Summary
T-lymphoblastic leukaemia (T-ALL) and T-lymphoblastic lymphoma (T-LBL) are neoplasms derived from immature lymphoid cells of T-cell lineage. These neoplasms are biologically similar, but significant differences may exist between the two given their clinical differences. Although ample data regarding the immunophenotypic characterization T-ALL are available, there is a paucity of such data in children and adolescents with T-LBL. We used flow cytometry and/or immunohistochemistry to characterize the immunophenotypic profile of 180 children and adolescents with newly diagnosed T-LBL enrolled in the Children’s Oncology Group 5971 study. Multiple T-cell, B-cell, myeloid, and other markers were evaluated. We identified diagnostically useful immunophenotypic features of T-LBL as well as distinct immunophenotypic subgroups, although none of these was statistically related to event-free or overall survival in this retrospective analysis. Further studies of biologically and immunophenotypically distinct subgroups of T-LBL, such as the early T-cell precursor phenotype, are warranted.
Keywords: T-lymphoblastic lymphoma, early T-cell precursor, immunophenotypic analysis, paediatric lymphoma, T-cell antigens
Precursor T-acute lymphoblastic leukaemia (T-ALL) and precursor T-lymphoblastic lymphoma (T-LBL) are biologically similar neoplasms derived from immature lymphoid cells of T-cell lineage. The precise nature of the relationship between T-ALL and T-LBL is the subject of debate (Hoelzer & Gokbuget, 2009). Although these neoplasms are closely related, significant differences may exist between the two given the differences in their clinical presentation and sites of relapse (Burkhardt, 2009). The initial clinical manifestation in T-LBL, which accounts for approximately one quarter of childhood non-Hodgkin lymphoma, usually takes the form of a mediastinal mass or lymphadenopathy whereas T-ALL patients present with predominantly bone marrow and peripheral blood disease manifestations (Reiter et al, 1995). T-LBL is distinguished from T-ALL based primarily on the degree of bone marrow involvement by T-lymphoblasts. Patients with <25% bone marrow involvement are classified as T-LBL while patients with 25% or more bone marrow blasts are diagnosed with T-ALL (Swerdlow et al, 2008). Many of the recurrent chromosomal translocations seen in paediatric T-ALL have also been reported in small series of cases of T-LBL (Kaneko et al, 1989; Graux et al, 2006; Lones et al, 2007). Array-based gene expression profiling studies have revealed similarities as well as noteworthy differences between these two entities (Raetz et al, 2006; Schraders et al, 2009). Although substantial overlap exists, immunophenotypic characterization T-ALL in the current literature is much more complete while there is a paucity of extensive immunophenotypic data in children and adolescents who present with T-LBL (Crist et al, 1988; Uckun et al, 1997a; Oschlies et al, 2011).
With intensive modern chemotherapy, cure rates for T-ALL and T-LBL approach 80% (Burkhardt et al, 2006; Pui & Evans, 2006; Abromowitch et al, 2008; Uyttebroeck et al, 2008). Currently, reliable pretreatment identification of the one-fifth of patients who are likely fail conventional therapy is not possible. Patients with T- and B-LBL who fail induction or develop subsequent relapse suffer from poor salvage rates and only rarely achieve long-term even-free survival (Burkhardt et al, 2009). Clinical predictors, such as older age at presentation and high white blood cell count, may be helpful but do not sufficiently account for the risk of treatment failure in one-fifth of patients (Pullen et al, 1999; Goldberg et al, 2003). Measurement of minimal residual disease (MRD) in T-ALL patients at the completion of therapy, however, is associated with negative prognostic significance (Willemse et al, 2002). In addition, in cases of T-LBL, a recent study showed that identification of minimal disseminated disease (MDD) identified in the blood of patients without leukaemic bone marrow disease may also predict unfavourable event-free survival (EFS) (Coustan-Smith et al, 2009a). Immunophenotypic features, such as stem cell and myeloid marker expression, have also been studied in this regard but definitive prognostic conclusions remain elusive (Pui et al, 1993; Vitale et al, 2007; Al Khabori et al, 2008). A previously unrecognized subtype of T-ALL with an early T-cell precursor phenotype (CD1a negative, CD8 negative, CD5 weak positive, CD34/myeloid antigen positive) appears to have a poor prognosis and high risk of treatment failure (Coustan- Smith et al, 2009b). The phenotypic data presented in this study, along with additional studies for determining diagnostic and prognostic utility of immunophenotypic subgroups, may provide a basis for classification and further study of unique subtypes of T-LBL, such as the early T-cell precursor subtype, with respect to prediction of clinical outcome.
We here present the immunophenotypic characterization of a large cohort of children and adolescent with T-LBL enrolled in the 5971 study of the Children’s Oncology Group (COG).
Materials and methods
Patient selection
The COG trial A5971 enrolled patients between 2000 and 2005 and was open to all newly diagnosed patients with lymphoblastic lymphoma, including both T-LBL and B-LBL. The clinical trial and associated studies were approved by the appropriate institutional review boards and informed consent was obtained for all patients prior to enrolment. A retrospective review of de-identified flow cytometric, pathological, and immunohistochemical staining and available clinical data from 186 patients with a diagnosis of T-LBL enrolled in on the trial was performed; 180 patients evaluated were eligible for the study and were included in this analysis. Patients were diagnosed with T-lymphoblastic lymphoma as defined by the current World Health Organization Classification (Swerdlow et al, 2008), and underwent T-ALL type therapy for disseminated T-LBL. As a study criterion, lack of bone marrow involvement or demonstration of <25% bone marrow lymphoblasts by morphologic analysis was required.
