, and adults. J Pediatr. 1978;93:62–66. doi: 10.1016/s0022-3476(78)80601-5. [DOI] [PubMed] [Google Scholar]
- 46.Thyss A, Suciu S, Bertrand Y, et al. Systemic effect of intrathecal methotrexate during the initial phase of treatment of childhood acute lymphoblastic leukemia. The European Organization for Research and Treatment of Cancer Children’s Leukemia Cooperative Group. J Clin Oncol. 1997;15:1824–1830. doi: 10.1200/JCO.1997.15.5.1824. [DOI] [PubMed] [Google Scholar]
- 47.Hempel G, Relling MV, de Rossi G, et al. Pharmacokinetics of daunorubicin and daunorubicinol in infants with leukemia treated in the interfant 99 protocol. Pediatr Blood Cancer. 2010;54:355–360. doi: 10.1002/pbc.22266. [DOI] [PubMed] [Google Scholar]