Abstract
Parent-only (PO) treatments for childhood obesity are feasible, more cost-effective and potentially easier to disseminate. The objective of this study was to determine whether a PO treatment is not inferior to a parent + child (PC) treatment for childhood obesity. Eighty parent–child dyads with an 8–12 year old overweight or obese child (>85th BMI-P) were recruited and randomized into PO or PC treatment for childhood obesity. Parents or parent–child dyads attended 5-month treatment groups. Child and parent body size, child caloric intake, and child physical activity were assessed at baseline, post-treatment, and 6-months follow-up. Noninferiority testing using mixed linear models was used to compare PO treatment with PC treatment. Results showed that the PO group was not inferior to the PC group in terms of child weight loss. Results also showed that the PO group was not inferior to the PC group in terms of parent weight loss and child physical activity, but not child caloric intake. This study suggests that a PO treatment could provide similar results to PC in child weight loss and other relevant outcomes, and potentially could be more cost-effective and easier to disseminate. Although further research is needed, this study suggests that PO groups are a viable method for providing childhood obesity treatment.
INTRODUCTION
Recent data suggest that 31% of children are overweight or obese (1), which affects 4–5 million children in the United States. The current gold standard treatment for childhood obesity is a program delivered to both parents and children which combines nutrition education, and exercise with behavior therapy techniques (2). Parents play a significant role in this program, and behavioral reinforcement of both child and parent behavior are associated with the most successful child outcomes (3). This treatment protocol is fairly intensive, and includes parent and child separate groups, and individual one-on-one meetings for goal setting and problem solving for 4–6 months on a weekly basis (2). Ten-year longitudinal data show that one third of children are no longer overweight in adulthood (4), which is more promising as compared to interventions for adults (5). For these reasons, it is important to identify efficacious methods for delivering this behavioral treatment to a greater proportion of the population.
As the most significant people in a child’s environment, parents serve as the first and most important teachers of their children, and reinforce and support the acquisition and maintenance of eating and exercise behaviors. It is possible that many of the needed parenting skills to assist the child in weight loss could be delivered to the parents without the child coming to clinic. Parent-only (PO) treatments are used to deliver treatments for other child behavioral issues, including tantrums, self-destructive behaviors, verbal aggression, excessive crying, thumb sucking, school phobia, and oppositional behavior (6), and naturally could be applied to obesity treatment. PO treatments are less expensive than parent + child (PC) treatments (7), and offer greater opportunities for dissemination because the meetings do not have to coordinate around both parent and child schedules.
The published data on PO treatments for childhood obesity suggest that PO treatments could be viable, but it is unclear whether PO treatments provide similar outcomes to PC. Three publications by Golan and colleagues showed that a PO treatment outperformed PC (8–10), whereas three other studies showed that PO performed similarly to PC (11–13). These data are further clouded by methodological issues, such as unmatched dose effects (8), lack of standardized protocols (i.e., cognitive-behavioral approach with the mothers, and relaxation training with the children) (11), families with multiple children who provided data for the study (14), and the provision of treatment to specific populations (rural families) (12). None of the published studies in the literature compared a PO version of the manualized behavior therapy treatment program for childhood obesity with a PC version in a clinic-based program. Moreover, in the aforementioned studies, many of the results were analyzed using only individuals that completed treatment. Testing for noninferiority of PO treatment to PC treatment could examine whether the former has comparable efficacy to the latter, the focus of the current paper.
Thus, the purpose of this project was to evaluate whether a standardized behavioral PO treatment program would not be inferior to a standardized behavioral PC program on child weight loss and other relevant markers of change.
METHODS AND PROCEDURES
Participants
Parents and their overweight or obese (>85 BMI-percentile) child aged 8–12 years were recruited through media announcements, advertisements, direct mailing, and physician referrals in Minneapolis and San Diego. At least one parent or guardian participated with the child. If two children in the family met criteria for the study, both were invited to attend the treatment groups but a coin-flip was used to determine which child’s data would be part of the study. Parent–child dyads were excluded if either the child or parent was currently involved in psychological or weight loss treatment, was using medications which affected weight or appetite, or had a psychiatric or physical condition (e.g., eating disorder, psychosis) that would interfere with participation. Participants provided written informed consent (participating parent) and assent (child). Eighty parent–child dyads (66 in MN/14 in CA) were randomly assigned to either PC or PO groups.
Study design
This randomized controlled trial was conducted with parent–child dyads randomized into the two conditions (PC or PO) within five cohorts (four in Minnesota, one in San Diego), with intervention provided in treatment groups. Random assignment was conducted after completing the initial assessment using a computer generated random numbers table. Parent–child dyads were treated and assessed in Minnesota between January 2007 and February 2009, and parent–child dyads were treated and assessed in San Diego between April 2008 and March 2009. Treatment sessions were conducted at University of Minnesota and University of California, San Diego. The Institutional Review Boards of both universities approved the study.