Flow cytometry
Immunophenotypic analysis, usually consisting of flow cytometric workup of the initial diagnostic specimen, was performed for each case at the referring institution’s clinical laboratory. As such, marker selection and interpretation were at the discretion of the performing laboratory and pathologist. Multiple T-cell, B-cell, myeloid, stem cell and other markers were evaluated. All cases were subject to centralized pathology diagnostic review by COG pathologists. The immunophenotypic data was compiled and analysed using Microsoft Excel software.
Immunohistochemical staining
Surgical pathology reports submitted with specimens were reviewed to identify pertinent immunohistochemical staining patterns performed at the primary institution. In addition, as part of central pathology review, a limited immunohistochemical staining panel consisting of CD3, CD20, CD79a, CD45RO, and TdT (Dako, Glostrup, Denmark and Supertech, Bethesda, ME, USA) was performed on all cases using formalin-fixed, paraffin embedded diagnostic tumour sections. In some cases, archived immunophenotypic data were incomplete with respect to certain markers of interest. In a subset of these cases, additional immunohistochemical staining was performed on available unstained slides cut from deidentified, formalin-fixed, paraffin embedded tumour sections. CD1a, CD8, and CD33 were performed using commercially available antibodies (Beckman Coulter/Immunotech, Miami, FL, USA and Novocastra, Norwell, MA, USA) on a Ventana ES automated stainer (Ventana, Tucson, AZ, USA) with appropriately staining positive and negative controls. Staining patterns were identified as either membranous or cytoplasmic. These additional studies were performed in order to identify cases with features otherwise suspicious for the early T-cell phenotype (e.g., to evaluate aberrant myeloid antigen expression in CD1a−, CD8−, CD5weak+, CD34+ cases). Positive staining was defined as at least 20% of the tumour cells showing definitive expression of the marker of interest.
Bone marrow disseminated disease
A subset of the patients (59/180 or 33% of the total patients) studied also had data available on the degree of disease dissemination in the bone marrow as assessed by flow cytometric analysis at diagnosis performed at St. Jude Children’s Research Hospital, Memphis, TN, as previously described (Coustan-Smith et al, 2009a). Disseminated disease at diagnosis was expressed as the percentage of T-lymphoblasts among bone marrow mononucleated cells.
Statistical analysis
We further examined three immunophenotypic subgroups of T-LBL, including early T-cell precursor, CD33 positive, and TdT negative cases, for differences in clinical outcome. Cases with available bone marrow disease dissemination at diagnosis by flow cytometry were also compared in terms of presence (≥0·01%) versus absence as well as degree of involvement (≥1% versus lower). EFS and overall survival estimates with 95% confidence intervals were made using the Kaplan–Meier method (median follow-up 4·8 years). Potential association between the immunophenotypic subgroups and patients that had bone marrow disease dissemination at diagnosis was examined using Fisher’s exact test. P values < 0·05 were considered statistically significant.
Results
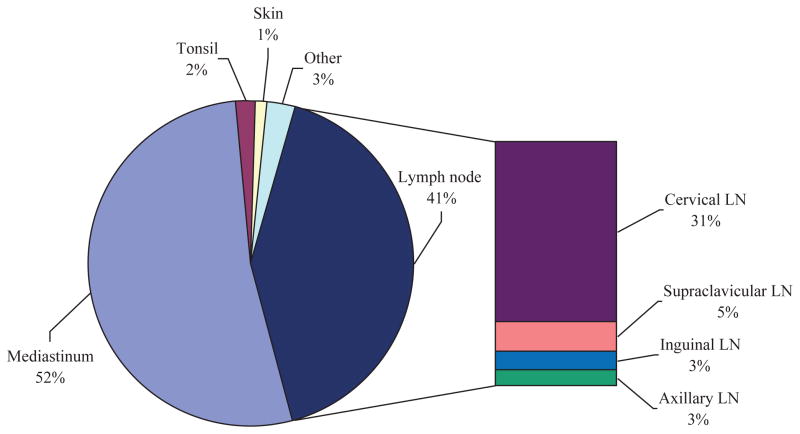
Clinical sites of involvement and diagnostic biopsy are shown in Fig 1. Patients were grouped by site of dominant clinical presentation but, notably, this designation does not exclude concurrent disease at other sites or organs listed. More than half of the T-LBL patients studied presented with a mediastinal mass, followed closely by lymphadenopathy of various sites. Rare cases involved the skin, tonsil, kidney, and bone as primary sites of disease.
Fig 1.
T-lymphoblastic lymphoma: sites of diagnostic biopsy. The majority of patients presented with T-lymphoblastic lymphoma involving mediastinal and/or nodal disease as the primary site. Head and neck lymph nodes showed highest incidence of involvement among lymph node groups, while visceral organs were rarely affected.
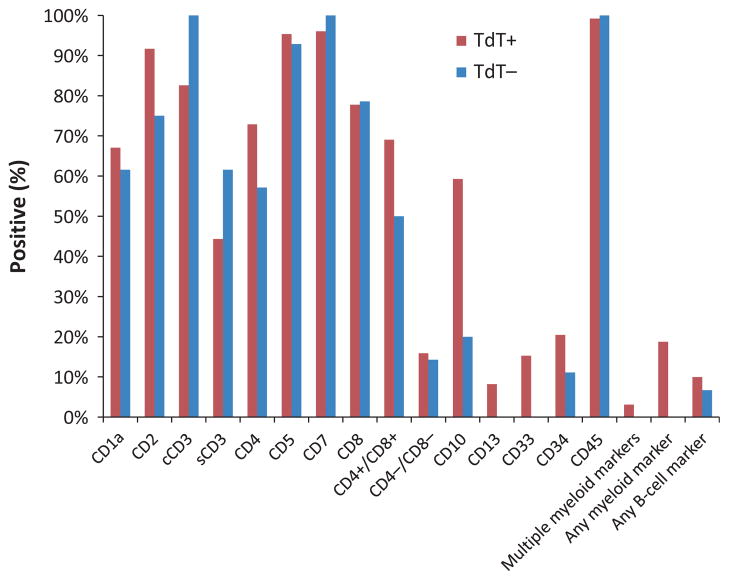
The vast majority (>90%) of T-LBL cases studied were characterized by expression of TdT, CD2, cytoplasmic and/or surface CD3, CD5, and CD7 (Table I). Most cases also expressed CD1a (67%) and CD43 (86%). CD10 was positive in a majority of cases (57%) while expression of the stem-cell marker CD34 was seen in 20%.
