Abstract
Background
The binding of drugs to catheters can be a source variation in dosing chemotherapeutics. Drug contamination from the dosing central venous line (CVL) can impact the reporting of pharmacokinetic (PK) results and analysis. Peripheral venipuncture avoids binding complications from the CVL but dissuades patients from enrolling. Our group has developed a catheter clearing procedure to minimize the extent of contamination so that dosing and sampling from the CVL can ensue, promoting patient willingness to participate in phase I pediatric oncology trials.
Objectives
To develop a population pharmacokinetic model of actinomycin-D (AMD) in children with cancer incorporating expressions for drug contamination from PK samples obtained via indwelling CVLs and to evaluate the efficiency of a catheter clearing procedure in removing contamination as well as the impact of contamination on PK results.
Methods
A dataset of 199 AMD plasma concentration measurements from 36 patients (age 1.6–20.3 years) was analyzed using nonlinear mixed-effects modeling. Quantitative modeling approaches, including baseline contamination model, covariate model, and catheter clearance model, were evaluated to describe catheter contamination. Monte Carlo simulations mimicking a prospective study in children with cancer were performed to assess the performance of the final model and impact of catheter contamination on PK reporting.
Results
The PK of AMD was best described by a linear 3-compartment model with first-order elimination. A baseline contamination model including a contamination factor proportional to the model-predicted concentration for samples obtained from central catheters was chosen as the most parsimonious and accurate among competing models. The final model parameters were allometrically scaled to a 70 kg person. The estimated mean parameter values were 11 L/h, 5.79, 24.2, 490 L, 17.7, and 42.8 L/h for total clearance, central volume of distribution, peripheral volume 1, peripheral volume 2, inter-compartmental clearance 1, and inter-compartmental clearance 2, respectively. The proportional contamination factor was 19.3 % immediately post-drug administration and decreased at a first-order rate of 0.0932 h−1. Simulations precisely re-estimated kinetic parameters with catheter contamination adjustment. Large uncertainty and poor estimation were observed when contamination was ignored.
Conclusions
Drug contamination from sampling catheter can impact AMD PK results and should be accounted for in the analysis. We provide a framework for evaluating catheter contamination and guidance on adjustment in the PK model.
Keywords: Actinomycin-D, Catheter, Contamination, Pediatric, Pharmacokinetics
Introduction
Survival rates for most childhood cancer have improved steadily since the 1970s as a result of more effective treatments and the emergence of new molecularly targeted agents [1] as well as a greater understanding of older, effective cytotoxic agents. Increased incentive to conduct pediatric studies for older chemotherapeutic agents has ensued since pediatric data are often lacking and dosing guidance is likewise still dependent on historical and empirical practices. Both actinomycin-D (AMD) and vincristine (VCR) have been longstanding components of many pediatric cancer treatment regimens, yet only a handful of pediatric PK studies have been published and very little is known about their exposure-toxicity profiles. There is a continued challenge in conducting pediatric PK studies, especially due to parental concerns with excessive needle sticks for blood sample collections. Sparse sampling strategies coupled with population pharmacokinetic analysis techniques have been implemented to reduce the burden on patients and to comply with blood sample volume restrictions in pediatric studies. Another approach to enhance study enrollment is to eliminate sampling from peripheral catheters and obtain PK samples directly from the indwelling central venous catheter from which intravenous chemotherapeutics are administered.
