Abstract
We speculated that some individuals with de novo acute myelogenous leukemia (AML) may have undiagnosed Fanconi Anemia (FA). Data from patients enrolled on AML protocol CCG-2961, published FA cohort studies, SEER, and Bayes rule were used to estimate the probability of FA among all newly diagnosed AML cases, and among those who had no or delayed recovery of the absolute neutrophil count following initial chemotherapy. We determined that the probability of undiagnosed FA in patients in a treatment trial for newly diagnosed patients was around 0.18%, and around 0.83% in the subset who had poor marrow recovery. We suggest that FA or other inherited bone marrow failure syndromes be considered prior to treatment, or certainly among those with poor recovery.
Keywords: Fanconi anemia, Acute myelogenous leukemia, Prevalence
1. Introduction
Fanconi anemia (FA) is an inherited bone marrow failure syndrome (IBMFS) characterized by varying degrees of bone marrow failure, birth defects, and high risks of myelodysplastic syndrome, acute myelogenous leukemia (AML) and specific solid tumors [1]. Analysis of several FA cohorts indicates that FA patients are more than 500-fold more likely to develop AML than the general population [2,3]. In addition, AML was the first presentation that led to the diagnosis of FA in approximately one-third of published cases of FA with AML [4]. Due to increased sensitivity to the cytotoxic effects of chemotherapy, patients with FA may have prolonged or failure of recovery of adequate neutrophil counts, which may lead to significant treatment-related morbidity or mortality. Indeed, in some of the published cases, FA was diagnosed because the patient failed to recover bone marrow function following chemotherapy. The phenotypic heterogeneity of FA, extraordinarily high incidence of AML among FA patients, and exceptionally elevated risk of treatment-related toxicity in this group raise the question of whether all newly diagnosed AML patients should be screened for FA. In this study, we analyzed the distribution of recovery of the absolute neutrophil count (ANC) in patients with de novo AML, and speculated that the group with delayed or no ANC recovery would be enriched with FA patients. We evaluated whether the probability of undiagnosed FA among patients with de novo AML exceeds zero.
2. Methods
A key quantity of interest is the probability of FA among AML patients [P(FA|AML)]. To estimate this probability, we used Bayes rule to relate P(FA|AML) to other quantities that can be estimated from existing data: P(FA|AML) = [P(AML|FA) × P(FA)]/P(AML). Results from published FA cohorts were used to estimate the probability of developing AML among patients with FA, P(AML|FA). The recently revised FA carrier frequency of 1 in 180 [5] and autosomal recessive pattern of inheritance were used to estimate the probability of an individual having FA, P(FA). Data from the Surveillance, Epidemiology and End Results (SEER) Program were used to estimate the probability of a person developing AML during childhood, P(AML), which was the group eligible for Children's Oncology Group (COG) treatment protocol CCG-2961. We used a simple parametric bootstrap to calculate a confidence interval for P(FA|AML) assuming independent normal distributions for our estimates of P(AML|FA), P(FA), and P(AML). In a comprehensive assessment of the world's literature cases, about one-third were not diagnosed until AML occurred [4]. Therefore, the value of p = P(FA|AML)/3 was used to estimate the probability that an AML case in a treatment trial for sporadic AML in fact had undiagnosed FA. Data on the time to recovery of ANC above 1000/μL were examined in 892 de novo AML patients enrolled on CCG-2961 between 1997 and 2002. From these data we determined the number M and proportion f = M/N of patients who failed to achieve an ANC >1000/μL after the first round of chemotherapy. We assumed that any patient with undiagnosed FA would be included in this group of treatment recovery failures. We also calculated the proportion of recovery failures who had undiagnosed FA as p/f. Finally, for hypothetical screening studies for FA, we used the binomial distribution to calculate the probability of detecting at least one undiagnosed patient with FA in the N trial participants assuming a true proportion p, and in the M patients who failed recovery assuming a true proportion p/f.
3. Results
Using data on the annual hazard rate of AML from published FA cohorts, we estimated that the probability of AML in FA patients, P(AML|FA), is 0.5% per year (Table 1A) [3]. The most plausible FA carrier frequency in North America was recently revised from the often cited 1 in 300, to be 1 in 180 [5]. Assuming an autosomal recessive mode of inheritance and the FA carrier frequency of 1/180, the probability of FA in the US population, P(FA), was estimated to be 1 in 129,600 births. SEER data were used to estimate the age-adjusted annual probability of AML, P(AML), in persons 0–18 years, as 0.72/100,000. Hence, the estimated probability that a newly diagnosed AML patient has FA [P(FA|AML)] is 0.54%. Assuming that the prior rate of presentation of undiagnosed FA with AML continues to be one-third of FA patients with AML, the probability of FA among patients being enrolled on a protocol for sporadic AML is 0.54%/3 = 0.18% (Table 1A). The 95% confidence interval (CI) for this quantity, not reflecting uncertainty about the scaling factor of 1/3, is 0.10–0.27%.