Table I.
Immunophenotypic features of T-lymphoblastic lymphoma, selected markers.
| Marker | N★ | Positive (%) |
|---|---|---|
| CD1a | 96 | 66·7 |
| CD2 | 136 | 90·4 |
| cCD3 | 125 | 84 |
| sCD3 | 134 | 46 |
| CD4 | 148 | 71·6 |
| CD5 | 148 | 95·3 |
| CD7 | 144 | 96·5 |
| CD8 | 145 | 77·9 |
| CD4+/CD8+ | 138 | 71 |
| CD4−/CD8− | 138 | 16·7 |
| CD10 | 128 | 57 |
| CD13 | 70 | 7·1 |
| CD30 | 31 | 3·2 |
| CD33 | 69 | 13 |
| CD34 | 106 | 19·8 |
| CD45 | 142 | 99·3 |
| CD79a | 135 | 5·2 |
| TdT | 170 | 90 |
| Multiple myeloid markers | 75† | 2·6 |
| Any myeloid marker | 92★ | 16·3 |
| Any B-cell marker | 172★ | 9·3 |
Number with at least one marker with data.
Number with at least two markers with data.
B-cell markers: CD19, CD20, CD22, CD24, CD45RA, CD79a.
Myeloid markers: CD13, CD14, CD15, CD33.
Aberrant antigen expression occurs in a subset of T-LBL. Nearly one-fifth of cases in this paediatric cohort showed positivity for at least one myeloid marker; <5% expressed multiple myeloid markers. CD33 was the most likely to be positive (13%), followed by CD13 (7%) and CD15 (6%). Up to 9% showed co-expression of a B-cell associated antigen, with CD79a and CD45RA each seen in approximately 5% of cases. Rare examples of T-LBL with expression of CD22 and CD24 were also noted.
Approximately 10% of T-LBL lacked expression of TdT by flow cytometry and immunohistochemistry. The TdT− cases (n = 16) retained an immunoprofile that was otherwise similar to their TdT+ counterparts, with positivity for CD2, CD5, and CD7 in most cases (Fig 2). Some notable differences were observed, however. TdT− T-LBL appeared less likely to express aberrant myeloid or B-cell associated antigens as well as CD10 or CD34. Double positivity for CD4 and CD8 was also decreased in TdT− cases relative to TdT+ (69% vs. 50%, respectively), although with the exception of CD10+ association with TdT+ expression (P = 0·02, Fisher’s exact test), these differences were not statistically significant.
Fig 2.
Expression of selected immunophenotypic markers in T-lymphoblastic lymphoma patients. All cases demonstrated positivity for cytoplasmic and/or surface CD3 and the pan T-cell antigens CD2, CD5, and CD7 were expressed in the vast majority. Aberrant myeloid antigens were observed in 16% of cases, with CD33 being the most likely antigen to be expressed. TdT was demonstrably positive in 90% of cases. TdT negative cases showed a similar immunoprofile, with notably decreased incidence of CD4/CD8 double positivity and aberrant myeloid antigen expression.
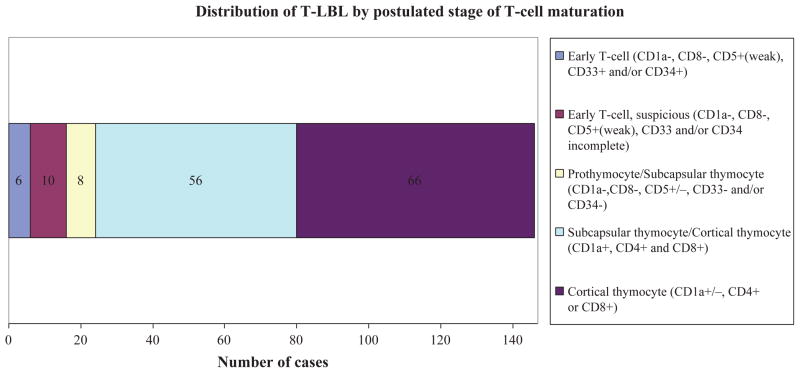
An attempt was made to divide cases into postulated normal counterpart T-cell maturation stages by immunophenotype. For the purposes of T-LBL, these included early T-cell, prothymocyte, subcapsular thymocyte, and cortical thymocyte (Fig 3). Overlap between these stages exists biologically and phenotypically, particularly with regard to the distinction between prothymocyte and subcapsular thymocyte as well as subcapsular and cortical thymocyte (Bene et al, 1995; Cauwelier et al, 2007; Han & Bueso-Ramos, 2007). This limitation is reflected in our results. Early T-cell subtype was defined by the following immunoprofile: CD1a−, CD8−, and CD5+/weak with positivity for one or more myeloid (e.g., CD13, CD33) or stem cell (e.g., CD34) antigens. In cases where relevant stem cell and/or myeloid antigens were not performed, but which otherwise fitted the early T-cell phenotype (e.g., CD1a−, CD8−, CD5+/weak) were designated suspicious. Seven cases met the criteria for early T-cell subtype while 10 were suspicious. The prothymocyte maturational stage is characterized by a CD1a−, CD7+, CD8−, and TdT+ phenotype with demonstrated negativity for myeloid and stem cell antigens in order to exclude possible early T-cell subtype. The subcapsular thymocyte stage was distinguished by negativity for CD1a and CD4+CD8+ double positivity, while cortical thymocytes were defined primarily by CD1a+, with CD4+ and/or CD8+ in most cases. Thirty-four cases were not classified due to incomplete data for necessary markers.