Adsorption of drug molecules to catheter tubing is a well-recognized phenomenon. The extent of contamination is influenced by multiple intrinsic and extrinsic factors, including physiochemical properties of drug molecules, physical properties of catheter materials and configurations, clearing vehicles and clearing procedures. As part of a cooperative study to quantify the PK of AMD and VCR in children with cancer, we previously reported on the extent of drug contamination from simultaneous dosing and sampling through a single catheter in an in vitro experimental system and concluded that five blood-draw return cycles using 5 mL of whole blood was the most effective method of clearing residual drug from the sampling catheter [2]. The initial catheter-bound drug accounted for more than 25.4 and 8.69 % of the total infused AMD and VCR, respectively. Both drugs were completely cleared from the catheter following the aforementioned clearing procedure, although residual VCR concentration was undetectable after only three blood-draw return cycles. The catheter clearing procedure was then evaluated for its clinical efficiency and feasibility in a pilot study where paired PK samples were collected from both the dosing central venous catheter and a separate peripheral catheter in three pediatric cancer patients. Samples from the central catheter were subjected to clearing on the first two samples (5, 10 or 30 min) with the view that contamination would be the greatest and therefore would have the most impact on PK parameters at earlier time points. On average, samples obtained from the central catheter were higher than the matching samples obtained through the peripheral catheter for both AMD and VCR. Moreover, systematic bias between the paired concentrations was larger for AMD (4.12 ± 6.34 ng/mL) than VCR (1.45 ± 3.74 ng/mL), suggesting that the clearing procedure was more efficient in removing catheter-bound VCR than AMD, consistent with the in vitro findings [2].
The ability to accurately report PK results is crucial in deriving dose–exposure and exposure–response relationships in order to establish rational pediatric dosing guidance for older chemotherapeutic agents. This report provides modeling results for AMD from the second project of a larger Best Pharmaceuticals for Children’s Act (BPCA)-funded study of AMD and VCR in children with cancer. Following up with our initial effort in addressing the issue of drug catheter contamination and devising a clearing method to minimize such contamination, we sought to investigate the impact of contamination on PK parameters. Following quantitative assessment of the clearing procedure, we sought to define a systematic approach to ensure accurate reporting of PK results. We applied population modeling and simulation approaches to address these goals. Our specific aims were to (1) develop population PK models which estimate the magnitude of systematic bias following central venous catheter sampling with and without a clearing procedure; (2) examine the impact of catheter contamination on PK parameters based on trial simulations of an ongoing prospective study in children with cancer; and (3) recommend a modeling procedure for reporting accurate PK results following the completion of the aforementioned study.
Methods
Data sources
The modeling dataset consisted of the current catheter study as well as previously published studies of AMD PK in pediatric cancer patients [2-4]. The historical dataset consisted of 145 and 19 AMD plasma concentrations collected from 31 patients enrolled in a United Kingdom (UK) Children’s Cancer Study Group study and two patients from a previous pilot study at the Children’s Hospital of Philadelphia (CHOP), respectively. PK samples from the UK study were collected from the same central line as the drug infusion catheter (e.g., single-lumen catheters, double-lumen catheters, and Port-A-Cath) at 15 and 30 min and 1-, 2-, 4-, 6-, and 24-h post-administration, and no systematic clearing procedure was implemented prior to sample withdrawal. The study design and results were described in detail by Veal et al. [3]. PK samples from the CHOP pilot study were obtained from a separate peripheral line at 5, 10, 30, 60, 90, and 150 min and 4–6, 8–12, 20–24, and 44–48 h post-administration [4]. The current pilot dataset was extracted from our proof-of-principle study aimed at evaluating the efficiency of the proposed catheter clearing procedure to clear drug from central venous lines in pediatric patients dosed with AMD and VCR. Paired PK samples from an indwelling central venous catheter (e.g., Broviac and Port-A-Cath) and a newly placed peripheral venous catheter were obtained from three patients at 5, 10, 30, 60, 90, and 150 min and 4–6, 8–12, and 20–24 h post-administration. Prior to the collection of the first and second samples from the central catheter, a catheter clearing procedure consisted of five blood-draw return cycles as described previously was carried out by the study nurse [2].
Modeling approach
Population PK modeling was performed using a mixed-effects approach with NONMEM Version VI, Level 2.0 (ICON Development Solutions, Ellicott City, Maryland). PK parameters were estimated using the first-order conditional estimation method with η-ε interaction (FOCE INT). A historical 3-compartment model with first-order elimination and allometric scaling on clearances and compartment volumes was chosen as the base structural model for this analysis (Eq. 1) [4].
| (1) |
where the typical value of a model parameter for an individual (TVPi) was described as a function of the typical value for a 70 kg adult (θTVP) and individual normalized weight (WTi/70). The allometric power exponent (θallo) was fixed to a value of ¾ for physiological processes and a value of 1 for anatomical volumes [5].
