Abstract
RNA-binding proteins and corresponding post-transcriptional controls play critical roles in gene expression. The poly-(C) binding proteins, PCBPs (αCPs, hnRNPEs), comprise a well-characterized family of abundant RNA-binding proteins that impact on RNA processing in the nucleus as well as mRNA stability and translation in the cytoplasm. Here we demonstrate that PCBP1 and PCBP2 are abundantly expressed in the gastric epithelium with prominent enrichment in specific cell types within the gastric glandular mucosa. The spatial and intracellular patterns of PCBP1 and PCBP2 expression in these regions are highly correlated. Remarkably, we observe that these proteins are present in the nuclear and cytoplasmic compartments of zymogenic chief cells while they are restricted to the nuclear compartment in acid-secreting parietal cells and poorly expressed in pit cells that line the gland exit. This specificity of expression patterns and subcellular localization of PCBP1 and PCBP2, along with their appearance in the precursor tissues of the gastric epithelium during early postnatal development, suggests these RNA-binding proteins play specific roles in cell differentiation and organismal development within the gastric glandular epithelium.
1. Introduction
Post-transcriptional controls play a central role in establishing specific profiles of eukaryotic gene expression. These controls are critical to somatic development and cell type specification. Current evidence suggests that post-transcriptional controls mediated by subsets of RNA-binding proteins impact regulation of gastrointestinal stem cell compartments, development of the vertebrate gastrointestinal tract (Byeong-Moo Kim, 2011; Gorgoni et al., 2011; McKenna et al., 2010; Yang et al., 2009), and gastric epithelial cell renewal and differentiation (Byeong-Moo Kim, 2011; Gorgoni et al., 2011; Takahashi et al., 2013; Yang et al., 2009). Of note, however, post-transcriptional controls remain essentially unexplored in the formation and function of specific cell types in the gastrointestinal epithelium.
The poly(C) binding proteins (PCBP’s), PCBP1 and PCBP2 (also known as hnRNP E1, hnRNP E2 and αCP1, αCP2), are widely distributed and multifunctional. These isoforms shuttle between the nucleus and cytoplasm and exert their impact on RNA processing and mRNA expression through sequence-specific interactions with C-rich determinants within target mRNAs (Chaudhury et al., 2010a; Makeyev and Liebhaber, 2002). These proteins have been identified and characterized as important mediators of multiple processes, including replication of viruses with gastrointestinal and hepatic tropism, hepatic collagen synthesis, globin expression, and cellular proliferation (Makeyev et al., 2002; Stefanovic et al., 1997; Waggoner et al., 2009). In addition, recent data has uncovered a central role for these proteins in intracellular iron transport, as sensors of folate deficiency, and as antagonists of metastasis in human colon carcinoma (Shi et al., 2008; Tang et al., 2011; H. Wang et al., 2010). The mRNAs encoding PCBP1 and PCBP2 have a widespread tissue distribution (Aasheim et al., 1994; Leffers et al., 1995). While it is established that this distribution includes tissue within the gastrointestinal tract (Diez-Roux et al., 2011; Makeyev et al., 1999), corresponding information on protein localization and function in the adult stomach is notably lacking.
The PCBPs are encoded by four dispersed loci. The two major protein isoforms, PCPB1 and PCBP2, maintain a highly conserved primary structure (PCBP1 vs PCBP2 amino acid homology - 83% in human and 82% in mouse) with complete sequence identity in their nuclear localization domains and remarkable conservation in their three RNA binding KH domains. Importantly, they maintain a shared binding specificity for poly-(C) determinants and therefore target closely aligned sets of mRNAs. Despite this similarity in structure and binding specificity, these two proteins do demonstrate a subset of distinct functions in a number of experimental and physiologic settings. For example, exclusive PCBP2 control of HIV gene expression, poliovirus translation, and tumor suppressor gene expression in chronic myelogenous leukemia has been demonstrated (Blyn et al., 1997; Perrotti and Calabretta, 2002; Woolaway et al., 2007). In contrast, capacities unique to PCBP1 include modulation of epithelial-mesenchymal transitions, stabilization of endothelial nitric oxide synthase, and functioning as a candidate sensor of physiological folate deficiency (Chaudhury et al., 2010b; Ho et al., 2013; Tang et al., 2011). The observation that the genes encoding these two PCBP paralogs have been maintained over a substantial evolutionary history (Makeyev et al., 1999) further supports the conclusion that the encoded PCBP1 and PCBP2 proteins support subsets of critical and non-redundant functions.
In the current report we determine patterns of PCBP1 and PCBP2 protein expression in the mouse stomach with a particular focus on the gastric epithelium and its four specialized cell types: the acid secreting parietal cells, the zymogenic chief cells, the mucus-producing cells (pit cells and neck cells), and cells that subserve enteroendocrine functions. Each of these cell types can be readily identified by standard histologic and immunologic approaches and can be isolated for future analytic and functional studies. The data reveal that PCBP1 and PCBP2 are abundantly expressed in the gastric epithelium and that their expression patterns are identical to each other in this tissue. PCBP1 and PCBP2 are robustly expressed in chief cells and high levels of expression were also observed in the enteroendocrine cells of the gastric antrum. This robust expression contrasted with trace levels of expression in pit cells found in both the antrum and gastric body. In addition to these cell-type differences in expression, the data reveal a remarkable divergence in the sublocalization of PCBP1 and PCBP2 in zymogenic and parietal cell lineages; while both isoforms are abundantly present in the nuclear and cytoplasmic compartments of chief cells they are both restricted in parietal cells to the nuclear compartment. The specific patterns of PCBP distributions and cellular sublocalizations contrast with ubiquitous and strictly nuclear expression patterns of three additional and abundant ribonucleoproteins (RNPs). These data lead us to conclude that PCBP1 and PCBP2 play critical roles in cell determination and function within the gastric epithelia.
2. Results and Discussion
2.1 PCBP1 and PCBP2 protein expression is enriched in the basal region of the gastric glandular epithelium
PCBP1 and PCBP2 protein expression patterns were determined in the stomachs of neonatal and adult CD1 mice. A set of mono-specific antiseras to PCBP1 and PCBP2 recognized their corresponding proteins in the fore-stomach, gastric body and gastric antrum (Fig 1, A–I). The specificity of immunohistochemical signals for PCBP1 and PCBP2 was validated by peptide blocking assay (Fig 2). The highest levels of PCBP expression were observed in the mucosal epithelium and lowest levels in the underlying submucosa and muscularis mucosa (Fig 1). Fluorescence intensity was highest at the base of each epithelial glandular compartment. The distribution of PCBP1 and PCBP2 in the glandular mucosa of gastric body, a region enriched for acid-secreting parietal cells, was notable in that a subset of cells had staining restricted to the nucleus while a distinct subset of cells displayed staining in both the nuclear and cytoplasmic compartments (Fig 3).