Intervention description
The intervention was the current state-of-the-art behavioral treatment for childhood obesity described by Epstein and colleagues (15–17). The weight loss treatment is founded on the hypothesis that children are obese due to an imbalance in caloric intake and expenditure. All of the following skills were taught using standardized manuals to the parents and children. The treatment program includes dietary modification (traffic-light diet), increases in physical activity, behavioral change skills, and parenting skills specific for children who are overweight. The traffic-light diet follows the food guide pyramid and focuses on decreasing energy intake while increasing the nutrient density of the diet (18). Energy expenditure was addressed by increasing physical activity (lifestyle and planned) and decreasing sedentary behavior. Behavioral change skills included self-monitoring of targeted behaviors, positive reinforcement, stimulus control, preplanning, and modeling. During weight monitoring, parents and children in the PC group were taught to reflect on the behaviors that influenced weight that week. Parents in the PO group were coached on how to assist their children in weight monitoring at home and reflect on the behaviors that influenced weight. PC treatment was delivered in 60-min separate child and parent groups, while PO treatment was delivered in 60-min parent groups. Group size ranged from 6–10 participants. The same parent group materials were used for PC and PO parent groups. The material taught in the child groups of the PC arm was similar in content to that taught to the parents, but presented in an age-appropriate manner (i.e., fun games). Child group sessions included games focused on the topic and didactic learning. For the PC treatment, all parent–child dyads also met with an interventionist either pre- or postgroup for family goal setting for a maximum of 10 min. Goal setting for the PO group occurred during the treatment groups. All parents and children completed quizzes each week to assure knowledge of the treatment protocol.
All treatment groups were co-led by two interventionists, who were either licensed clinical psychologists, or advanced clinical psychology trainees. All interventionists attended a 3-day training regarding behavioral intervention for the study, and were supervised by the first author on a weekly basis during treatment.
Measurements
All measurements were completed at baseline (randomization), post-treatment (month 5), and at follow-up (month 11).
Weight of child and parent
Standardized protocols were used to evaluate weight and height of both children and parents at each assessment point. Height was measured using a portable Schorr height board (Schorr, Olney, MD) and body weight measured in kilograms on a Tanita Digital Scale (model WB-110A). Height and weight were converted to BMI (kg/m2) for both children and parents. BMI was standardized for age and gender (BMI-Z) and expressed as a percentile (BMI-P) using the US Centers for Disease Control and Prevention growth curves (19). BMI, BMI-Z, and BMI-P are useful for indexing adiposity and weight change in children. Cole and colleagues (20) are critical of the use of BMI-Z and BMI-P in that these indexes are less sensitive to weight change at heavier weights. Although the argument has merit on theoretical grounds, in that more weight change is typically required to change BMI-Z and BMI-P scores, this “insensitivity” may translate to greater validity from a health standpoint. For example, heavier kids may need to lose more weight than less heavier kids in order to obtain comparable health benefits. We chose to present all three to be comprehensive and because the demonstration of Cole and colleagues is sample-specific and might not be unconditionally true of all samples. Indeed, in the current sample, analyses related to weight change from baseline across both treatments indicated significant weight loss at post-treatment for BMI, BMI-Z, and BMI-P, but only BMI-Z at follow-up. The tests of significance for these results are not reported in this article because of a focus on noninferiority, but these results can be inferred from the means presented in Table 2. Given these considerations, the results of all three indexes are reported.
Table 2.
Means (s.d.) and number of respondents (N) at each time point by condition
| Outcome | Pre-treatment | Post-treatment | Follow-up |
|---|---|---|---|
| Child BMI-P | |||
| Parent-only | 98.37 (1.85) N = 40 | 96.82 (5.49) N = 24 | 95.08 (11.18) N = 24 |
| Parent–child | 98.34 (1.37) N = 40 | 97.21 (2.75) N = 28 | 97.23 (3.01) N = 28 |
| Child BMI-Z | |||
| Parent-only | 2.29 (0.38) N = 40 | 2.16 (0.54) N = 24 | 2.10 (0.68) N = 24 |
| Parent–child | 2.25 (0.34) N = 40 | 2.06 (0.40) N = 28 | 2.08 (0.41) N = 28 |
| Child BMI | |||
| Parent-only | 30.48 (6.08) N = 40 | 30.07 (7.01) N = 24 | 30.55 (7.48) N = 24 |
| Parent–child | 28.26 (4.64) N = 40 | 26.99 (4.08) N = 28 | 28.04 (4.14) N = 28 |
| Child caloric intake/day | |||
| Parent-only | 1,373.13 (647.56) N = 32 | 1058.32 (540.74) N = 23 | 1008.17 (324.48) N = 24 |
| Parent–child | 1469.88 (608.60) N = 37 | 1041.09 (412.46) N = 28 | 1002.76 (361.15) N = 27 |
| Child physical activity | |||
| Parent-only | 2.69 (0.61) N = 20 | 2.80 (0.61) N = 14 | 4.18 (3.69) N = 15 |
| Parent–child | 2.80 (0.77) N = 22 | 2.74 (0.99) N = 13 | 2.81 (0.71) N = 21 |
| Parent BMI | |||
| Parent-only | 32.47 (8.25) N = 39 | 31.81 (9.13) N = 24 | 32.53 (9.76) N = 24 |
| Parent–child | 31.47 (7.46) N = 40 | 31.35 (7.41) N = 28 | 31.80 (6.89) N = 28 |
Note: The number of respondents at each time were less for daily caloric intake and physical activity than for the weight outcomes because of nonresponse on some scale items. As indicated in the Analyses section, a multiple imputation approach was used as a form of sensitivity analysis, which yielded nonsubstantive differences with full-likelihood approach to analyzing the data that are reported.