Inter-individual variability (IIV) terms were included for total systemic clearance (CL) and central compartment (V1) using an exponential error model (Eq. 2).
| (2) |
where Pi is the estimated parameter value for individual i, TVP is the typical population value of the parameter, and ηPi are individual-specific IIV for individual i and parameter P which are assumed to be normally distributed η ~ N(0, ω2) with covariance matrix for the IIV (Ω).
The residual error was described by a proportional error model (Eq. 3).
| (3) |
where Cij is the jth measured observation in individual i, is the jth model-predicted value in individual i, and εij is the proportional residual random error for individual i and measurement j and is assumed to be normally distributed ε ~ N(0, σ2).
Full covariate analysis was previously investigated in the original historical model [4]. Additional covariates, specifically age and gender, were analyzed in this investigation based on exploratory graphics and physiological plausibility. Univariate analysis was only performed if plots of eta-covariate showed obvious trend. Due to the small number of covariates in the dataset, only forward selection was employed and a covariate was included in the final model if the objective function value (OFV) was decreased by at least 6.64 points (P < 0.01 for χ2 distribution with 1 degree of freedom). A normalized power model was used to describe the effects of continuous variables (Eq. 4).
| (4) |
where TVPi is the typical value of the model parameter for an individual with covariates (covi) equal to the reference covariate values (covref).
Three different modeling approaches were evaluated to assess the effects of catheter contamination on AMD PK, including (1) covariate model where the sampling catheter [central (CVL) or peripheral (PIV)] was treated as a categorical covariate on CL or V1; (2) baseline contamination model which assumed drug contamination from the sampling catheter as a baseline concentration and was accounted at the level of the individual predictions. Fixed amounts of contamination, concentration-dependent contamination, and saturable binding contamination were also explored using this approach; (3) mechanistic clearance model which incorporated a catheter binding compartment and kinetic parameters describing the “pull–push” catheter clearing procedure. Final model selection was based on the evaluation of goodness-of-fit diagnostic plots, successful convergence, plausibility and precision of parameter estimates, minimum objective function values and Akaike’s information criterion (AIC).
Model evaluation and simulation strategy
The final model was evaluated using a visual predictive check (VPC) by simulating concentrations from 1,000 replicate datasets of the UK study and CHOP pilot study separately. The same simulation approach was applied to evaluate the impact of catheter contamination in an ongoing prospective trial of AMD and VCR in children with cancer. Simulated patient population was obtained from the Centers for Disease Control/National Health and Nutritional Survey (CDC/NHANES) database which contained demographic information for 21,623 children and 26,789 adults collected from 1998 to 2008 [6]. The database was extracted using SAS to contain only relevant demographic variables (age, weight, and gender) and patients were randomly selected based on the trial age stratification scheme. The simulation populations were created from the database to reflect target enrollment (n = 140) with 35 children per pre-defined age strata. AMD dosing was calculated based on current Children’s Oncology Group (COG) studies in patients with Wilms’ tumor and rhabdomyosarcoma: 0.025 mg/kg if <1 year of age and 0.045 mg/kg if ≥1 year of age with a maximum dose of 2.5 mg [7]. Simulated patients were assigned to one of two sampling schemes per trial protocol (Scheme 1: 5, 10 min, 2, 24 and 48 h (25 % of patients had 48 h sampling time); Scheme 2: 5 min, 1, 5, 24, and 48 h (25 % of patients had 48 h sampling time)). One thousand trials were simulated and population PK parameters for each dataset were estimated using NONMEM. Empirical 95 % confidence intervals (CI) were constructed by obtaining the 2.5th and 97.5th percentiles of the parameter distributions for all successful runs.