Fig 3.
Distribution of T-LBL by postulated stage of T-cell maturation. Six cases (4%) clearly met the criteria, shown above, for early T cell subtype [CD1a−, CD8−, and CD5+/weak with positivity for one or more myeloid (e.g., CD13, CD33) or stem cell (e.g., CD34) antigens] while an additional 10 cases (7%) showed phenotypic features in keeping with early T cell but lacked myeloid antigen data. The great majority of cases showed an immunoprofile consistent with that of subcapsular or cortical thymocytes (84%). Thirty-four cases could not be classified due to incomplete immunophenotypic data.
Correlation of specimen site with phenotypic features showed a significant negative association between mediastinal disease and early T-cell phenotype, with only four cases (5% of total evaluable cases) presenting with primary mediastinal disease (P = 0·031) Fischer’s exact test) compared with presentation in the lymph nodes or other sites. There was no significant association of CD33 or TdT expression with primary site of disease (mediastinal versus lymph node/other site).
Bone marrow involvement was not uniformly reported for the entire patient cohort, nor were bone marrow aspirates available for central pathology morphological review to document bone marrow involvement. A subset of the patients (59/180 or 33%) underwent centrally performed flow cytometric analysis for documentation of bone marrow involvement as an indicator of bone marrow disseminated disease. Approximately 75% (44 patients) in this subset demonstrated a measurable degree of bone marrow involvement while the remaining 25% (15 cases) were free of detectable bone marrow disease (defined as <0·01% involvement). The median level of involvement was 0·041% bone marrow leucocytes, being represented by T-lymphoblasts and the maximum level was 28·465%. The patients with bone marrow involvement were initially stratified to very low-level involvement (>0·01% but <1% T-lymphoblasts), low-level disease (defined as ≥1% but <5% T-lymphoblasts) and high-risk disease (defined as ≥5% T-lymphoblasts). Very low-level involvement was seen in 49% of patients (29/59 cases) and low-level involvement was seen in 17% (10/59 cases). Only five patients (8%) in the subset analysis were defined as having high-risk disseminated bone marrow disease at diagnosis. The low numbers of high risk patients in this subset analysis precluded further meaningful statistical analysis in this group, so all subsequent analyses were performed on cases felt to have significant bone marrow involvement, defined as ≥1% bone marrow lymphoblasts.
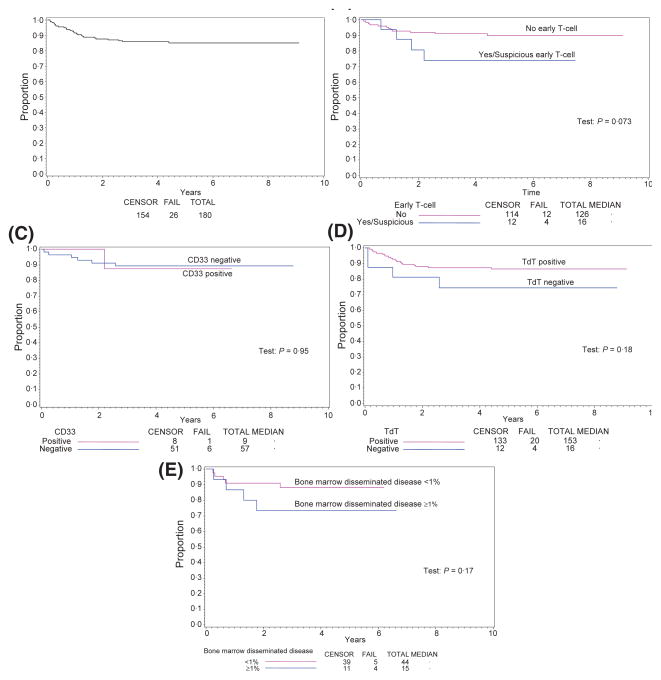
Estimated 4-year EFS and overall survival for all patients was 86% and 88%, respectively (Fig 4, panel A, only EFS data shown). Although there appeared to be a trend towards decreased EFS and overall survival in early T-cell precursor (Fig 4, panel B) and TdT negative cases (Fig 4, panel C), outcomes comparisons showed no statistically significant differences in EFS or overall survival (data not shown) between the subgroups studied. CD33 expression alone did not show independent prognostic significance (Fig 4, panel D). Bone marrow disseminated disease at diagnosis ≥1% positive cases also showed no statistically significant association with EFS (Fig 4, panel E) or overall survival (data not shown) in this selected patient cohort, possibly reflecting the small numbers of cases that had both immunophenotypic and bone marrow disseminated disease data available for analysis. However, increasing degrees of bone marrow disseminated disease at diagnosis appeared to trend towards decreased survival. Notably, a bone marrow disseminated disease at diagnosis level of ≥1% was significantly correlated with the early T cell phenotype. Other immunophenotypic variables did not show statistical correlation with bone marrow disseminated disease status (Table II).
Fig 4.