BMI-Z, BMI standardized for age and sex; BMI-P, BMI-Z expressed as a percentile.
Parent weight was included in this study as an outcome because previous studies have shown that one of the best predictors of child weight loss is parent weight loss (2,21). Results for BMI are presented for parents.
Dietary intake of child
Usual dietary intake of the child was assessed with the Block Kids Questionnaire (22). The questionnaire includes 77 food items and uses the USDA Nutrient Database for Dietary Studies. Results for total caloric intake are presented.
Physical activity of child
Children completed the Physical Activity Questionnaire for Older Children (PAQ-C) (23).The PAQ-C is a self-administered 7-day recall measure designed to assess general physical activity levels during the school year for children. The scale consists of nine items, with each rated on a five-point scale, with the anchor points varying by item. For instance, one item asks how often in the last 7 days the child was very active after school (e.g., playing sports): “None”, “1 time last week”, “2 or 3 times last week”, “4 times last week”, “5 times last week”. The scale score is calculated as an item average and is presented.
Analyses
The analyses are based on linear-mixed models implemented in SAS, version 9.2, using Proc Mixed. Repeated measures were modeled with an unstructured covariance matrix, and an additional random-effect error term was included to account for the possible additional variation resulting from parents/children being treated in groups (i.e., cohorts).
Results are presented using a full-likelihood approach to handling missing data that make use of all observed values at each time point (24). These results were compared to those under multiple imputation (not reported) (25–27), yielding nonsubstantive differences in the results. Study sample size was determined by pragmatic factors, including budget and investigator time commitments. No interim analyses were done.
The hypotheses tested relate to noninferiority of the PO treatment to the PC treatment on child and parent weight loss and child daily caloric intake and physical activity. A form of equivalence testing (28–30) known as noninferiority testing (31) was used to test the hypothesis that the PO intervention is not inferior to the PC intervention. Hypotheses are tested using contrasts that compare the intervention conditions at post-treatment and follow-up on change from baseline. The statistical null hypothesis for all tests is that PO is inferior to PC. Noninferiority is supported if the upper bound of the two-sided 90% confidence interval of the mean difference (PO-PC) is less than the prespecified noninferiority bound. Using the upper bound of a two-sided 90% confidence interval leads to probability of type I error of ≤0.05. A 90% confidence interval is typically associated with a 10% error rate, rather than 5% on each side; however, in this case interest is only on one side of the interval, therefore the error rate is 5%. In addition to testing for noninferiority, confidence intervals are reported and the upper bound is interpreted independent of the noninferiority bound in order to address the subjectivity inherent in selecting a bound when conducting noninferiority testing. Use of the upper bound of the confidence interval can in part address the subjectivity introduced by this method by indicating that any number greater than the upper bound would be unlikely in the population, and the choice is left to the reader to determine whether this is evidence of noninferiority. Reporting and interpreting the upper bound of the confidence interval is also more informative than simply making dichotomous statements about the presence or absence of noninferiority.
For child weight-related outcomes, the design of the trial was based on only testing noninferiority using weight standardized for age and sex and expressed as a percentile (BMI-P), which was considered a more valid indicator of child weight than BMI (see above). Moreover, the choice to use BMI-P rather than BMI-Z was that percentiles are on a more meaningful metric than z-scores, which is needed in order to construct a substantively meaningful noninferiority bound. BMI noninferiority tests were included post hoc, based on the recommendations of an anonymous reviewer.