Results
Patient characteristics and AMD dosing
Thirty-six children and adolescents receiving AMD were included in the pooled dataset. Basic patient demographics were similar between the current pilot study and historical study population (Comparison of study demographics provided in supplemental files, Table S1). Male patients accounted for two-thirds of the total population for both datasets which was consistent with the gender distribution pattern for certain childhood cancers [8, 9]. Maximum AMD dose was higher for the pilot study (2.5 mg) than the historical study (2.0 mg), reflecting differences in dose capping rules from different institutions. However, maximum dose based on body weight was higher for the historical study (0.072 mg/kg) than the pilot study (0.044 mg/kg), which was due to older dosing regimen when body surface area was used to calculate AMD dosing.
Model development
Base model
A total of 199 AMD plasma concentrations were included in the modeling dataset, of which 17 and 145 concentrations were sampled via a central venous line with (pilot study) and without catheter clearing procedure (historical study), respectively. Observed AMD plasma concentrations versus time after dose showed a multi-phasic exponential decay pattern, and data from the pilot study were comparable with the historical data (comparability of analysis dataset with historical data provided in supplemental files, Figure S1).
The published 3-compartment population PK model developed from historical data, which did not address catheter contamination, was used as the initial model for the pooled dataset. While the model successfully converged with reasonable agreement between observed and population-predicted values, a trend toward under-prediction was observed for concentrations greater than 30ng/mL, supporting the concern for addressing contamination at early sampling times (Fig. 1). A single set of paired central and peripheral samples from the pilot study was speculated as mislabeled during the analytical process since both measured AMD and VCR concentrations were higher from the peripheral catheter than the dosing central line, which was inconsistent with the contamination pattern seen with the remaining paired samples from the same patient. While these concentrations were excluded in the analysis, the objective function value (OFV) was decreased from 599 to 583 (Table 1).
Fig. 1.
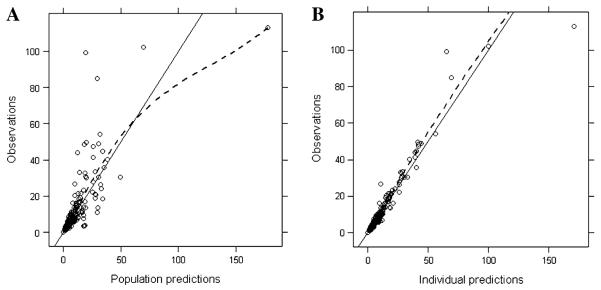
Diagnostic plots for the base AMD 3-compartment model. a Observed concentrations versus population-predicted concentrations. b Observed concentrations versus individual-predicted concentrations
Table 1.
Summary of model progression
| Model description | Baseline contamination | CTM (h) | OFV | AIC | |
|---|---|---|---|---|---|
| Base model | |||||
| 1 | Allometric model on V1, V2, V3, CL, Q2, Q3 | – | – | 599 | 619 |
| 2 | As model 1, 2 outliers from pilot excluded | – | – | 583 | 603 |
| 3 | As model 2, | – | – | 576 | 598 |
| Contamination model | |||||
| 4 | CTM = θ9 on CVL | 1 parameter: fixed | 0.486 ng/mL | 557 | 583 |
| 5 | CTM = θ9 · IPRED on CVL | 1 parameter: proportional | 0.192 | 529 | 555 |
| 6 | CTM1 = θ9 · IPRED on CVL (no clearing) CTM2 = θ10 · IPRED on CVL (with clearing) |
2 parameters: proportional | θ9 = 0.149 θ10 = 0.436 |
527 | 557 |
| 7 | As model 5, CTM = (θ9 · exp(-Ke · TIME)) · IPRED on CVL |
1 parameter: proportional, declines with time |
0.193 | 516 | 544 |