Event-free survival curves for all patients by selected immunophenotypic parameters and bone marrow disseminated disease. Kaplan–Meier plots showing (A) event-free survival (EFS) for all eligible patients, (B) early T cell subtype versus non early T cell, (C) CD33 positive versus negative, (D) TdT positive versus negative, (E) Bone marrow disseminated disease <1% vs. ≥1% bone marrow T-lymphoblasts by flow cytometric analysis. Statistically significant differences in EFS were not observed.
Table II.
Associations between bone marrow disseminated disease and selected immunophenotypic variables.
| Bone marrow T-lymphoblasts ≥1% N (%) |
Bone marrow T-lymphoblasts <1% N (%) |
P-value | ||
|---|---|---|---|---|
| Early T-cell | No | 34 (97) | 5 (50) | 0·0011 |
| Yes/Suspicious | 1 (3) | 5 (50) | ||
| CD33 | Negative | 17 (94) | 5 (71) | 0·18 |
| Positive | 1 (6) | 2 (29) | ||
| TdT | Negative | 2 (5) | 0 (0) | 1·0 |
| Positive | 37 (95) | 15 (100) |
Discussion
We studied the immunophenotype T-LBL in a large cohort of paediatric patients. Our findings indicate, similar to other small case series reports, that the overall immunoprofile of T-LBL is generally similar to that described in T-ALL (Lewis et al, 2006; Uyttebroeck et al, 2007; Hoelzer & Gokbuget, 2009), with a few notable exceptions that are discussed below. T-LBL is characterized by TdT positivity in at least 90% of cases. The combination of T-cell associated antigens CD2, CD5, and CD7 shows high sensitivity and typifies at least 90% of T-LBL but is not lineage-specific and may be seen in rare natural killer cell precursor neoplasms (Spits et al, 1995). CD3 is lineage-specific and cytoplasmic and/or surface positivity is seen in nearly all cases of T-LBL. Co-expression with the B-cell antigen receptor complex associated protein CD79a occurs less often than has been previously reported and was seen in approximately 5% of cases (Pilozzi et al, 1998). The frequency of CD4/CD8 double positive cases supports the notion that T-LBL is derived in most cases from a more mature thymocyte counterpart relative to T-ALL (Weiss et al, 1986; Uyttebroeck et al, 2007). Indeed, when classified according to T-cell maturation stage, over 75% of cases demonstrated subcapsular or cortical thymocyte-like phenotypes. The distribution of thymic maturational stages in our study roughly mirrors those recently presented in another large series of T lymphoblastic lymphomas (Oschlies et al, 2011).
Early T-cell precursor ALL is a recently described pathobiological subtype of T-ALL that confers poor prognosis when treated with standard chemotherapy (Coustan-Smith et al, 2009b). Biologically, the early T-cell phenotype represents a subset of prothymocytes that appear to be directly derived from haematopoietic stem cells and are characterized by retention of lineage plasticity, particularly myeloid lineage potential (Bell & Bhandoola, 2008; Wada et al, 2008). This phenotype accounted for approximately 12% of patients in a cohort of 239 T-ALL patients in which it was described. Our data show that phenotype occurs in T-LBL and likely does so with similar frequency. Together, early T-cell and suspicious early T-cell phenotype cases totalled approximately 14% of cases in this cohort.
The incidence of aberrant myeloid antigen expression in T-LBL is around 20%, and appears slightly lower but near to the rates previously described in T-ALL (Uckun et al, 1997b; Putti et al, 1998). CD33 is the most likely myeloid marker to be aberrantly expressed, followed by CD13. Multiple myeloid antigens are rarely present. Biologically, myeloid antigen expression in T-LBL may indicate greater lineage plasticity in this subset, and perhaps confers decreased tumour susceptibility to lymphoid-specific therapeutic regimens currently employed in T-ALL/T-LBL (Kalina et al, 2005; Al Khabori et al, 2008). Clinical outcome studies in these patients, however, have shown conflicting data with regard to the prognostic significance, or lack thereof, of myeloid antigen positivity (Khalidi et al, 1999; Vitale et al, 2007).
The TdT− subset is also interesting, as these often represent diagnostically challenging cases. Although the absolute number of these cases is small (n = 16 or 8·9% of total cases), TdT− LBL appears less likely to express aberrant B-cell markers, myeloid antigens, CD10, and CD34. These cases are likely to retain expression of cytoplasmic CD3, CD5, and CD7 with either CD4 and/or CD8. These phenotypic features are consistent with a relatively mature cortical or medullary thymocyte phenotype.
The immunophenotypic subgroups studied showed no statistically significant differences in EFS or overall survival. Moreover, the lack of sufficient numbers of cases with bone marrow disseminated disease at diagnosis and full immunophenotypic analysis available prevented us to conclusively determine whether immunophenotype or the extent of disease dissemination can be considered independent prognostic variables. In the early T-cell precursor group, it remains unclear whether the failure to show a survival difference suggests lack of prognostic significance in T-LBL (as opposed to T-ALL) or whether the lack of a demonstrable difference may be due to impurity of the subgrouping due to incomplete immunophenotypic data in some cases and lack of availability of tissue samples for definitive identification of the early T-cell precursor gene expression signature. In addition, due to the low number of early T-cell precursor cases and relatively few treatment failures, the study may have been inadequately powered to reveal differences in survival in this small group of patients. In light of these deficiencies, T-LBL patients with an early T-cell precursor phenotype may yet suffer a relatively poor prognosis and could benefit from early recognition, though further study and clinical correlation is necessary. The association between early T cell precursor phenotype and bone marrow disseminated disease positivity ≥1% may support such an interpretation.