The bound for noninferiority hypotheses related to BMI-P was set to 1. This is the maximum value the PC group could do better than PO, below which noninferiority would be concluded. This bound could correspond to an average-aged child in this sample having BMI of 26 in the PC group and 28.5 in the PO group at post-treatment/follow-up, assuming equivalence at baseline. Because children in this sample are so overweight, moderate group differences in BMI correspond to relatively small differences in BMI-P. This presented a problem because the noninferiority bound for BMI-P is specified a priori, therefore it cannot be modified after viewing the data (e.g., using a lower BMI-P bound to correspond to a lower BMI). However, as mentioned above, our presentation and interpretation of the upper bounds of confidence intervals independent of the noninferiority bound can help address the problem. For a noninferiority bound for child BMI, which was selected post hoc, we considered choosing a BMI that would correspond to the BMI-P noninferiority bound (i.e., BMI = 2.5), but instead chose a more rigorous value of BMI = 1, which corresponds to the same value selected for parents (see below). Noninferiority hypotheses related to parent weight are tested using a bound of 1 BMI. Noninferiority bounds related to child daily caloric intake and physical activity were set to 300 and 1, respectively. For the physical activity measure, the individual items are on a five-point ordinal scale (see Methods and Procedures) and the scale score is expressed as an item average. A value of 1 is the maximum value the PC group could do better than PO, below which noninferiority would be concluded. For instance, if all items had the scaling of the item described in the Methods and Procedures section, noninferiority would be concluded if in the population of the PO group had a scale score >2 (corresponding to a rating of “2 or 3 time last week”) and the PC group had a score of 3 (corresponding to a rating of “4 times last week”) at post-treatment/follow-up, assuming equivalence at baseline.
RESULTS
Recruitment and completion
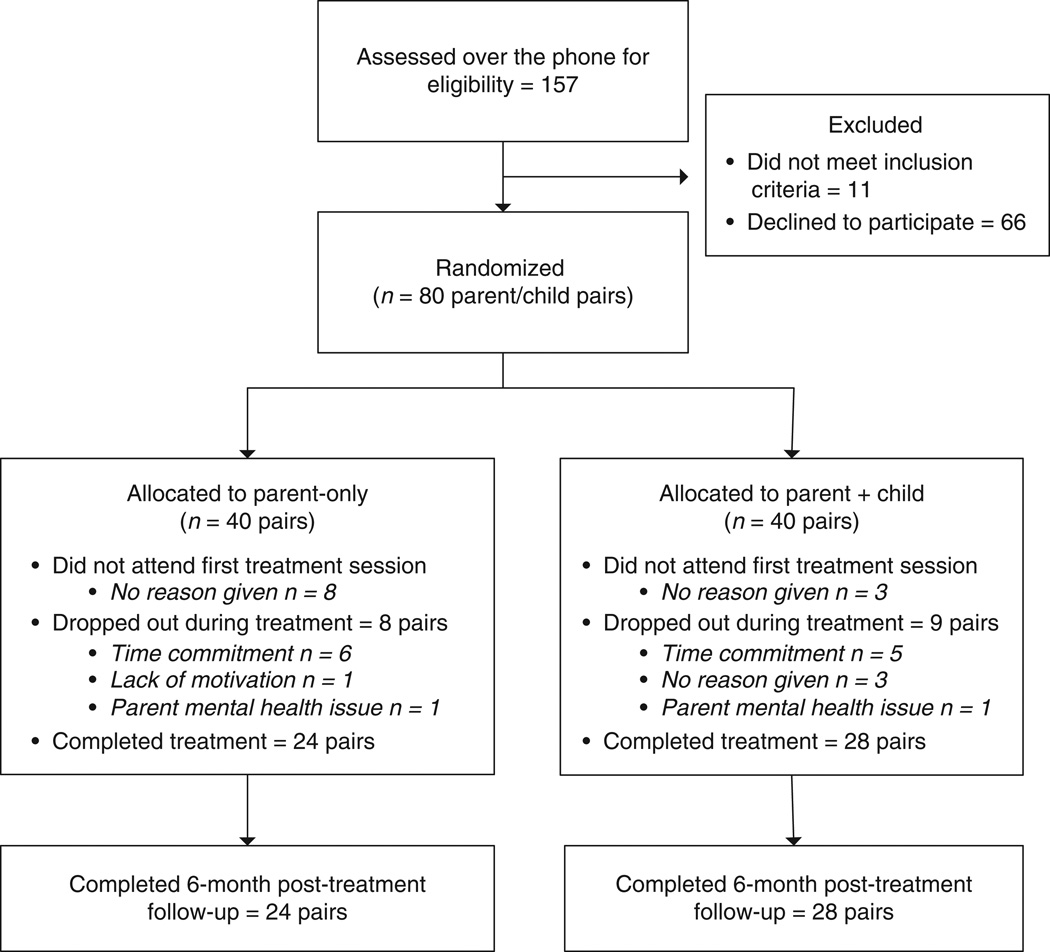
Figure 1 shows the study recruitment and completion rate of parent–child dyads in this study. If a family responded to an advertisement, and had a child that could qualify, they were invited in for an assessment where they would have their height and weight measured and complete surveys. As Figure 1 shows, more of the parent–child dyads randomized to the PO treatment dropped between initial assessment and first treatment visit compared to PC. However, once parent–child dyads attended at least one treatment session, the dropout rates became more similar, suggesting that once parent–child dyads became involved, they were as likely to stay in the treatment in both arms. Age, gender, and education attainment at baseline for each condition are presented in Table 1 and weight at baseline by condition is presented in Table 2. The difference between the conditions in the point estimates is relatively small, suggesting that the groups were roughly equivalent at baseline with respect to these variables. Tests of significance of group differences on baseline characteristics are not conducted because this method of attempting to identify confounders is conflated with the sample size (32), only addresses the relation between treatment assignment and covariates (not the relationship to the response that also contributes to the presence of confounding) (33), and depending on the nature of the inferences desired equivalence testing may be more appropriate than conventional significance tests. In general, given the experimental design, concern about the presence of confounders should be minimal.