AIC Akaike’s information criteria, CL clearance, CTM contamination factor, CVL central venous line, DV observed concentration, IPRED individual predictions, Ke rate constant for change in contamination factor, OFV objective function value, Q2 and Q3 inter-compartmental clearance, TVV1 typical population value of volume of central compartment, V1 volume of central compartment, V2 and V3 volume of peripheral compartment
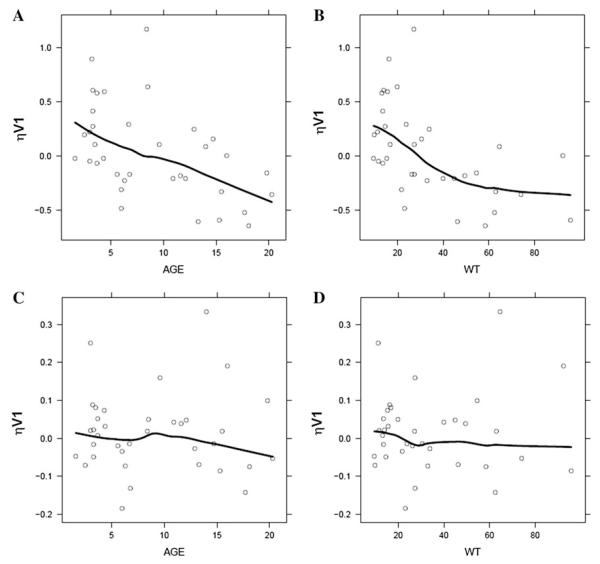
The effect of different measures of body size was extensively evaluated previously in the historical model and body weight was the significant covariate explaining variability in AMD PK parameters [4]. Despite inclusion of allometric model with normalized weight on all relevant PK parameters (CL, Q2, Q3 exponent fixed to ¾; V1, V2, V3 exponent fixed to 1), correlation between variability for central volume (V1) and age or body weight was observed in the pooled dataset (Fig. 2a, b). Although age and weight are highly correlated, inclusion of age covariate as a power function on V1 abolished trend in random effects and OFV dropped by 7 points (Fig. 2c, d). Hence, this model served as the base model for subsequent development of catheter contamination models (Table 1, Model 3).
Fig. 2.
Exploratory graphics from the historical 3-compartment model and base model from current analysis for covariate relationships. a, b Relationship between inter-individual variability of central volume of distribution (ηV1) and age or body weight (WT) in the historical model including weight as a significant covariate. c, d Relationship between inter-individual variability of central volume of distribution (ηV1) and age or body weight (WT) in the base model including both weight and age as significant covariates. Solid line is a loess smooth fit
Baseline contamination model
To account for residual catheter-bound drugs in PK samples collected via the central dosing line, a baseline contamination model was developed which included estimation of a basal drug level or contamination factor (CTM) in the model prediction for CVL samples but not PIV samples. The contamination factor was not well estimated (95 % confidence interval encompasses zero) when assuming the magnitude of contamination remained static during the entire sampling period (Model 4). Contamination parameter estimation did not improve with either time or concentration constraints. When a proportional contamination model was applied where catheter contamination was viewed as a fraction of the model-predicted concentration, model fitting improved significantly with 47 points drop in OFV (Model 5). To test the hypothesis that catheter clearing minimizes contamination during PK sampling, two separate proportional contamination factors were included in the model based on whether or not catheter clearing was implemented. Although model converged successfully, both contamination factors were poorly estimated with large relative standard error (RSE>50 %) and 95 % confidence intervals. The single proportional contamination model was then refined to include a first-order rate parameter (Ke) describing time-dependent decline in contamination. Model fitting was further improved and all parameters were well estimated. CVL catheter contamination was found to be 19.3 % (95 % CI: 12.5–26.1 %) that of the model-predicted concentration at the initial time point immediately after the drug administration and declining at a first-order rate of 0.0932 h−1 (95 % CI: 0.0601–0.126 h−1). A summary of model progression is listed in Table 1.