One limitation of our study is that the flow cytometric analysis of patient specimens was performed at various referring laboratories rather than in a central laboratory. Hence, selection of markers interpretation criteria was, to some degree, at the discretion of the primary pathologists that made the initial diagnosis of T-LBL. Markers of potential interest, such as CD117, could not be evaluated in many cases due to lack of reporting or evidence of testing from the submitting institution.
In summary, we provide an immunophenotypic characterization of T-LBL in a large cohort of paediatric patients. The data reported will serve as a reference for further studies aimed at fully elucidating the prognostic significance of the subgroups that identified here.
Footnotes
Author contributions
JLP, JA, MA, DC, JJ, MAL, TGG, MSC, and SLP designed the research study and performed the research. JA, MA, DC, JJ, MAL TGG, MSC and SLP were all involved in the clinical trial from which the clinical/pathologic data and patient samples were derived. JLP, LMS and SLP performed the specific immunophenotypic research subset analysis presented in the manuscript. LMS performed the statistical analysis for both the clinical trial and the pathologic analysis. JLP, LMS, and SLP wrote the paper with input from the other authors.
References
- Abromowitch M, Sposto R, Perkins S, Zwick D, Siegel S, Finlay J, Cairo MS. Shortened intensified multi-agent chemotherapy and non-cross resistant maintenance therapy for advanced lymphoblastic lymphoma in children and adolescents: report from the Children’s Oncology Group. British Journal of Haematology. 2008;143:261–267. doi: 10.1111/j.1365-2141.2008.07320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Khabori M, Samiee S, Fung S, Xu W, Brandwein J, Patterson B, Brien W, Chang H. Adult precursor T-lymphoblastic leukemia/lymphoma with myeloid-associated antigen expression is associated with a lower complete remission rate following induction chemotherapy. Acta Haematologica. 2008;120:5–10. doi: 10.1159/000146081. [DOI] [PubMed] [Google Scholar]
- Bell JJ, Bhandoola A. The earliest thymic progenitors for T cells possess myeloid line-age potential. Nature. 2008;452:764–767. doi: 10.1038/nature06840. [DOI] [PubMed] [Google Scholar]
- Bene MC, Castoldi G, Knapp W, Ludwig WD, Matutes E, Orfao A, Van’t Veer MB. Proposals for the immunological classification of acute leukemias. European Group for the Immunological Characterization of Leukemias (EGIL) Leukemia. 1995;9:1783–1786. [PubMed] [Google Scholar]
- Burkhardt B. Paediatric lymphoblastic T-cell leukaemia and lymphoma: one or two diseases? British Journal of Haematology. 2009;149:653–668. doi: 10.1111/j.1365-2141.2009.08006.x. [DOI] [PubMed] [Google Scholar]
- Burkhardt B, Woessmann W, Zimmermann M, Kontny U, Vormoor J, Doerffel W, Mann G, Henze G, Niggli F, Ludwig WD, Janssen D, Riehm H, Schrappe M, Reiter A. Impact of cranial radiotherapy on central nervous system prophylaxis in children and adolescents with central nervous system-negative stage III or IV lymphoblastic lymphoma. Journal of Clinical Oncology. 2006;24:491–499. doi: 10.1200/JCO.2005.02.2707. [DOI] [PubMed] [Google Scholar]
- Burkhardt B, Reiter A, Landmann E, Lang P, Lassay L, Dickerhoff R, Lakomek M, Henze G, von Stackelberg A. Poor outcome for children and adolescents with progressive disease or relapse of lymphoblastic lymphoma: a report from the berlin-frankfurt-muenster group. Journal of Clinical Oncology. 2009;27:3363–3369. doi: 10.1200/JCO.2008.19.3367. [DOI] [PubMed] [Google Scholar]
- Cauwelier B, Cave H, Gervais C, Lessard M, Barin C, Perot C, Van den Akker J, Mugneret F, Charrin C, Pagès MP, Grégoire MJ, Jonveaux P, Lafage-Pochitaloff M, Mozzicconacci MJ, Terré C, Luquet I, Cornillet-Lefebvre P, Laurence B, Plessis G, Lefebvre C, Leroux D, Antoine-Poirel H, Graux C, Mauvieux L, Heimann P, Chalas C, Clappier E, Verhasselt B, Benoit Y, Moerloose BD, Poppe B, Van Roy N, Keersmaecker KD, Cools J, Sigaux F, Soulier J, Hagemeijer A, Paepe AD, Dastugue N, Berger R, Speleman F. Clinical, cytogenetic and molecular characteristics of 14 T-ALL patients carrying the TCRbeta-HOXA rearrangement: a study of the Groupe Francophone de Cytogenetique Hematologique. Leukemia. 2007;21:121–128. doi: 10.1038/sj.leu.2404410. [DOI] [PubMed] [Google Scholar]