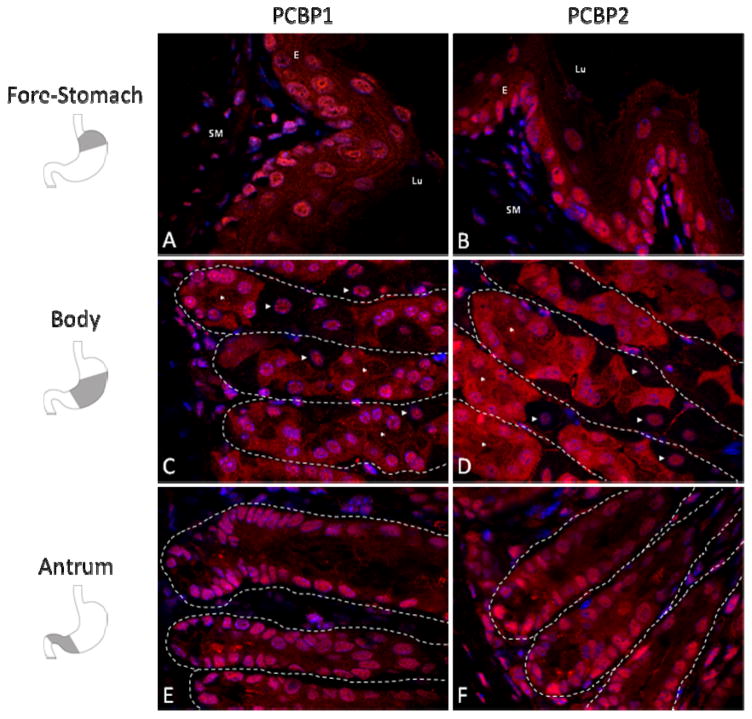
Figure 1. PCBP1 and PCBP2 proteins are enriched in the gastric glandular epithelium.
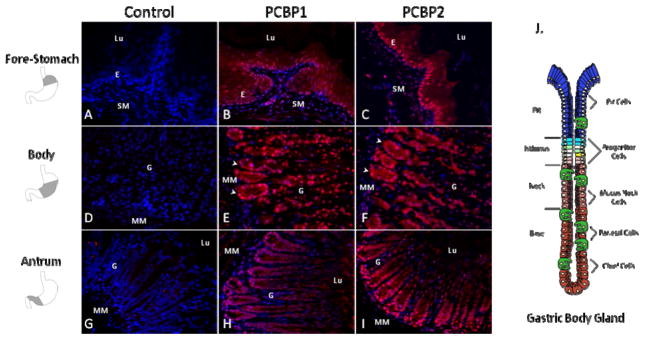
Confocal sections (30x) of the adult CD1 mouse stomach stained for PCBP1 (B, E, H), PCBP2 (C, F, I), or no primary control (A, D, G). Sections represent the 3 distinct mucosal regions anatomically segregated into fore-stomach (A, B, C), gastric body (D, E, F) and gastric antrum (G, H, I). Mucosa of the murine fore-stomach is characterized by a well-keratinized squamous epithelium. The gastric body and gastric antrum contain a glandular epithelium each with tubular glands that terminate in shallow pits at the luminal surface. Expression of both PCBP1 and PCBP2 is most abundant in the epithelium of each compartment, with strongest expression in the epithelial glands of the gastric body (arrowheads in frame E and F). Nuclei are counterstained with DAPI (blue). Abbreviations: E, squamous epithelium, G, glandular epithelium, Lu, Lumen, MM, muscularis mucosa, SM, submucosa. Shaded region in the diagrams to the left of the figure denote the anatomic region corresponding to each tissue section. J. Anatomical schematic of the gastric gland unit in longitudinal axis with specialized cells surrounding a central lumen (modified from (Karam, 2010)). Four regions can be distinguished by histology (pit, isthmus, neck, and base). Putative stem cells and highly proliferative precursor cells reside in the isthmus. Mucus producing cells are abundant in the neck and pit regions. Parietal cells can be found throughout the gland and chief cells are the major cell type of the gland base.
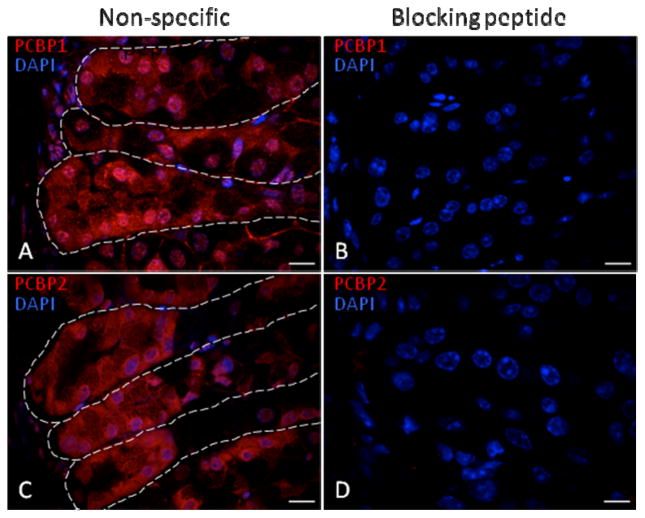
Figure 2. Peptide blocking assay demonstrates specificity of PCBP1 and PCBP2 immunohistochemical signals.
Confocal sections (60x) of the gastric body glandular epithelium are depicted. This region contains the highest PCBP expression levels in the stomach. The degree of signal blockade in the frame is representative of all gastric tissues surveyed. Antibodies (indicated in red) were pre-incubated with immunizing peptides (B, D) corresponding to the PCBP1 (B) and PCBP2 (D). The PCBP1 peptide served as a non-specific control for anti-PCBP2 (A) and vice versa (C). Dashed lines outline individual gland units within the gastric body epithelium. Nuclei were counterstained with DAPI. Nuclear and cytoplasmic staining were both efficiently blocked. White scale bars = 10 microns.
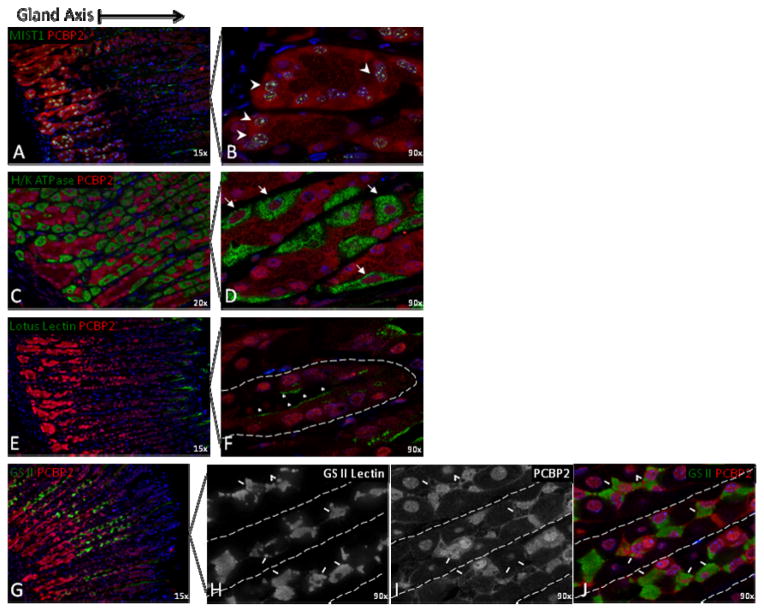
Figure 3. PCBP1 and PCBP2 intracellular expression patterns overlap and are cell-type specific in gastric body glands.
High magnifications (60x) of stained confocal sections reveal parallels in PCBP1 (A, C, E) and PCBP2 (B, D, F) subcellular localization. (A, B) Forestomach epithelium contains high levels of nuclear and cytoplasmic PCBP protein expression. (C, D) Two distinct PCBP intracellular patterns are present in the gastric glandular epithelium of the stomach body. Arrowheads denote exclusively nuclear staining in cells with parietal cell-like features (rhomboidal shape, centrally placed nuclei) intercalated between chief cells (pyramidal shape, basolateral nuclei, zymogen vesicle network in apical cytoplasm, gland base location). Asterisks (*) demonstrate cell clusters with abundant nuclear and cytoplasmic staining and chief cell-like features. (E, F) Nuclear and cytoplasmic staining with nuclear enrichment is the predominant pattern of both PCBPs in glands of the gastric antrum. Dashed lines outline epithelial glands. Nuclei were counterstained with DAPI (blue). Abbreviations: E, squamous epithelium, Lu, Lumen, SM, submucosa.