Figure 1.
Study recruitment, randomization and completion of parent/child pairs in Minnesota and San Diego.
Table 1.
Characteristics of the sample at baseline by study condition
| Parent-only (N = 40) |
Parent–child (N = 40) |
|
|---|---|---|
| Child | ||
| Mean age (s.d.) | 10.81 (1.31) | 10.08 (1.15) |
| Femalea (%) | 50% | 70% |
| Parent | ||
| Mean age (s.d.) | 44.61 (4.73) | 41.03 (5.29) |
| Femalea (%) | 87% | 90% |
| Education-highest parenta | ||
| Less than high school | 0.0% | 2.5% |
| High school | 7.9% | 12.5% |
| Vocational school | 7.9% | 5.0% |
| Some college | 23.7% | 25.0% |
| College graduate | 42.1% | 35.0% |
| Advanced degree | 18.4% | 20.0% |
| Total household incomea | ||
| Below $20,000 | 2.6% | 5.0% |
| $20,001–$40,000 | 7.9% | 10.0% |
| $40,001–$60,000 | 18.4% | 15.0% |
| Above $60,000 | 63.2% | 65.0% |
| Don’t know | 7.9% | 5.0% |
Expressed as percent of total or condition.
Child weight changes by group
Descriptive statistics for each outcome at each time point separated by condition are presented in Tables 2 and 3. Results for child weight loss are presented for BMI, BMI-Z, and BMI-P in Table 3. Examination of the point estimate for BMI-P suggests a slight advantage of PO at post-treatment, but a more moderate advantage of PO at follow-up. The upper bound of the 90% confidence interval is less than the noninferiority bound of 1 at post-treatment and follow-up, indicating noninferiority in both cases. Furthermore, the upper bounds of the confidence intervals suggest that differences between the groups of 0.620 or greater at post-treatment and 0.274 or greater at follow-up are unlikely in the population. That is, PC is unlikely to have weight loss that is better than PO by 0.620 or greater at post-treatment and 0.274 or greater at follow-up. Although BMI-Z is not tested for noninferiority, the upper bounds of the confidence intervals are used. Specifically, the upper bounds suggest PC is unlikely to have weight loss that is better than PO by 0.084 or greater at post-treatment and 0.035 or greater at follow-up. For BMI, the upper bound of the 90% confidence interval is less than the noninferiority bound of 1 at post-treatment and follow-up, indicating noninferiority in both cases. Furthermore, the upper bounds suggest PC is unlikely to have weight loss that is better than PO by 0.894 or greater at post-treatment and 0.479 or greater at follow-up. Collectively, these results suggest noninferiority of PO to PC on child weight loss.
Table 3.
Tests of noninferiority of parent-only treatment to parent + child treatment
| Outcome | Estimate (PO-PC) | s.e. | 90% CI | Noninferiority bound |
Noninferiority present |
|---|---|---|---|---|---|
| Post-treatment | |||||
| Child BMI-P | −0.500 | 0.680 | −1.619 to 0.619 | 1 | Yes |
| Child BMI-Z | −0.010 | 0.045 | −0.064 to 0.084 | – | – |
| Child BMI | 0.280 | 0.373 | −0.334 to 0.894 | 1 | Yes |
| Child caloric intake/day | 109.34 | 172.64 | −174.636 to 393.316 | 300 | No |
| Child physical activity | 0.205 | 0.314 | −0.311 to 0.721 | 1 | Yes |
| Parent BMI | −0.565 | 0.333 | −1.113 to −0.017 | 1 | Yes |
| Follow-up | |||||
| Child BMI-P | −2.316 | 1.574 | −4.905 to 0.273 | 1 | Yes |
| Child BMI-Z | −0.077 | 0.068 | −0.189 to 0.035 | – | – |
| Child BMI | −0.327 | 0.490 | −1.133 to 0.479 | 1 | Yes |
| Child caloric intake/day | 95.53 | 157.31 | −163.229 to 354.289 | 300 | No |
| Child physical activity | 1.557 | 0.860 | 0.142 to 2.972 | 1 | Yes |
| Parent BMI | −0.294 | 0.499 | −1.115 to 0.527 | 1 | Yes |
Note: Negative values in the estimate column for all outcomes except physical activity indicate result favors PO group (i.e., more weight loss or fewer calories consumed), positive values indicate result favors PC group. The opposite is true for physical activity.