Parameter estimates resulting from the historical model, base model, and contamination model were shown in Table 2. There were no appreciable differences in the PK parameters between the historical model and base model with the inclusion of the catheter pilot study data. Modest changes in CL (+21 %) and V1 (+83 %) were observed when catheter contamination was accounted for in the model. Typical values for volumes and inter-compartmental clearances were estimated with better precision. Moreover, unexplained inter-individual variability in V1 was reduced in the contamination model (24.6 % CV) when compared to the base model (41.6 % CV), which reflects the greater impact of contamination at early time points around Cmax. The residual variance between the contamination-free model and contamination model was similar at 24.6 and 20.8 % CV, respectively. Since the majority of the historical data were from the UK study and only three subjects were included in the CHOP pilot study, the potential impact of different analytical assays on PK parameters could not be fully evaluated. When separate residual error models were applied to the historical data and pilot data, no improvement in model fit was observed.
Table 2.
Parameter estimates from AMD population pharmacokinetic models
| Historical model | Base model | Contamination model | |
|---|---|---|---|
| Parameter (Point estimate, % RSE) | |||
| CL, L/h/70 kg | 9.99 (11.2) | 9.82 (12.2) | 11 (9.36) |
| V1, L/70 kg | 3.56 (39.3) | 3.17 (61.8) | 5.79 (18.3) |
| V2, L/70 kg | 16.1 (80.7) | 16.1 (116) | 24.2 (69.4) |
| V3, L/70 kg | 367 (19.7) | 367 (28.1) | 490 (8.29) |
| Q2, L/h/70 kg | 11.4 (58.8) | 10.9 (93.6) | 17.7 (38) |
| Q3, L/h/70 kg | 25.2 (23.3) | 24.1 (35.1) | 42.8 (13.6) |
| CL, Q2, Q3 ~ WT/70 power | 3/4 fixed | 3/4 fixed | 3/4 fixed |
| V1, V2, V3 ~ WT/70 power | 1 fixed | 1 fixed | 1 fixed |
| V1 ~ AGE/8.8 power | - | -0.329 (30.4) | -0.448 (26.6) |
| CTM | - | - | 0.193 (17.9) |
| Ke, h−1 | - | - | 0.0932 (18.1) |
| IIV (% CV, % RSE) | |||
| 40.4 (56.3) | 39.9 (60.8) | 48.4 (28.1) | |
| 49 (36.2) | 41.6 (54.5) | 24.6 (64.2) | |
| - | - | 127 (40.9) | |
| COVCL,VI | -0.057a | -0.0412a | -0.0507a |
| COVCL,CTM | - | - | -0.198a |
| COVV1,CTM | - | - | -0.0601a |
| Residual variance (% CV, % RSE) | |||
| 24.7 (34.5) | 24.6 (33.2) | 19.6 (27) |
IIV inter-individual variability, CL clearance, COVCL,Vl covariance between random effect of CL and V, CTM contamination factor, CV coefficient of variation, Ke rate constant for change in contamination factor, Q2 and Q3 inter-compartmental clearance, RSE relative standard error of the estimate, VI volume of central compartment, V2 and V3 volume of peripheral compartment; WT body weight, and variances of random effect of CL and V1, respectively, proportional residual error model
Point estimate
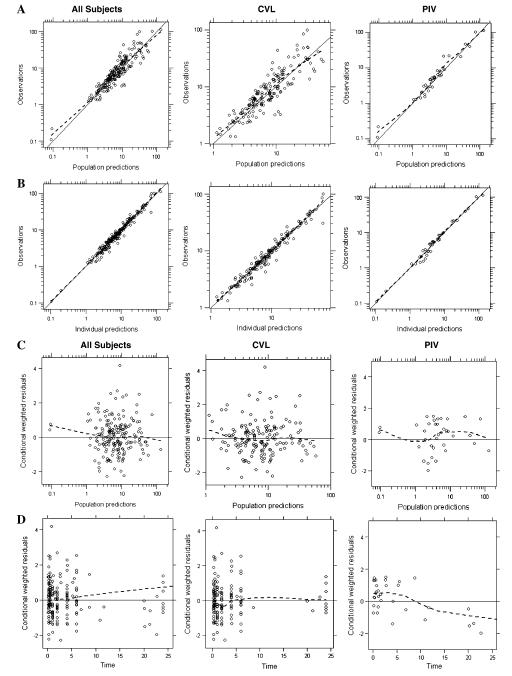
Goodness-of-fit and diagnostic plots for the final model are shown in Fig. 3. Population-predicted concentrations were in good agreement with the observed data showing little systematic bias for both CVL and PIV samples. Conditional weighted residuals versus time plot showed slight trending toward under-prediction at early time points for CVL samples, suggesting potential inadequacy of the model to accommodate highest level of contamination immediately post-dosing.