The highest levels of PCBP1 and PCBP2 expression in the gastric body were detected in cells morphologically categorized as chief cells. These cells have a pyramidal shape, nuclei basolateral to the glandular lumen, an apical cytoplasm with an extensive network of zymogen-containing vesicles, and basal location along the glandular axis (Fig 3C, D). While PCBP1 and PCBP2 were predominantly localized in the nucleus in the majority of cells in the parietal cell enriched gastric body glands, the cells in base of these glands and those nearest the isthmus demonstrated both nuclear and cytoplasmic staining (Fig 1E–F, Fig 8b). The gland isthmus is of particular interest because it comprises the putative stem cell zone and site of precursor cell expansion. These findings demonstrate widespread PCBP expression in the gastric mucosa with evidence for distinct localizations within defined subregions and cell types. These initial observations were next explored in greater detail.
Figure 8. PCBP’s localize to the glandular proliferative zone and are expressed within putative antral stem cells.
(A) Ki67 staining marks the progenitor compartment at the isthmus of the gastric body glands. (B) PCBP2 is enriched in both the nucleus and cytoplasm of Ki67 positive cells in the proliferative zone (90x magnification). A parietal cell (PC) is identified for contrast. (C) The Ki67 positive proliferative zone in antrum resides at the gland base. PCBP2 also has a nuclear and cytoplasmic distribution in antral Ki67 positive cells. (D) GFP staining of the Lgr5-GFP fusion protein, a marker of putative gastric stem cells. This staining localizes this population to the antral gland base in Lgr5 transgenic mice. (E–G) Co-staining demonstrates that PCBP1 is expressed in gastric stem cells (60x magnification, arrows). Nuclei are counterstained with DAPI (blue). Abbreviations: G, glandular epithelium, Lu, Lumen, MM, muscularis mucosa, PC, parietal cell, SM, submucosa. Dashed lines outline individual gastric glands. Shaded region in stencils denote the corresponding anatomic region of tissue section shown. Reference arrows appended to frames indicate the orientation of the gland axis.
2.2 PCBP1 and PCBP2 co-localize within the gastric glandular epithelium
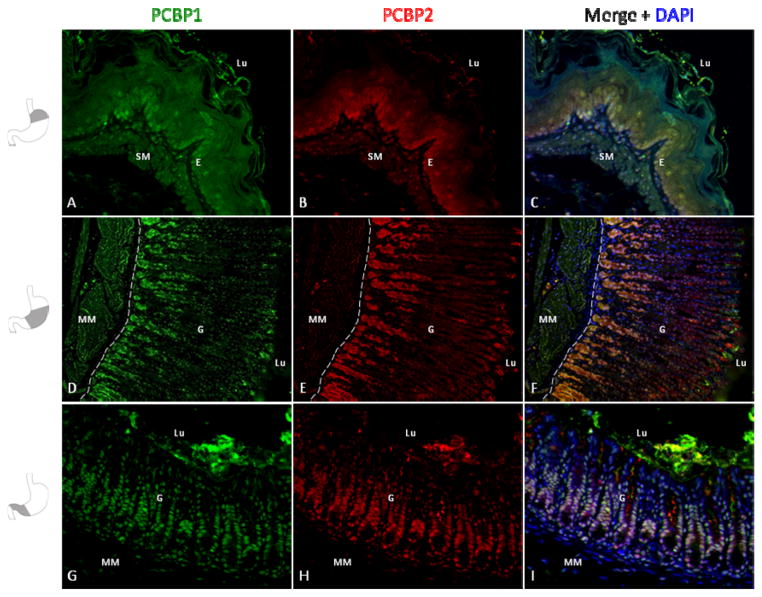
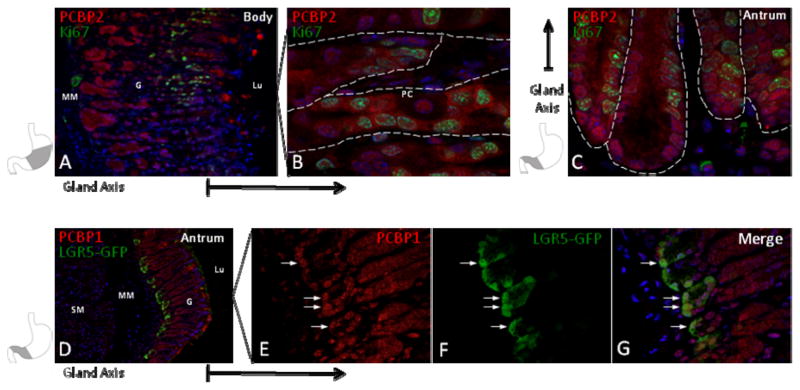
The initial studies, carried out by parallel stains with PCBP1 and PCBP2 antiseras, revealed evidence for cell-type specific intracellular distribution and abundance. Since PCBP1 and PCBP2 have been documented in other settings to mediate distinct as well as overlapping functions in post-transcriptional controls, we next determined if PCBP1 and PCBP2 have identical or distinct patterns of protein distribution within the gastric epithelium. Since both PCBP antibodies were of rabbit origin, we biotinylated our affinity purified anti-PCBP2 antibodies to facilitate two color co-localization studies. These studies revealed significant overlapping of PCBP1 and PCBP2 protein expression patterns within the gastric mucosa from fore-stomach to antrum (Fig 4). There were no regions in which we could identify a relative predominance of one isotype over the other. These data led us to conclude that the distributions of PCBP1 and PCBP2 are highly correlated in the gastric mucosa.
Figure 4. The distributions of PCBP1 and PCBP2 in the gastric mucosa are highly correlated.
Forestomach (20x magnification), gastric body (15x) and antrum (20x) tissue sections were stained in series with anti-PCBP1 (A, D, G) and biotinylated anti-PCBP2 (B, E, H). Merged images (C, F, I) reveal extensive co-localization in the forestomach epithelium, gastric glandular mucosa with enrichment at the gland base and within antral glands. Dashed lines demarcate the boundary between the glandular epithelium (G) and muscularis mucosa (MM) in the gastric body (D, E, F). Nuclei counterstained with DAPI are depicted in the merged image. Green signal in the forestomach submucosa (frames (A) and (C)), gastric body muscularis mucosa (frames (D) and (F)), and luminal portions of frames (A) and (G) represent technical artifact. Abbreviations: E, squamous epithelium, G, glandular epithelium, Lu, Lumen, MM, muscularis mucosa, SM, submucosa.