- Coustan-Smith E, Sandlund JT, Perkins SL, Chen H, Chang M, Abromowitch M, Compana D. Minimal disseminated disease in childhood T-cell lymphoblastic lymphoma: a report from the children’s oncology group. Journal of Clinical Oncology. 2009a;27:3533–3539. doi: 10.1200/JCO.2008.21.1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coustan-Smith E, Mullighan CG, Onciu M, Behm FG, Raimondi SC, Pei D, Cheng C, Su X, Rubnitz JE, Basso G, Biondi A, Pui CH, Downing JR, Compana D. Early T-cell precursor leukaemia: a subtype of very high-risk acute lymphoblastic leukaemia. The lancet oncology. 2009b;10:147–156. doi: 10.1016/S1470-2045(08)70314-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crist WM, Shuster JJ, Falletta J, Pullen DJ, Berard CW, Vietti TJ, Alvarado CS, Roper MA, Prasthofer E, Grossi CE. Clinical features and outcome in childhood T-cell leukemia-lymphoma according to stage of thymocyte differentiation: a Pediatric Oncology Group Study. Blood. 1988;72:1891–1897. [PubMed] [Google Scholar]
- Goldberg JM, Silverman LB, Levy DE, Dalton VK, Gelber RD, Lehmann L, Cohen HJ, Sallan SE, Asselin BL. Childhood T-cell acute lymphoblastic leukemia: the Dana-Farber Cancer Institute acute lymphoblastic leukemia consortium experience. Journal of Clinical Oncology. 2003;21:3616–3622. doi: 10.1200/JCO.2003.10.116. [DOI] [PubMed] [Google Scholar]
- Graux C, Cools J, Michaux L, Vandenberghe P, Hagemeijer A. Cytogenetics and molecular genetics of T-cell acute lymphoblastic leukemia: from thymocyte to lymphoblast. Leukemia. 2006;20:1496–1510. doi: 10.1038/sj.leu.2404302. [DOI] [PubMed] [Google Scholar]
- Han X, Bueso-Ramos CE. Precursor T-cell acute lymphoblastic leukemia/lymphoblastic lymphoma and acute biphenotypic leukemias. American Journal of Clinical Pathology. 2007;127:528–544. doi: 10.1309/2QE3A6EKQ8UYDYRC. [DOI] [PubMed] [Google Scholar]
- Hoelzer D, Gokbuget N. T-cell lymphoblastic lymphoma and T-cell acute lymphoblastic leukemia: a separate entity? Clinical lymphoma & myeloma. 2009;9:S214–S221. doi: 10.3816/CLM.2009.s.015. [DOI] [PubMed] [Google Scholar]
- Kalina T, Vaskova M, Mejstrikova E, Madzo J, Trka J, Stary J, Hrusak O. Myeloid antigens in childhood lymphoblastic leukemia: clinical data point to regulation of CD66c distinct from other myeloid antigens. BMC Cancer. 2005;5:38. doi: 10.1186/1471-2407-5-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko Y, Frizzera G, Shikano T, Kobayashi H, Maseki N, Sakurai M. Chromosomal and immunophenotypic patterns in T cell acute lymphoblastic leukemia (T ALL) and lymphoblastic lymphoma (LBL) Leukemia. 1989;3:886–892. [PubMed] [Google Scholar]
- Khalidi HS, Chang KL, Medeiros LJ, Brynes RK, Slovak ML, Murata-Collins JL, Arber DA. Acute lymphoblastic leukemia. Survey of immunophenotype, French-American-British classification, frequency of myeloid antigen expression, and karyotypic abnormalities in 210 pediatric and adult cases. American Journal of Clinical Pathology. 1999;111:467–476. doi: 10.1093/ajcp/111.4.467. [DOI] [PubMed] [Google Scholar]
- Lewis RE, Cruse JM, Sanders CM, Webb RN, Tillman BF, Beason KL, Lam J, Koehler J. The immunophenotype of pre-TALL/LBL revisited. Experimental and Molecular Pathology. 2006;81:162–165. doi: 10.1016/j.yexmp.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Lones MA, Heerema NA, Le Beau MM, Sposto R, Perkins SL, Kadin ME, Kjeldsberg CR, Meadows A, Siegel S, Buckley J, Abromowitch M, Kersey J, Bergeron S, Cairo MS, Sanger WG. Chromosome abnormalities in advanced stage lymphoblastic lymphoma of children and adolescents: a report from CCG-E08. Cancer Genetics and Cytogenetics. 2007;172:1–11. doi: 10.1016/j.cancergencyto.2006.07.011. [DOI] [PubMed] [Google Scholar]
- Oschlies I, Burkhardt B, Chassagne-Clement C, D’Amore ES, Hansson U, Hebeda K, Mc Carthy K, Kodet R, Maldyk J, Müllauer L, Porwit A, Schmatz AI, Tinguely M, Abramov D, Wotherspoon A, Zimmermann M, Reiter A, Klapper W. Diagnosis and immunophenotype of 188 pediatric lymphoblastic lymphomas treated within a randomized prospective trial: experiences and preliminary recommendations from the European Childhood Lymphoma Pathology Panel. American Journal of Surgical Pathology. 2011;35:836–844. doi: 10.1097/PAS.0b013e318213e90e. [DOI] [PubMed] [Google Scholar]
- Pilozzi E, Pulford K, Jones M, Muller-Hermelink HK, Falini B, Ralfkiaer E, Pileri S, Pezzella F, De Wolf-Peeters C, Arber D, Stein H, Mason D, Gatter K. Co-expression of CD79a (JCB117) and CD3 by lymphoblastic lymphoma. The Journal of pathology. 1998;186:140–143. doi: 10.1002/(SICI)1096-9896(1998100)186:2<140::AID-PATH149>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Pui CH, Evans WE. Treatment of acute lymphoblastic leukemia. New England Journal of Medicine. 2006;354:166–178. doi: 10.1056/NEJMra052603. [DOI] [PubMed] [Google Scholar]
- Pui CH, Hancock ML, Head DR, Rivera GK, Look AT, Sandlund JT, Behm FG. Clinical significance of CD34 expression in childhood acute lymphoblastic leukemia. Blood. 1993;82:889–894. [PubMed] [Google Scholar]