BMI-P, BMI-Z expressed as a percentile; BMI-Z, BMI standardized for age and sex; PC, parent–child; PO, parent-only.
Parent weight, child caloric intake and physical activity changes by group
Results for child daily caloric intake, child physical activity, and parent weight loss are presented in Tables 2 and 3. Examination of the point estimates of daily caloric intake suggest a small advantage of PC at post-treatment and follow-up, but the results do not support noninferiority at either time point because the upper bound of the 90% confidence interval is greater than the noninferiority bound of 300. Nevertheless, the upper bounds of the confidence intervals suggest PC is unlikely to have daily caloric intake reduction that is better than PO by 393.317 or greater at post-treatment and 354.290 or greater at follow-up. In contrast, the estimates for physical activity suggest a small advantage for PO at post-treatment, but a larger advantage for PO at follow-up. For both time points noninferiority is indicated by a lower bound of the 90% confidence interval that is less than the noninferiority bound of 1. The lower bounds of the confidence intervals suggest PC is unlikely to have physical activity increase that is better than PO by 0.311 or greater at post-treatment, whereas at follow-up not only is PC unlikely to do better than PO, but PO will likely do better than PC in the range of 0.143 to 2.971. For parent BMI a small advantage for PO was observed at both post-treatment and follow-up, with noninferiority indicated at both time points by upper bounds of the 90% confidence interval that are less than the noninferiority bound of 1. The upper bounds of the confidence intervals suggest PC is unlikely to have parent BMI reduction that is better than PO by 0.722 or greater at post-treatment and 0.528 or greater at follow-up. Collectively, these results suggest noninferiority of PO to PC on child physical activity and parent BMI but not child caloric intake.
DISCUSSION
This study showed that a PO group was not inferior to the PC group in terms of child weight loss, suggesting that PO groups could viably be used to treat children who are overweight. Although other studies have reported comparing PC and PO approaches for the treatment of childhood obesity, this is the first to formally test whether a standardized PO treatment is not inferior to a PC treatment. In this study, we also found that the PO group was not inferior to the PC group in terms of parent weight loss and child physical activity, but not child caloric intake. It should be noted that the latter result does not imply that PO is inferior to PC on caloric intake, rather only that we fail to conclude the presence of noninferiority. Overall, this project suggests that a PO treatment could provide similar results to PC in child weight loss and other relevant outcomes, and potentially could be more cost-effective and easier to disseminate.
A priori, we made specific efforts in this study to provide a treatment protocol in a manner that would be more similar to that in a clinic in the community. Participants only attended one assessment session where they were randomized, which differs from other more complicated trials that require attendance at more than one pre-treatment meeting (34–37). This was not done in the current study, and not surprisingly, a larger dropout than is reported in other trials was observed (34–37). However, dropout was more similar to that seen in other community-based trials (12). In addition, we only excluded parent–child dyads when there were major psychiatric illnesses or severe behavioral issues reported on the phone screen; standardized interviews were not conducted to determine unreported behavioral issues.
It is interesting that we saw a larger dropout in the PO group compared to the PC group from assessment visit to first treatment visit. However, we saw a similar level of dropout in both groups following the first treatment visit. One hypothesis is that parents may have wanted the PC group, as a method for having another adult teach the child weight loss skills. Parents could potentially view this as a way of reducing conflict around eating and weight. An alternative hypothesis is that parents might be looking for a more collaborative treatment setting with their children, where their children can learn from and be supported by other children making similar lifestyle changes. These preferences should be examined in future studies with PO treatments for childhood obesity.
This study has a number of strengths and weaknesses that need to be noted. This was a randomized, clinical intervention study that compared a standardized treatment of PO to PC. The strengths of this study include a clinically relevant protocol in selecting participants for this study, a moderate number of parent–child dyads randomized, and 6-month follow-up. Weaknesses include high dropout rates. We believe that the high dropout rates and the modest weight losses seen are related to a heterogeneous sampling procedure, which included only one assessment prior to randomization. Another weakness to consider is power for noninferiority tests related to child caloric intake, which was the only outcome that was not significant at both post-treatment and follow-up.
Considering these strengths and weaknesses, this study contributes to a fairly dearth body of literature on the use of parents as interventionists for childhood obesity. Further studies are needed to confirm these results, including studies in different contexts, with different sampling methods, and with larger sample sizes and longer follow-up. If PO continues to perform similarly to PC, the standardized protocol for delivering childhood obesity treatment could be revised.