2.3. Specific distributions of PCBP1 and PCBP2 proteins are identified within the gastric mucosa
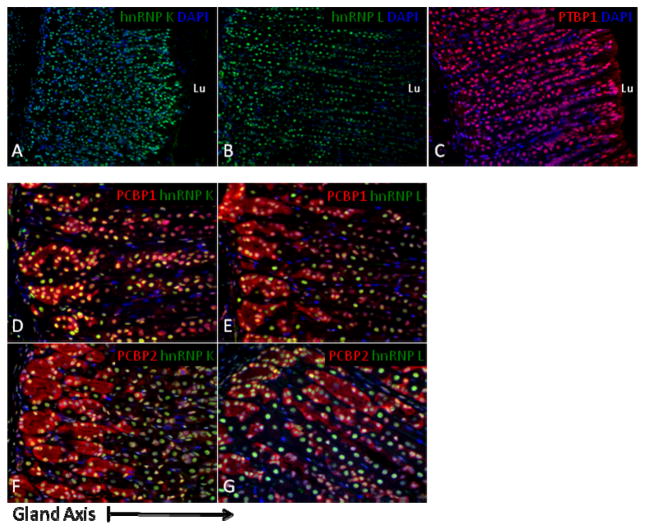
To further assess the specificity of PCBP1 and PCBP2 distributions within the gastric mucosa, we compared their staining patterns to those of three other abundant RNA-binding proteins, PTBP1, hnRNP L, and hnRNP K. As is the case for the PCBPs, all three of these proteins can shuttle between nucleus and cytoplasm (Kim et al., 2000). hnRNP K shares a triple KH domain structure with the PCBPs along with a shared poly-(C) binding specificity (Makeyev and Liebhaber, 2002). PTBP1 has a more relaxed binding specificity for polypyrimidine motifs, whereas hnRNP L interacts with C/A-rich elements (Chaudhury et al., 2010a). All three of these proteins have been demonstrated to interact with PCBP’s as well as each other (Kim et al., 2000). In marked contrast to the specificity of PCBP localizations, the immuno-staining studies revealed uniform expression patterns throughout all regions of the stomach for each of these three additional RBPs (Fig 5 and data not shown). Furthermore, the expressions of all three were strictly nuclear in all glandular regions of the gastric mucosa (Fig 5). The general RNP ‘packaging’ functions of major RNA binding proteins such as hnRNP L and hnRNP K and the general splicing functions of PTBP1 are consistent with their ubiquitous distributions. These observations further support a model in which the PCBPs underlie specific functional roles critical to cellular differentiation and functions in the gastric mucosa.
Figure 5. PCBP1 and PCBP2 protein expression in the gastric mucosa contrasts with that of three interacting RNA-binding proteins.
Confocal sections of gastric body glandular mucosa were stained with antiseras to (A) hnRNP K, (B) hnRNP L, and (C) PTBP1 (15x magnification). The exclusively nuclear staining pattern for each of these three RNA-binding proteins was uniform throughout the gastric gland axis. Co-localization of PCBP isoforms with hnRNP K (D, F) and hnRNP L (E, G) demonstrate overlapping nuclear expression and highlight the abundant and selective expression of PCBP’s in the cytoplasm of cells within the glandular base (magnification 30x). Nuclei were counterstained with DAPI (blue). The reference arrow beneath frames D–G denotes the orientation of the gland axis.
2.4. PCBP1 and PCBP2 are present within four specialized cell types in the gastric epithelial gland
The preceding studies revealed a high-level enrichment of PCBP1 and PCBP2 within the chief cells of the gastric body. This observation was based on cellular morphology and glandular location. The three major cell types of the glandular epithelium, the foveolar Pit cells, the acid-secreting parietal cells, and the zymogenic chief cells, each arises from a distinct progenitor lineage in the gastric body (Karam, 1999). In contrast, parietal cells and chief cells are not present in the simple mucus glands of the antrum. With this in mind, we next used a series of co-localization markers to determine whether these three lineages have distinct patterns and levels of PCBP1 and PCBP2 protein expression. MIST1, a nuclear transcription factor critical for chief cell differentiation (Bredemeyer et al., 2009; Lennerz et al., 2010), was localized to the gastric gland base (Fig 6A–B). These MIST1 positive zymogenic cells contained PCPB1 and PCBP2 in both the nuclear and cytoplasmic compartments. The antibody to H+/K+-ATPase, the gastric proton pump, marked the parietal cell population. In clear distinction to the chief cells, the localization of PCBP1 and PCBP2 in parietal cells was restricted to the nucleus (Fig 6C–D). The lowest levels of PCBP1 and PCBP2 staining was observed for the foveolar Pit cells abutting the gastric lumen (Fig 6E–F) where PCBP1/2 expression was both nuclear and cytoplasmic.
Figure 6. PCBPs are enriched within gastric chief cells.
(A, B) Gastric body confocal sections co-stained for PCBP2 and the nuclear transcription factor MIST1, a chief cell marker, demonstrate co-localization within cells of the gastric gland base (arrowheads identify 4 of several positive cells). (C, D) The parietal cell marker H/K ATPase (“proton-pump”), a cytoplasmic protein, demonstrates gastric gland cells with exclusive PCBP2 nuclear staining (arrows). (E, F) Lotus lectin staining identifies gastric pit cells that harbor low levels of PCBP2 in both nucleus and cytoplasm (F, asterisks). (G) PCBP2 co-localizes with GS II lectin, a mucus neck cell marker. (H–J) PCBP2 protein expression is nuclear and cytoplasmic in mucus neck cells. PCPB2 immunofluorescence signal is evident surrounding mucus filled granules in the apical cytoplasm of this population (block arrows). Dashed lines outline gland borders. Nuclei (blue) are counterstained with DAPI. Section magnifications are noted.
A fourth cell type, the mucous-secreting neck cell, shares a common progenitor with the chief cells (Hanby et al., 1999; Karam and Leblond, 1993; Quante et al., 2010; Ramsey et al., 2007). Mucous neck cells can be specifically identified by GSII lectin staining which binds to mucins found in the large and numerous apical secretory granules in this population. Consistent with its shared origin with chief cells, the PCBP1 and PCBP2 staining pattern in the mucous neck cells was both nuclear and cytoplasmic (Fig 6G–J). The cell-type dependent abundance and intracellular distribution of PCBP’s within the glandular unit of the gastric body suggests that the PCBPs impact on both nuclear and cytoplasmic controls over gene expression in the gastric mucosa. Furthermore, the pattern of abundant PCBP1/2 nuclear and cytoplasmic expression in mucous neck and zymogenic cells, coupled with low levels in pit cells and exclusively nuclear expression in parietal cells supports a model in which PCBP’s constitute an important determinant of cell fate decisions in mature glands of the murine gastric mucosa.
Enteroendocrine cells of the gastric epithelium secrete hormonal proteins in response to signals generated in the gastrointestinal tract. Gastric enteroendocrine cells have poorly defined progenitors and are scattered throughout the gastric mucosa. These cells include the gastrin secreting cells prominently located within the gastric antrum. PCBP1 and PCBP2 staining co-localized with gastrin-positive cells in antrum tissue. In these gastrin-positive cells, staining for the PCBPs was observed in both the nuclear and cytoplasmic compartments (Fig 7 and data not shown). Consistent with this finding is the report by others that PCBP1 and its closely-related paralog, hnRNP K, bind to and stabilize gastrin mRNA in human gastric adenocarcinoma cells (Lee et al., 2007). Co-localization of PCBP’s in murine gastrin producing cells is consistent with a model in which they contribute to differentiation of the enteroendocrine lineage via post-transcriptional control of gastrin gene expression.
Figure 7. PCBP2 is abundant in gastric mucosa enteroendocrine cells.