- Pullen J, Shuster JJ, Link M, Borowitz M, Amylon M, Carroll AJ, Land V, Look AT, McIntyre B, Camitta B. Significance of commonly used prognostic factors differs for children with T cell acute lymphocytic leukemia (ALL), as compared to those with B-precursor ALL. A Pediatric Oncology Group (POG) study. Leukemia. 1999;13:1696–1707. doi: 10.1038/sj.leu.2401555. [DOI] [PubMed] [Google Scholar]
- Putti MC, Rondelli R, Cocito MG, Arico M, Sainati L, Conter V, Guglielmi C, Cantú-Rajnoldi A, Consolini R, Pession A, Zanesco L, Masera G, Biondi A, Basso G. Expression of myeloid markers lacks prognostic impact in children treated for acute lymphoblastic leukemia: Italian experience in AIEOP-ALL 88–91 studies. Blood. 1998;92:795–801. [PubMed] [Google Scholar]
- Raetz EA, Perkins SL, Bhojwani D, Smock K, Philip M, Carroll WL, Minn DJ. Gene expression profiling reveals intrinsic differences between T-cell acute lymphoblastic leukemia and T-cell lymphoblastic lymphoma. Pediatric Blood & Cancer. 2006;47:130–140. doi: 10.1002/pbc.20550. [DOI] [PubMed] [Google Scholar]
- Reiter A, Schrappe M, Parwaresch R, Henze G, Muller-Weihrich S, Sauter S, Sykora KW, Ludwig WD, Gadner H, Riehm H. Non-Hodgkin’s lymphomas of childhood and adolescence: results of a treatment stratified for biologic subtypes and stage – a report of the Berlin-Frankfurt-Munster Group. Journal of Clinical Oncology. 1995;13:359–372. doi: 10.1200/JCO.1995.13.2.359. [DOI] [PubMed] [Google Scholar]
- Schraders M, van Reijmersdal SV, Kamping EJ, van Krieken JH, van Kessel AG, Groenen PJ, Hoogerbrugge PM, Kuiper RP. High-resolution genomic profiling of pediatric lymphoblastic lymphomas reveals subtle differences with pediatric acute lymphoblastic leukemias in the B-lineage. Cancer Genetics and Cytogenetics. 2009;191:27–33. doi: 10.1016/j.cancergencyto.2009.01.002. [DOI] [PubMed] [Google Scholar]
- Spits H, Lanier LL, Phillips JH. Development of human T and natural killer cells. Blood. 1995;85:2654–2670. [PubMed] [Google Scholar]
- Swerdlow SHCE, Harris NL, Jaffe ES, Pileri SA, Thiele J, Vardiman JW, editors. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. IARC; Lyon: 2008. [Google Scholar]
- Uckun FM, Gaynon PS, Sensel MG, Nachman J, Trigg ME, Steinherz PG, Hutchinson R, Bostrom BC, Sather HN, Reaman GH. Clinical features and treatment outcome of childhood T-lineage acute lymphoblastic leukemia according to the apparent maturational stage of T-lineage leukemic blasts: a Children’s Cancer Group study. Journal of Clinical Oncology. 1997a;15:2214–2221. doi: 10.1200/JCO.1997.15.6.2214. [DOI] [PubMed] [Google Scholar]
- Uckun FM, Sather HN, Gaynon PS, Arthur DC, Trigg ME, Tubergen DG, Nachman J, Steinherz PG, Sensel MG, Reaman GH. Clinical features and treatment outcome of children with myeloid antigen positive acute lymphoblastic leukemia: a report from the Children’s Cancer Group. Blood. 1997b;90:28–35. [PubMed] [Google Scholar]
- Uyttebroeck A, Vanhentenrijk V, Hagemeijer A, Boeckx N, Renard M, Wlodarska I, Vandenberghe P, Depaepe P, De Wolf-Peeters C. Is there a difference in childhood T-cell acute lymphoblastic leukaemia and T-cell lymphoblastic lymphoma? Leukaemia & Lymphoma. 2007;48:1745–1754. doi: 10.1080/10428190701509772. [DOI] [PubMed] [Google Scholar]
- Uyttebroeck A, Suciu S, Laureys G, Robert A, Pacquement H, Ferster A, Marguerite G, Mazingue F, Renard M, Lutz P, Rialland X, Mechinaud F, Cavé H, Baila L, Bertrand Y. Treatment of childhood T-cell lymphoblastic lymphoma according to the strategy for acute lymphoblastic leukaemia, without radiotherapy: long term results of the EORTC CLG 58881 trial. European Journal of Cancer. 2008;44:840–846. doi: 10.1016/j.ejca.2008.02.011. [DOI] [PubMed] [Google Scholar]
- Vitale A, Guarini A, Ariola C, Meloni G, Perbellini O, Pizzuti M, De Gregoris C, Mettivier V, Pastorini A, Pizzolo G, Vignetti M, Mandelli F, Foà R. Absence of prognostic impact of CD13 and/or CD33 antigen expression in adult acute lymphoblastic leukemia. Results of the GIMEMA ALL 0496 trial. Haematologica. 2007;92:342–348. doi: 10.3324/haematol.10385. [DOI] [PubMed] [Google Scholar]
- Wada H, Masuda K, Satoh R, Kakugawa K, Ikawa T, Katsura Y, Kawamoto H. Adult T-cell progenitors retain myeloid potential. Nature. 2008;452:768–772. doi: 10.1038/nature06839. [DOI] [PubMed] [Google Scholar]
- Weiss LM, Bindl JM, Picozzi VJ, Link MP, Warnke RA. Lymphoblastic lymphoma: an immunophenotype study of 26 cases with comparison to T cell acute lymphoblastic leukemia. Blood. 1986;67:474–478. [PubMed] [Google Scholar]
- Willemse MJ, Seriu T, Hettinger K, d’Aniello E, Hop WC, Panzer-Grumayer ER, et al. Detection of minimal residual disease identifies differences in treatment response between T-ALL and precursor B-ALL. Blood. 2002;99:4386–4393. doi: 10.1182/blood.v99.12.4386. [DOI] [PubMed] [Google Scholar]