Fig. 3.
Diagnostic plots for the final AMD 3-compartment contamination model. a Observed concentrations versus population-predicted concentrations. b Observed concentrations versus individual-predicted concentrations; conditional weighted residuals versus c population-predicted concentrations or d time after dose for the final model
Additional contamination models
Alternative modeling approaches to address the issue of catheter contamination was explored. For example, the type of PK sampling catheter (CVL or PIV) was considered as a categorical covariate and its effect on PK parameters (CL and V1) was evaluated. Although this covariate model approach was computationally simplistic, the model parameters lacked physiological relevance or afforded estimation of the extent of contamination. A more mechanistic approach where catheter space and kinetic rate processes associated with the catheter clearing “pull–push” actions were incorporated into a catheter clearance model. The model consisted of five compartments, three bioavailability terms and five additional kinetic rate constants associated with drug catheter binding phenomenon. The estimation of all parameters was not entirely feasible due to the limited dataset; thus several parameters were derived based on projections from the catheter in vitro experiments as well as sensitivity analysis [2]. Simulation of the clearance model indicated progressive removal of drug in the catheter-bound compartment with each additional blood-draw return cycle and almost complete clearing of bound drug was achieved after four cycles at the second sampling time (37 min). Supplemental files (Figure S2 and Table S2) provide a schematic of the mechanistic contamination model along with the simulated profiles in each of the sampling compartments following the clearance procedure and a summary of the various modeling approaches assessed to examine AMD catheter contamination.
Simulation
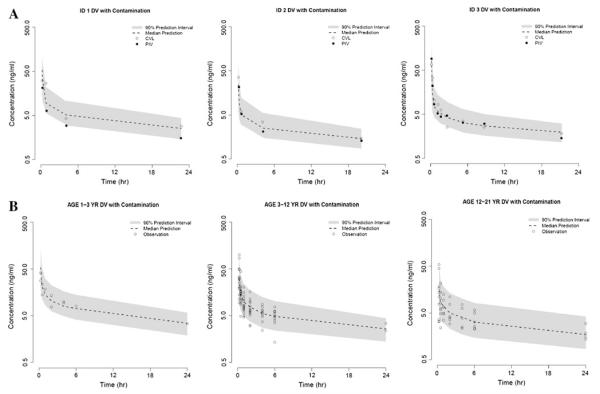
Evaluation of the final contamination model included visual predictive checks, which revealed that the model provided a reliable description of the observed data from both the pilot study and UK study (shown in Fig. 4). AMD concentrations collected via the CVL route in the pilot study fell more closely around the median of the prediction intervals in comparison with PIV samples which were either similar to or lower than CVL samples. The majority of the observed data from the UK study are captured within the 90 % prediction intervals, with the exception of a few points around Cmax for ≥3 years age group.
Fig. 4.
Visual predictive checks comparing observed AMD plasma concentrations and model predictions. Patients from a CHOP and b UK studies were simulated 1,000 times using the final contamination model. Predicted medians (dotted line) and 90 % prediction intervals (shaded region) were plotted as a function of time after dosing
Patient demographics of the simulated population for the prospective trial are summarized in Table 3. The final model yielded simulated AMD concentrations that were significantly higher for CVL samples than PIV samples. As expected, the absolute catheter contamination from CVL route was more evident at early PK time points (5 and 10 min post-dosing) and remained detectable to a lesser extent for samples collected after 1 h of administration (Table 3).
Table 3.