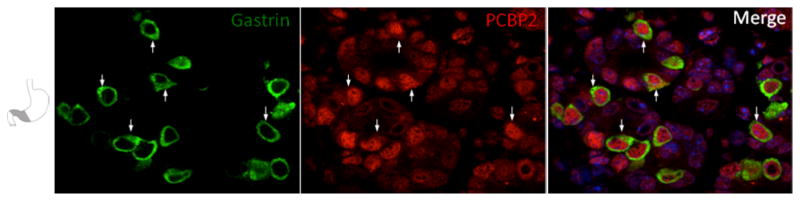
Confocal sections (90x) from gastric antrum are co-stained with PCBP2 and the cytoplasm-localized enteroendocrine marker gastrin. PCBP2 is observed in both nuclear and cytoplasmic (arrows) compartments. Nuclei are counterstained with DAPI (blue in merged image).
2.5 PCBP1 and PCBP2 proteins are abundant in the gastric stem cell and progenitor cell compartments and demonstrate nuclear and cytoplasmic localization
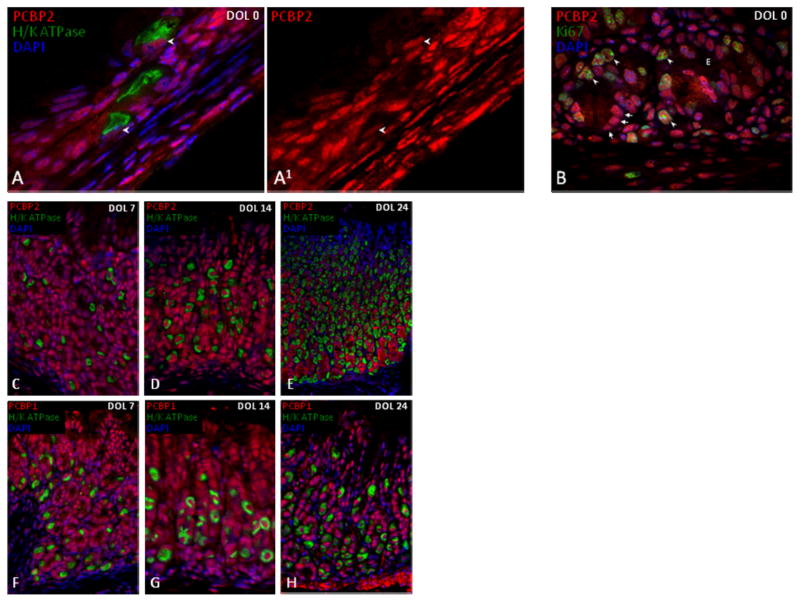
PCBP1 and PCBP2 have been functionally linked to cell cycle control (Waggoner et al., 2009). For this reason, we next determined whether these proteins co-localize with stem cells and proliferating progenitor cells of the glandular epithelium. Co-staining with Ki67, a marker of cellular proliferation, revealed co-PCBP1 and PCBP2 co-localization in cells at the gland isthmus, the site of bi-directional expansion of the three main gastric progenitor lineages. PCBP1 and PCBP2 staining was positive in both the nuclear and cytoplasmic compartments in these cells (Fig 8A–C). To specifically assess PCBP1 and PCBP2 expression in the stem cell population, we utilized Lgr5-EGFP transgenic animals. These C57Bl/6 mice contain an EGFP open reading frame inserted within the LGR5 locus (Barker et al., 2007). First characterized as an intestinal stem cell marker, LGR5 positive cells also localize to the base of mature pyloric glands and have pyloric gland renewing capability (Barker et al., 2010). LGR5 stem cells (GFP+) were limited to the adult gastric antrum and colocalized with PCBP1 and PCBP2 (Fig 7D–F). GFP staining was absent from the gastric body and fore-stomach tissue which is consistent with prior observations demonstrating that Lgr5 expression is restricted to stem cells in mature antral glands (data not shown) (Barker et al., 2010). The high levels of PCBP1/2 proteins observed in antral stem cells and proliferative cells throughout the gastric mucosa supports a model in which they contribute to the differentiation and function of progenitors in these compartments.
2.6 PCBP1 and PCBP2 expression correlates with gastric gland elongation in the post-natal period
The murine gastric epithelium consists of a simple short tubular structure at birth. During the first month of postnatal life it rapidly elongates, invaginates, and forms the characteristic morphology of a mature glandular network (Karam, 1999). To determine the timing of PCBP regionalization within the glands of the gastric body, we examined the gastric mucosa in neonatal mice on days of life 0, 7, and 14, then again on day 24, when mice had been weaned from breast milk and were maintained on standard mouse chow. PCBP1 and PCBP2 staining was detected in all cells of the primitive epithelium (Day 0, Fig 9 and data not shown). On the day of birth (DOL 0), the PCBPs were restricted to the nuclei in the cells located at the luminal surface and within the lamina propria. In contrast, cells within the middle layers of the epithelium revealed combined nuclear and cytoplasmic staining. PCBP1 and PCBP2 co-localized in DOL 0 sections with the large population of proliferating cells identified by Ki67 staining (Fig 9B and data not shown). PCBP1 and PCBP2 were nuclear restricted in parietal cells in the neonatal epithelium (Fig 9A–A1, and data not shown) suggesting that this cell-type nuclear restriction characteristic of adult parietal cells is already established prior to birth. PCBP1 and PCBP2 remain abundant throughout the gastric epithelium on days 7 and 14 (i.e., prior to weaning) (Fig 9C, D, F, G). In contrast, on Day 24 we noted enrichment of PCBP1 and PCBP2 in the gland base (Fig 9E, 9H). These data lead us to conclude that the adult pattern of PCBP accumulation within the epithelial glandular regions is a temporal event that coincides with gland elongation and correlates with the dietary changes that are associated with weaning from breast milk to standard mouse chow between the 3rd and 4th week of life.
Figure 9. The distinct nuclear/cytoplasmic distributions of PCBPs in the chief cells and the parietal cells are established in the neonatal gastric epithelium. Post-natal gastric gland elongation is associated with enrichment of PCBP’s in the gland base.
(A) PCBP2 staining in neonatal gastric epithelium. Exclusive PCPB2 nuclear staining (A1, arrowheads in monochromatic) in parietal cells marked by H/K ATPase indicates PCBP intracellular partitioning in this lineage is a prenatal event. (B) Neonatal epithelium co-staining of PCBP2 and Ki67 in proliferative cells (arrowheads). Three chief cells within the immature epithelium (arrows) demonstrate PCBP2 nuclear and cytoplasmic expression in the chief-cell lineage. PCBP1 and PCBP2 remain widespread during early gland elongation prior to weaning (C, D, F, G – 30x magnification). (E, H) PCBP1 and PCBP2 regionalize to the gland base in juvenile mice weaned to standard chow (20x magnification). Nuclei counterstained with DAPI. Abbreviations: DOL, day of life.
2.7. Conclusions
Our analysis of PCBP1 and PCBP2 expression demonstrates robust expression of these two proteins within the gastric epithelium with high concordance for both proteins in their cell-type specificities and intracellular distributions. Importantly, the distribution patterns for the PCPBs were distinct from that of a structurally-related RNP with the same poly(C)-binding specificity (hnRNP K), from that of an RNA-binding protein with a similar pyrimidine-rich binding specificity but distinct structure (PTBP1), and from a phylogenetically unrelated RNA-binding protein known to interact with PCBP proteins (hnRNP L) (Kim et al., 2000). hnRNP K, PTBP1, and hnRNP L were all observed to be ubiquitously expressed in the gastric glands and were all uniformly restricted to the nucleus. These observations support the conclusion that, unlike these ‘house-keeping’ RNA-binding proteins, PCBPs play specific roles in gastric cell differentiation and function.