ACKNOWLEDGMENTS
We thank the children and parents for participating in the study. As in all behavioral treatment studies, a large team was necessary to complete this study. We would like to thank all the interventionists in MN: Mary Alm, Heather Libbey, Diane Rubright, Aimee Arikian, Robyn Birkeland and in CA: Tammy Maginot, Roxanne Rockwell, Taya Cromley, Kristy Center and the research assistants Cathryn Johnson and Hanaah Fannin. We would also like to thank Dr Epstein for all of his training, assistance and support. This research was funded in part by University of Minnesota Obesity Center (NIH NIDDK/5P30-DK050456-14) and University of California, San Diego, Academic Senate Award.
Footnotes
DISCLOSURE
The authors declared no conflict of interest.
REFERENCES
- 1.Ogden CL, Carroll MD, Curtin LR, Lamb MM, Flegal KM. Prevalence of high body mass index in US children and adolescents, 2007–2008. JAMA. 2010;303:242–249. doi: 10.1001/jama.2009.2012. [DOI] [PubMed] [Google Scholar]
- 2.Epstein LH. Family-based behavioural intervention for obese children. Int J Obes Relat Metab Disord. 1996;20(Suppl 1):S14–S21. [PubMed] [Google Scholar]
- 3.Epstein LH, McCurley J, Wing RR, Valoski A. Five-year follow-up of family-based behavioral treatments for childhood obesity. J Consult Clin Psychol. 1990;58:661–664. doi: 10.1037//0022-006x.58.5.661. [DOI] [PubMed] [Google Scholar]
- 4.Epstein LH, Valoski A, Wing RR, McCurley J. Ten-year outcomes of behavioral family-based treatment for childhood obesity. Health Psychol. 1994;13:373–383. doi: 10.1037//0278-6133.13.5.373. [DOI] [PubMed] [Google Scholar]
- 5.Epstein LH, Valoski AM, Kalarchian MA, McCurley J. Do children lose and maintain weight easier than adults: a comparison of child and parent weight changes from six months to ten years. Obes Res. 1995;3:411–417. doi: 10.1002/j.1550-8528.1995.tb00170.x. [DOI] [PubMed] [Google Scholar]
- 6.Johnson CA, Katz RC. Using parents as change agents for their children: a review. J Child Psychol Psychiatry. 1973;14:181–200. doi: 10.1111/j.1469-7610.1973.tb01186.x. [DOI] [PubMed] [Google Scholar]
- 7.Janicke DM, Sallinen BJ, Perri MG, et al. Comparison of program costs for parent-only and family-based interventions for pediatric obesity in medically underserved rural settings. J Rural Health. 2009;25:326–330. doi: 10.1111/j.1748-0361.2009.00238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Golan M, Weizman A, Apter A, Fainaru M. Parents as the exclusive agents of change in the treatment of childhood obesity. Am J Clin Nutr. 1998;67:1130–1135. doi: 10.1093/ajcn/67.6.1130. [DOI] [PubMed] [Google Scholar]
- 9.Golan M, Fainaru M, Weizman A. Role of behaviour modification in the treatment of childhood obesity with the parents as the exclusive agents of change. Int J Obes Relat Metab Disord. 1998;22:1217–1224. doi: 10.1038/sj.ijo.0800749. [DOI] [PubMed] [Google Scholar]
- 10.Golan M, Crow S. Targeting parents exclusively in the treatment of childhood obesity: long-term results. Obes Res. 2004;12:357–361. doi: 10.1038/oby.2004.45. [DOI] [PubMed] [Google Scholar]
- 11.Munsch S, Roth B, Michael T, et al. Randomized controlled comparison of two cognitive behavioral therapies for obese children: mother versus mother-child cognitive behavioral therapy. Psychother Psychosom. 2008;77:235–246. doi: 10.1159/000129659. [DOI] [PubMed] [Google Scholar]
- 12.Janicke DM, Sallinen BJ, Perri MG, et al. Comparison of parent-only vs family-based interventions for overweight children in underserved rural settings: outcomes from project STORY. Arch Pediatr Adolesc Med. 2008;162:1119–1125. doi: 10.1001/archpedi.162.12.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kingsly RG, Shapiro J. A comparison of three behavioral programs for the control of obesity in children. Behav Ther. 1977;8:30–36. [Google Scholar]
- 14.Golan M, Kaufman V, Shahar DR. Childhood obesity treatment: targeting parents exclusively v. parents and children. Br J Nutr. 2006;95:1008–1015. doi: 10.1079/bjn20061757. [DOI] [PubMed] [Google Scholar]
- 15.Epstein LH, Myers MD, Raynor HA, Saelens BE. Treatment of pediatric obesity. Pediatrics. 1998;101:554–570. [PubMed] [Google Scholar]