Table 1.
Incidence of undiagnosed FA among newly diagnosed AML patients ≤18 yo.
| A |
| Probability of AML among FA patients, derived from published cohorts: |
| P(AML|FA) = 0.5%/year (95% CIa: 0.33–0.71%) |
| Probability of FA based on carrier frequency, derived from FARFb data: |
| P(FA) = 1/180 × 1/180 × 1/4 = 1/129,600 (95% CI: 1/174,700–1/97,300) |
| Annual probability of AML ≤18 yo derived from SEERc data: |
| P(AML) = 0.72/100,000 (95% CI: 0.68–0.76/100,000) |
| Probability of FA among AML patients: |
| P(FA|AML) = [P(AML|FA) × P(FA)]/P(AML) = 0.005 × (1/129,600)/(0.72/100,000) = 0.54% (95% CI: 0.30–0.82%) |
| P(FA|enrolled in a treatment trial for sporadic AML) = 0.54%/3 = 0.18% (95% CI: 0.10–0.27%) |
| B |
| N = 892 patients enrolled on CCG 2961. Undiagnosed FA = p = 0.18%. |
| X = number of FA in the N patients. |
| P(X > 0|p = 0.18%) = 1 – P(X = 0|p = 0.18%) = 0.7995 or about 80%. |
| M = 193 patients who failed to achieve ANC recovery on the study protocol. |
| f = M/N = 193/892 = 0.22. |
| Undiagnosed FA = p/f = 0.18%/0.22 = 0.83%. |
| Z = number of FA in the M patients who failed to achieve ANC recovery on study. |
| P(Z > 0|p = 0.83%) = 1 – P(Z = 0|p = 0.83%) = 0.7998 or about 80%. |
CI, confidence interval.
FARF, Fanconi Anemia Research Fund.
Surveillance, Epidemiology, and End Results Program, 13 Registries Database, 1992–2008.
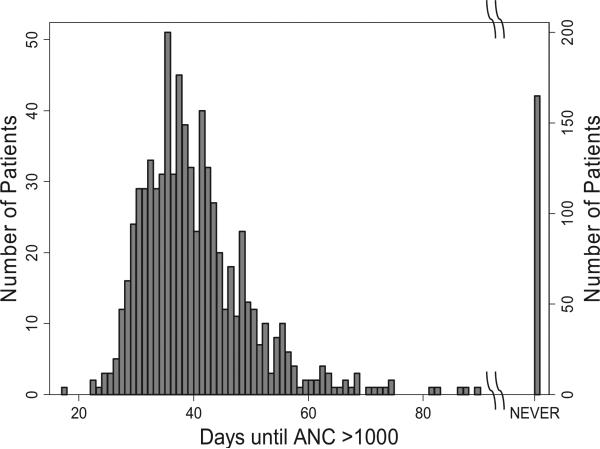
Among 892 de novo AML patients enrolled on CCG-2961 between 1997 and 2002, the mean time for recovery of the ANC to >1000/μL after the first round of chemotherapy was 40 ± 9.6 [1 standard deviation (SD)] days with a slightly left-skewed distribution (Fig. 1). One hundred and sixty-five patients never achieved an ANC >1000/μL (these patients died or went to transplant), and 28 recovered after 60 days (40 + 2 SD), for a total of 193, or 22% of the total. Based on our estimate that 0.18% of newly diagnosed patients with AML have undiagnosed FA, we speculate that 1.6 out of 892 de novo AML cases might have FA. Since many of the literature cases of FA were reported a posteriori to have failed to recover bone marrow function following chemotherapy, the estimate of 0.18% of all AML patients with FA (i.e. 1.6 patients with FA among 892) could represent 0.8% (1.6/193) of the subset of patients who had delayed or had no ANC recovery. Thus, FA testing would have an 80% chance of finding at least one patient with undiagnosed FA among 892 cases with apparently sporadic AML (Table 1B).
Fig. 1.
Time to recovery of absolute neutrophil count (ANC) >1000/μL in 892 de novo AML patients on CCG-2961. Raw data are shown by the bars. Note the change to the right Y-axis for the patients who failed to recover their ANC. There were 28 patients who recovered after 60 days, and 165 who never recovered.