The distinct patterns of subcellular localization of PCBP1 and PCBP2 in cell types within the gastric epithelium are of particular interest. These proteins are known to have both nuclear and cytoplasmic functions in post-transcriptional controls in a number of experimental models. These activities include the control of transcript splicing (Ji et al., 2007) and 3’ cleavage/polyadenylation (Ji et al., 2011; 2013) in the nucleus and in the control of mRNA stability and translational efficiency of targeted mRNAs in the cytoplasm (Ji et al., 2003; X. Wang et al., 1995). Thus, the localization to one or both compartments in various cells implies distinct subsets of functions in the specialized cells of the gastric epithelium.
The three well-characterized progenitor lineages in the gastric mucosa generate terminally differentiated pit cells, parietal cells, and the two zymogenic lineage cells -- chief cells and neck cells. Expression of the PCBPs was notable for robust enrichment in the zymogenic cell lineages and low levels of expression in foveolar pit cells. While easily detectable in parietal cells, PCBP expression in these cells was restricted to the nucleus, contrasting with the nuclear and cytoplasmic distribution observed in zymogenic and pit cell lineages. PCBP1 and PCBP2 protein expression was also robust in the stem cell and progenitor cell niche where it was found in both nuclear and cytoplasmic compartments. These findings support functions of PCBPs in both nuclear and cytoplasmic controls and suggest that these controls are modulated during development and/or during lineage specification in the gastric body mucosa. The prior demonstration that gastrin mRNA is targeted for stabilization by PCBP proteins is consistent with this model (Lee et al., 2007).
Mechanistic support for specific compartmentalized PCBP functions in development and in response to physiologic stimuli comes from studies of epithelial-mesenchymal transitions in secretory epithelium where reversible phosphorylation of PCBP1 has been demonstrated to regulate its cytoplasmic localization and its ability to silence the translation of epithelial-mesenchymal transition genes (Chaudhury et al., 2010b; Hussey et al., 2011). Based on the abundance and intracellular distribution of PCBP1 and PCBP2 in the mouse stomach, we hypothesize that these proteins coordinate a related subset of RNA’s important for zymogenic lineage differentiation and function through direct protein:RNA interactions that regulate RNA processing and stability. Functional analysis of these potential mechanisms can now be initiated to test and delineate the roles played by PCBP1/2 in zymogenic cell lineage differentiation and maintenance.
3. Experimental Procedures
3.1 Materials
A. Antibodies
Rabbit polyclonal antibodies were raised against peptides (GenScript, Piscataway, NJ) corresponding to human PCBP1 (Residues 229–243 (NCBI: NP_006187, UniProt: Q15365): TISPLDLAKLNQVAR) and human PCBP2 (Residues 237–251 (NCBI: NP_005007, UniProt: Q15366): AIPQPDLTKLHQLAM). The PCBP2 sequence is common to the PCBP2 full-length and KL isoforms, but distinct from PCBP1. Anti-PCBP2 was affinity purified using the immunizing polypeptide. Anti-PCBP1 rabbit serum was used without fractionation. Primary antibodies and working dilutions utilized in this study are listed in Table 1.
Table 1.
| Antibody | Source | Dilution | Host |
|---|---|---|---|
| PCBP1 | Stephen A. Liebhaber, Philadelphia, PA | 1:1000 | Rabbit, p |
| PCBP2 | Stephen A. Liebhaber, Philadelphia, PA | 1:15000 | Rabbit, p |
| PCBP2-bt | Stephen A. Liebhaber, Philadelphia, PA | 1:1000 | Rabbit, p |
| hnRNP K/J | Millipore, clone 3C2, Cat# 05-1519 | 1:20,000 | Mouse, m |
| hnRNP L | Abcam, Cat# 6106 | 1:2500 | Mouse, m |
| H+/K+ ATPase | MBL - Medical and Biological Labs, Cat# D031-3H | 1:2000 | Mouse, m |
| Mist1 | Stephen F. Konieczny, West Lafayette, IN(Pin et al., 2000) | 1:100 | Rabbit, p |
| PTBP1 | Douglas Black, Los Angeles, CA (Markovtsov et al., 2000) | 1:1000 | Rabbit, p |
| Ki-67 | BD Pharmingen Cat# 556003 | 1:1000 | Mouse, m |
| Gastrin | Santa-Cruz Cat# SC-7783 | 1:2500 | Goat, p |
| GFP | Abcam Cat# Ab6673 | 1:250 | Goat, p |
| GSII lectin-Alexa 488 | Invitrogen Cat# L-21415 | 1:2000 | NA |
| Lotus Lectin | Vector Labs Cat# B-1325 | 1:10000 | NA |
p, polyclonal; m, monoclonal; NA, not applicable.
B. Animals
All experiments were conducted in accordance with protocols approved by the Institutional Animal Care and Use Committee at the Perelman School of Medicine at the University of Pennsylvania. All mice were euthanized by carbon dioxide inhalation followed by cervical dislocation. Immunohistochemical analyses are representative of gastrointestinal tissue dissected from at least 4 non-fasted, wild-type CD1 adult or juvenile mice and Lgr5-EGFP-IRES-creERT2 mice (The Jackson Laboratory, Bar Harbor, ME).
3.2 Methods
A. Immunofluorescence
Gastrointestinal viscera from sacrificed mice were dissected and immediately flushed with ice cold PBS, fixed in neutral buffered formalin (Fisher Cat# L14449) overnight, washed with PBS, and stored in 70% ethanol until embedding in paraffin. All studies utilized 5-μm sections. Samples were deparaffinized with xylene and ethanol, washed in water and heated to 121°C in a pressure cooker for 20 minutes in 10 mM sodium citrate pH 6.0 for antigen retrieval. Specimens were rinsed in water, equilibrated with PBS then blocked for 15 minutes at room temperature (CAS block; Invitrogen Cat # 00-8120). All primary antibodies were diluted in CAS block to a working concentration detailed in Table 1. Tissues were incubated with primary antibodies overnight at 4°C in a humidified box, washed twice with PBS and incubated for 30 minutes at 37°C with Cy2 or Cy3 conjugated secondary antibodies (Jackson Immunoresearch: Anti-rabbit, Anti-goat or Anti-mouse) diluted to 1:600 in CAS block. Tissues were washed twice with PBS, rinsed in water, and then counterstained for 30 seconds with DAPI (0.1 mcg/mL). Tissues were mounted in aqueous mounting medium (KPL, Cat No. 71-00-16). Images were acquired using a Nikon Eclipse Ti Confocal Microscope and processed with iVision software.
B. Peptide blocking assay
Primary antibodies (Anti-PCBP1 or Anti-PCBP2) were diluted in CAS block (Table 1). PCBP1 and PCBP2 peptides diluted in sterile water and used for antibody generation as noted were added to 4ug/mL final concentration based upon titration analysis. Antibody:peptide mixtures were incubated overnight with rotation at 4°C. PCBP1 peptides served as non-specific control peptides for PCBP2 antibody. Similarly, PCBP2 peptides served as non-specific control peptides for PCBP1 antisera. Samples were centrifuged for 2 minutes at 20,800 RCF and the top 95% of supernatant used for immunostaining as described.