- 16.Raynor HA. Evidence-based treatments for childhood obesity. In: Jelalian E, Steele RG, editors. Handbook of Childhood and Adolescent Obesity. New York: Springer; 2008. pp. 201–220. [Google Scholar]
- 17.Johnston CA, Tyler C, Foreyt J. Behavioral approaches to childhood overweight treatment. In: O’Donohue WT, Moore BA, Scott BJ, editors. Handbook of Pediatric and Adolescent Obesity Treatment. New York: Routledge; 2008. pp. 195–204. [Google Scholar]
- 18.Epstein LH, Masek BJ, Marshall WR. A nutritionally based school program for control of eating in obese children. Behav Ther. 1978;9:766–778. [Google Scholar]
- 19.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, et al. CDC growth charts: United States. Adv Data. 2000:1–27. [PubMed] [Google Scholar]
- 20.Cole TJ, Faith MS, Pietrobelli A, Heo M. What is the best measure of adiposity change in growing children: BMI, BMI %, BMI z-score or BMI centile? Eur J Clin Nutr. 2005;59:419–425. doi: 10.1038/sj.ejcn.1602090. [DOI] [PubMed] [Google Scholar]
- 21.Wrotniak BH, Epstein LH, Paluch RA, Roemmich JN. Parent weight change as a predictor of child weight change in family-based behavioral obesity treatment. Arch Pediatr Adolesc Med. 2004;158:342–347. doi: 10.1001/archpedi.158.4.342. [DOI] [PubMed] [Google Scholar]
- 22.Block G. Block Kids Questionnaire. < http://www.nutritionquest.com/>. [Google Scholar]
- 23.Crocker PR, Bailey DA, Faulkner RA, Kowalski KC, McGrath R. Measuring general levels of physical activity: preliminary evidence for the Physical Activity Questionnaire for Older Children. Med Sci Sports Exerc. 1997;29:1344–1349. doi: 10.1097/00005768-199710000-00011. [DOI] [PubMed] [Google Scholar]
- 24.Siddique J, Brown CH, Hedeker D, et al. Missing Data in Longitudinal Trials - Part B, Analytic Issues. Psychiatr Ann. 2008;38:793–801. doi: 10.3928/00485713-20081201-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Allison PD. Missing Data. Thousand Oaks, CA: Sage; 2001. [Google Scholar]
- 26.Rubin D. Multiple Imputation for Nonrespone in Surveys. New York: Wiley; 1987. [Google Scholar]
- 27.Little RJA, Rubin DA. Statistical Analysis With Missing Data. 2nd edn. Hoboken, NJ: Wiley; 2002. [Google Scholar]
- 28.Rogers JL, Howard KI, Vessey JT. Using significance tests to evaluate equivalence between two experimental groups. Psychol Bull. 1993;113:553–565. doi: 10.1037/0033-2909.113.3.553. [DOI] [PubMed] [Google Scholar]
- 29.Schuirmann DJ. A comparison of the two one-sided tests procedure and the power approach for assessing the equivalence of average bioavailability. J Pharmacokinet Biopharm. 1987;15:657–680. doi: 10.1007/BF01068419. [DOI] [PubMed] [Google Scholar]
- 30.Westlake WJ. Symmetrical confidence intervals for bioequivalence trials. Biometrics. 1976;37:589–594. [PubMed] [Google Scholar]
- 31.Pocock SJ. The pros and cons of noninferiority trials. Fundam Clin Pharmacol. 2003;17:483–490. doi: 10.1046/j.1472-8206.2003.00162.x. [DOI] [PubMed] [Google Scholar]
- 32.Stuart EA, Marcus SM, Horvitz-Lennon MV, Gibbons RD, Normand SL. Using Non-experimental data to estimate treatment effects. Psychiatr Ann. 2009;39:41451. doi: 10.3928/00485713-20090625-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kleinbaum DG, Kupper LL, Muller KE, Nizam A. Applied Regression Analysis and Multivariable Methods. London: Duxbury; 2008. [Google Scholar]
- 34.Wilfley DE, Stein RI, Saelens BE, et al. Efficacy of maintenance treatment approaches for childhood overweight: a randomized controlled trial. JAMA. 2007;298:1661–1673. doi: 10.1001/jama.298.14.1661. [DOI] [PubMed] [Google Scholar]
- 35.Epstein LH, Paluch RA, Gordy CC, Dorn J. Decreasing sedentary behaviors in treating pediatric obesity. Arch Pediatr Adolesc Med. 2000;154:220–226. doi: 10.1001/archpedi.154.3.220. [DOI] [PubMed] [Google Scholar]
- 36.Epstein LH, Paluch RA, Kilanowski CK, Raynor HA. The effect of reinforcement or stimulus control to reduce sedentary behavior in the treatment of pediatric obesity. Health Psychol. 2004;23:371–380. doi: 10.1037/0278-6133.23.4.371. [DOI] [PubMed] [Google Scholar]
- 37.Epstein LH, Paluch RA, Gordy CC, Saelens BE, Ernst MM. Problem solving in the treatment of childhood obesity. J Consult Clin Psychol. 2000;68:717–721. [PubMed] [Google Scholar]