4. Discussion
This study is the first to estimate the potential prevalence of FA among patients with apparently sporadic AML. Our calculations used broadly representative data from published FA cohorts to estimate the probability of AML among FA patients. We also used data on recovery of ANC from a recent large COG trial to further explore the potential clinical impact of screening for FA in all AML patients. We estimated that 0.18% (95% CI: 0.10–0.27%) of newly diagnosed patients in treatment trials for sporadic AML might, in fact, have FA. It is possible that the incidence of AML among FA patients based on cohort data may be affected by ascertainment bias; if so, this estimate may be too high. However, although the relative statistical uncertainty is substantial, i.e. around plus or minus 50%, the lower confidence limit has the same order of magnitude as the point estimate.
Strength of our study is that we applied the estimate of the incidence of FA among AML patients to data on ANC recovery from a large cohort. The rationale for doing so stems from the fact that the diagnosis of AML was the first presentation in approximately one-third of published cases of FA, often associated with therapy-induced, prolonged ANC suppression. The large number of AML patients enrolled on CCG-2961 allowed us to estimate times to ANC recovery. We found that 22% of AML patients did not achieve an ANC >1000/μL by day 60 or never. We speculated that this group may be enriched with undiagnosed FA. A limitation of this assumption is that there are many other reasons underlying treatment failures, including death, sepsis, multiorgan failure, toxicity of the AML treatment due to polymorphisms in genes involved in the metabolism of chemotherapy agents and other, undiagnosed, AML-prone genetic syndromes.
A prospective study, in which all newly diagnosed AML patients were screened for FA, would allow accurate estimation of the prevalence of FA among patients with apparently de novo AML. A retrospective analysis of all patients who failed to adequately recover from the first round of chemotherapy would validate our hypothesis that this group includes a non-zero proportion with undiagnosed FA. FA screening of the magnitude proposed here will require new technologies. While blood is the usual test material, consideration might be given to skin fibroblasts as a source of non-malignant DNA. The standard test, chromosome breakage in T lymphocytes after culture with a DNA crosslinking agent, may be unreliable in patients with malignant circulating myeloblasts prior to chemotherapy, or following cytoreduction subsequent to treatment. Even when applied to cultured fibroblasts, the test is expensive and labor intensive. However, the development of next generation whole genome sequencing may emerge soon enough to be used in this context.
Early identification of patients with undiagnosed FA who present with AML would provide insight into the mechanisms of treatment failure, and permit timely adjustment of chemotherapy in order to minimize toxicities, as well as subsequent modification of preparative regimens for hematopoietic stem cell transplantation. Diagnosis of such patients would also lead to appropriate genetic counseling of patients and family members. It is important to consider that what appears to be “sporadic” may occur on a genetic background that requires investigation and altered management.
Acknowledgements
This research was supported in part by Children's National Medical Center, the Children's Oncology Group Grants U10 CA98543 and U10 CA98413 from the National Cancer Institute (NCI), National Institutes of Health (NIH), and the Intramural Research Program of the NIH and the NCI. The authors gratefully thank an anonymous reviewer for detailed comments that led to significant improvements to our study.
Footnotes
Contributions. AR and BPA conceived and designed the study and wrote the manuscript, PSR performed the statistical analyses, and TAA, RBG, and BJL provided the data from CCG 2961. All authors edited and approved the manuscript.
Conflicts of Interest
No one has a conflict of interest.
Contributor Information
Philip S. Rosenberg, Biostatistics Branch, Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD, USA
Todd A. Alonzo, Department of Preventive Medicine, University of Southern California, Los Angeles, CA, USA
Robert B. Gerbing, Children's Oncology Group, Arcadia, CA, USA
Beverly J. Lange, Children's Hospital of Philadelphia, Philadelphia, PA, USA
Blanche P. Alter, Clinical Genetics Branch, Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD, USA.
References
- 1.Shimamura A, Alter BP. Pathophysiology and management of inherited bone marrow failure syndromes. Blood Rev. 2010;24:101–22. doi: 10.1016/j.blre.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosenberg PS, Greene MH, Alter BP. Cancer incidence in persons with Fanconi anemia. Blood. 2003;101:822–6. doi: 10.1182/blood-2002-05-1498. [DOI] [PubMed] [Google Scholar]
- 3.Alter BP, Giri N, Savage SA, et al. Malignancies and survival patterns in the National Cancer Institute inherited bone marrow failure syndromes cohort study. Br J Haematol. 2010;150:179–88. doi: 10.1111/j.1365-2141.2010.08212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alter BP. Cancer in Fanconi anemia, 1927–2001. Cancer. 2003;97:425–40. doi: 10.1002/cncr.11046. [DOI] [PubMed] [Google Scholar]
- 5.Rosenberg PS, Tamary H, Alter BP. How high are carrier frequencies of rare recessive syndromes? Contemporary estimates for Fanconi anemia in the United States and Israel. Am J Med Genet A. 2011;155:1877–83. doi: 10.1002/ajmg.a.34087. [DOI] [PMC free article] [PubMed] [Google Scholar]