C. PCBP2 biotinylation and co-localization assay
To facilitate co-localization analysis of PCBP1 and PCBP2, anti-PCBP2 antibodies were biotinylated (Anti-PCBP2-bt) using the Chromalink Biotin one-shot antibody-labeling kit according to the manufacturer’s instructions (Solulink, B-9007-009K). Deparaffinized and antigen retrieved tissues were blocked with CAS block for 15 minutes, incubated with Anti-PCBP1 (1:1000) overnight at 4°C, washed twice with PBS, then incubated with anti-rabbit Cy3 conjugated secondary antibodies as described above. Tissue sections were washed with PBS, blocked with Avidin, washed, and then Biotin blocked (Sigma-Aldrich) for 15 minutes to mask non-specific sites. Samples were then incubated with anti-PCBP2-bt diluted to 1:1000 in CAS-block for 2 hours at 37°C, washed twice with PBS and incubated for 2 hours with Cy3 conjugated streptavidin diluted to 1:400 in CAS block. Samples were washed twice in PBS, counterstained with DAPI and mounted.
Highlights.
PCBP1 and PCBP2 co-localize within the gastric glandular epithelium
PCBP1/2 are specifically enriched in chief cells.
PCBP1/2 are found in the nucleus and cytoplasm of chief cells
PCBP1/2 are nucleus restricted in the parietal cell lineage
PCBP1/2 expression correlates with gastric gland elongation in the post-natal period.
Acknowledgments
We are grateful for the assistance provided by the Molecular Pathology and Imaging core facility and productive discussions with members of the Liebhaber laboratory. This work was supported by NIH MERIT HL 65449 (S.A.L.), NIDDK T32-DK007066 Training grant in gastrointestinal sciences (L.G.) and Core facilities of the NIH/NIDDK P30-DK050306 Center for Molecular Studies in Digestive and Liver Diseases (Molecular Pathology and Imaging, Molecular Biology/Gene Expression, Cell Culture, and Transgenic and Chimeric Mouse Cores).
Footnotes
We declare we have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aasheim HC, Loukianova T, Deggerdal A, Smeland EB. Tissue specific expression and cDNA structure of a human transcript encoding a nucleic acid binding [oligo(dC)] protein related to the pre-mRNA binding protein K. Nucleic Acids Res. 1994;22:959–964. doi: 10.1093/nar/22.6.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker N, Huch M, Kujala P, van de Wetering M, Snippert HJ, van Es JH, Sato T, Stange DE, Begthel H, van den Born M, Danenberg E, van den Brink S, Korving J, Abo A, Peters PJ, Wright N, Poulsom R, Clevers H. Lgr5(+ve) stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell. 2010;6:25–36. doi: 10.1016/j.stem.2009.11.013. [DOI] [PubMed] [Google Scholar]
- Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, Clevers H. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- Blyn LB, Towner JS, Semler BL, Ehrenfeld E. Requirement of poly(rC) binding protein 2 for translation of poliovirus RNA. J Virol. 1997;71:6243–6246. doi: 10.1128/jvi.71.8.6243-6246.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredemeyer AJ, Geahlen JH, Weis VG, Huh WJ, Zinselmeyer BH, Srivatsan S, Miller MJ, Shaw AS, Mills JC. The gastric epithelial progenitor cell niche and differentiation of the zymogenic (chief) cell lineage. Dev Biol. 2009;325:211–224. doi: 10.1016/j.ydbio.2008.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byeong-Moo Kim JWCKRAS. Regulation of mouse stomach development and Barx1 expression by specific microRNAs. Development. 2011;138:1081. doi: 10.1242/dev.056317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhury A, Chander P, Howe PH. Heterogeneous nuclear ribonucleoproteins (hnRNPs) in cellular processes: Focus on hnRNP E1’s multifunctional regulatory roles. RNA. 2010a;16:1449–1462. doi: 10.1261/rna.2254110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhury A, Hussey GS, Ray PS, Jin G, Fox PL, Howe PH. TGF-beta-mediated phosphorylation of hnRNP E1 induces EMT via transcript-selective translational induction of Dab2 and ILEI. Nature Cell Biol. 2010b;12:286–293. doi: 10.1038/ncb2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diez-Roux G, Banfi S, Sultan M, Geffers L, Anand S, Rozado D, Magen A, Canidio E, Pagani M, Peluso I, Lin-Marq N, Koch M, Bilio M, Cantiello I, Verde R, De Masi C, Bianchi SA, Cicchini J, Perroud E, Mehmeti S, Dagand E, Schrinner S, Nürnberger A, Schmidt K, Metz K, Zwingmann C, Brieske N, Springer C, Hernandez AM, Herzog S, Grabbe F, Sieverding C, Fischer B, Schrader K, Brockmeyer M, Dettmer S, Helbig C, Alunni V, Battaini MA, Mura C, Henrichsen CN, Garcia-Lopez R, Echevarria D, Puelles E, Garcia-Calero E, Kruse S, Uhr M, Kauck C, Feng G, Milyaev N, Ong CK, Kumar L, Lam M, Semple CA, Gyenesei A, Mundlos S, Radelof U, Lehrach H, Sarmientos P, Reymond A, Davidson DR, Dollé P, Antonarakis SE, Yaspo ML, Martinez S, Baldock RA, Eichele G, Ballabio A. A high-resolution anatomical atlas of the transcriptome in the mouse embryo. PLoS Biol. 2011;9:e1000582. doi: 10.1371/journal.pbio.1000582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorgoni B, Richardson WA, Burgess HM, Anderson RC, Wilkie GS, Gautier P, Martins JPS, Brook M, Sheets MD, Gray NK. Poly(A)-binding proteins are functionally distinct and have essential roles during vertebrate development. Proc Natl Acad Sci USA. 2011;108:7844–7849. doi: 10.1073/pnas.1017664108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanby AM, Poulsom R, Playford RJ, Wright NA. The mucous neck cell in the human gastric corpus: a distinctive, functional cell lineage. J Pathol. 1999;187:331–337. doi: 10.1002/(SICI)1096-9896(199902)187:3<331::AID-PATH241>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Ho JJD, Robb GB, Tai SC, Turgeon PJ, Mawji IA, Man HSJ, Marsden PA. Active stabilization of human endothelial nitric oxide synthase mRNA by hnRNP E1 protects against antisense RNA and microRNAs. Mol Cell Biol. 2013;33:2029–2046. doi: 10.1128/MCB.01257-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussey GS, Chaudhury A, Dawson AE, Lindner DJ, Knudsen CR, Wilce MCJ, Merrick WC, Howe PH. Identification of an mRNP complex regulating tumorigenesis at the translational elongation step. Mol Cell. 2011;41:419–431. doi: 10.1016/j.molcel.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji X, Kong J, Carstens RP, Liebhaber SA. The 3′ untranslated region complex involved in stabilization of human alpha-globin mRNA assembles in the nucleus and serves an independent role as a splice enhancer. Mol Cell Biol. 2007;27:3290–3302. doi: 10.1128/MCB.02289-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji X, Kong J, Liebhaber SA. In vivo association of the stability control protein alphaCP with actively translating mRNAs. Mol Cell Biol. 2003;23:899–907. doi: 10.1128/MCB.23.3.899-907.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji X, Kong J, Liebhaber SA. An RNA-protein complex links enhanced nuclear 3′ processing with cytoplasmic mRNA stabilization. EMBO J. 2011;30:2622–2633. doi: 10.1038/emboj.2011.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji X, Wan J, Vishnu M, Xing Y, Liebhaber SA. αCP Poly(C) Binding Proteins Act as Global Regulators of Alternative Polyadenylation. Mol Cell Biol. 2013;33:2560–2573. doi: 10.1128/MCB.01380-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karam SM. Lineage commitment and maturation of epithelial cells in the gut. Front Biosci. 1999;4:D286–98. doi: 10.2741/karam. [DOI] [PubMed] [Google Scholar]
- Karam SM. A focus on parietal cells as a renewing cell population. World J Gastroenterol. 2010;16:538–546. doi: 10.3748/wjg.v16.i5.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karam SM, Leblond CP. Dynamics of epithelial cells in the corpus of the mouse stomach. III. Inward migration of neck cells followed by progressive transformation into zymogenic cells. Anat Rec. 1993;236:297–313. doi: 10.1002/ar.1092360204. [DOI] [PubMed] [Google Scholar]
- Kim JH, Hahm B, Kim YK, Choi M, Jang SK. Protein-protein interaction among hnRNPs shuttling between nucleus and cytoplasm. J Mol Biol. 2000;298:395–405. doi: 10.1006/jmbi.2000.3687. [DOI] [PubMed] [Google Scholar]
- Lee PT, Liao PC, Chang WC, Tseng JT. Epidermal growth factor increases the interaction between nucleolin and heterogeneous nuclear ribonucleoprotein K/poly(C) binding protein 1 complex to regulate the gastrin mRNA turnover. Mol Biol Cell. 2007;18:5004–5013. doi: 10.1091/mbc.E07-04-0384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leffers H, Dejgaard K, Celis JE. Characterisation of two major cellular poly(rC)-binding human proteins, each containing three K-homologous (KH) domains. Eur J Biochem. 1995;230:447–453. [PubMed] [Google Scholar]
- Lennerz JKM, Kim SH, Oates EL, Huh WJ, Doherty JM, Tian X, Bredemeyer AJ, Goldenring JR, Lauwers GY, Shin YK, Mills JC. The transcription factor MIST1 is a novel human gastric chief cell marker whose expression is lost in metaplasia, dysplasia, and carcinoma. Am J Pathol. 2010;177:1514–1533. doi: 10.2353/ajpath.2010.100328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makeyev AV, Chkheidze AN, Liebhaber SA. A set of highly conserved RNA-binding proteins, alphaCP-1 and alphaCP-2, implicated in mRNA stabilization, are coexpressed from an intronless gene and its intron-containing paralog. J Biol Chem. 1999;274:24849–24857. doi: 10.1074/jbc.274.35.24849. [DOI] [PubMed] [Google Scholar]
- Makeyev AV, Eastmond DL, Liebhaber SA. Targeting a KH-domain protein with RNA decoys. RNA. 2002;8:1160–1173. doi: 10.1017/s135583820202808x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makeyev AV, Liebhaber SA. The poly(C)-binding proteins: a multiplicity of functions and a search for mechanisms. RNA. 2002;8:265–278. doi: 10.1017/s1355838202024627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markovtsov V, Nikolic JM, Goldman JA, Turck CW, Chou MY, Black DL. Cooperative assembly of an hnRNP complex induced by a tissue-specific homolog of polypyrimidine tract binding protein. Mol Cell Biol. 2000;20:7463–7479. doi: 10.1128/mcb.20.20.7463-7479.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna LB, Schug J, Vourekas A, McKenna JB, Bramswig NC, Friedman JR, Kaestner KH. MicroRNAs control intestinal epithelial differentiation, architecture, and barrier function. Gastroenterology. 2010;139:1654–64. 1664.e1. doi: 10.1053/j.gastro.2010.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrotti D, Calabretta B. Post-transcriptional mechanisms in BCR/ABL leukemogenesis: role of shuttling RNA-binding proteins. Oncogene. 2002;21:8577–8583. doi: 10.1038/sj.onc.1206085. [DOI] [PubMed] [Google Scholar]
- Pin CL, Bonvissuto AC, Konieczny SF. Mist1 expression is a common link among serous exocrine cells exhibiting regulated exocytosis. Anat Rec. 2000;259:157–167. doi: 10.1002/(SICI)1097-0185(20000601)259:2<157::AID-AR6>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Quante M, Marrache F, Goldenring JR, Wang TC. TFF2 mRNA Transcript Expression Marks a Gland Progenitor Cell of the Gastric Oxyntic Mucosa. Gastroenterology. 2010;139:2018–2027. e2. doi: 10.1053/j.gastro.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey VG, Doherty JM, Chen CC, Stappenbeck TS, Konieczny SF, Mills JC. The maturation of mucus-secreting gastric epithelial progenitors into digestive-enzyme secreting zymogenic cells requires Mist1. Development. 2007;134:211–222. doi: 10.1242/dev.02700. [DOI] [PubMed] [Google Scholar]
- Shi H, Bencze KZ, Stemmler TL, Philpott CC. A cytosolic iron chaperone that delivers iron to ferritin. Science. 2008;320:1207–1210. doi: 10.1126/science.1157643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanovic B, Hellerbrand C, Holcik M, Briendl M, Aliebhaber S, Brenner DA. Posttranscriptional regulation of collagen alpha1(I) mRNA in hepatic stellate cells. Mol Cell Biol. 1997;17:5201–5209. doi: 10.1128/mcb.17.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Suzuki H, Imai T, Shibata S, Tabuchi Y, Tsuchimoto K, Okano H, Hibi T. Musashi-1 post-transcriptionally enhances phosphotyrosine-binding domain-containing m-Numb protein expression in regenerating gastric mucosa. PLoS ONE. 2013;8:e53540. doi: 10.1371/journal.pone.0053540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang YS, Khan RA, Zhang Y, Xiao S, Wang M, Hansen DK, Jayaram HN, Antony AC. Incrimination of heterogeneous nuclear ribonucleoprotein E1 (hnRNP-E1) as a candidate sensor of physiological folate deficiency. Journal of Biological Chemistry. 2011;286:39100–39115. doi: 10.1074/jbc.M111.230938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waggoner SA, Johannes GJ, Liebhaber SA. Depletion of the poly(C)-binding proteins alphaCP1 and alphaCP2 from K562 cells leads to p53-independent induction of cyclin-dependent kinase inhibitor (CDKN1A) and G1 arrest. J Biol Chem. 2009;284:9039–9049. doi: 10.1074/jbc.M806986200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Vardy LA, Tan CP, Loo JM, Guo K, Li J, Lim SG, Zhou J, Chng WJ, Ng SB, Li HX, Zeng Q. PCBP1 suppresses the translation of metastasis-associated PRL-3 phosphatase. Cancer Cell. 2010;18:52–62. doi: 10.1016/j.ccr.2010.04.028. [DOI] [PubMed] [Google Scholar]
- Wang X, Kiledjian M, Weiss IM, Liebhaber SA. Detection and characterization of a 3′ untranslated region ribonucleoprotein complex associated with human alpha-globin mRNA stability. Mol Cell Biol. 1995;15:1769–1777. doi: 10.1128/mcb.15.3.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolaway K, Asai K, Emili A, Cochrane A. hnRNP E1 and E2 have distinct roles in modulating HIV-1 gene expression. Retrovirology. 2007;4:28. doi: 10.1186/1742-4690-4-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Chan CY, Jiang B, Yu X, Zhu GZ, Chen Y, Barnard J, Mei W. hnRNP I inhibits Notch signaling and regulates intestinal epithelial homeostasis in the zebrafish. PLoS Genet. 2009;5:e1000363. doi: 10.1371/journal.pgen.1000363. [DOI] [PMC free article] [PubMed] [Google Scholar]