Simulated patient demographics and simulated concentrations of the prospective trial
| Age group (years) | n | Demographics |
Simulated concentrations (ng/mL) |
|||
|---|---|---|---|---|---|---|
| Variable | Mean (range) | Time (min) | PIV, mean (95 % CI) | CVL, mean (95 % CI) | ||
| <1 | 35 | Age (years) | 0.46 (0.01-0.88) | 5 | 43 (9.0-77) | 62 (11-170) |
| Weight (kg) | 7.13 (2.77-11.4) | 10 | 24 (7.1-39) | 34 (8.8-92) | ||
| Dose (mg) | 0.17 (0.06-0.28) | 60 | 4.5 (2.5-7.3) | 6.4 (2.8-16) | ||
| 1 ≤ Age < 3 | 35 | Age (years) | 2.01 (1.13-2.96) | 5 | 113 (58-168) | 162 (68-409) |
| Weight (kg) | 12.2 (8.18-17.1) | 10 | 53 (16-86) | 76 (20-195) | ||
| Dose (mg) | 0.52 (0.37-0.77) | 60 | 7.6 (4.4-12) | 11 (4.9-27) | ||
| 3 ≤ Age < 12 | 35 | Age (years) | 7.43 (3.21-11.2) | 5 | 131 (48-204) | 188 (62-477) |
| Weight (kg) | 24.8 (14.5-53.8) | 10 | 49 (11-95) | 69 (13-182) | ||
| Dose (mg) | 1.06 (0.65-2.42) | 60 | 7.7 (4.5-11) | 11 (5.0-27) | ||
| 12 ≤ Age < 17 | 35 | Age (years) | 14.2 (12.0-17.0) | 5 | 140 (44-225) | 200 (54-515) |
| Weight (kg) | 56.0 (30.3-86.2) | 10 | 47 (8.8-102) | 67 (10-191) | ||
| Dose (mg) | 2.48 (1.36-2.5) | 60 | 7.4 (4.2-11) | 10 (4.6-26) | ||
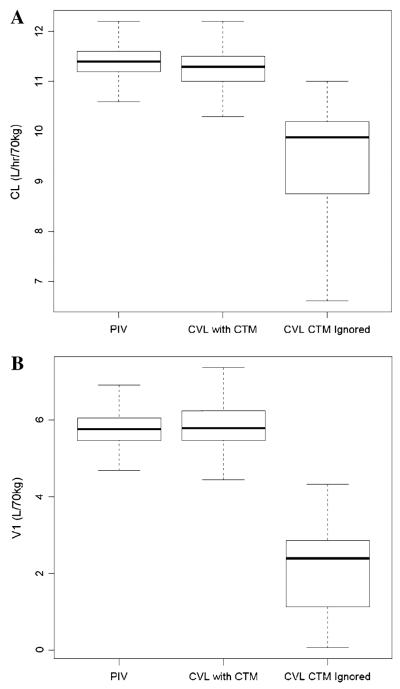
The impact of catheter contamination on AMD PK parameters was then examined by re-estimating kinetic parameters from trial simulation datasets. Clearance (CL) estimates were similar between PIV samples (11.4, 95 % confidence interval: 10.8–11.9 L/h/70 kg) and CVL samples (11.3, 95 % confidence interval: 10.7–12 L/h/kg) in which contamination was accounted for in the final model. However, using the same CVL simulation data and disregard the issue of catheter contamination in the model, CL was substantially lower than the “true” value from PIV samples and was poorly estimated with a large 95 % confidence interval (9.88, 4.05–10.7 L/h/70 kg) (see Fig. 5a). Similarly, model performance was robust in re-estimating central volume of distribution (V1) from the PIV dataset (5.76, 95 % confidence interval: 4.98–6.74 L/70 kg) and CVL dataset (5.79, 95 % confidence interval: 4.87–7.14 L/70 kg) adjusting for contamination, but not under the scenario when CVL contamination was ignored (2.39, 95 % confidence interval: 0.11–3.92 L/70 kg) (shown in Fig. 5b). The magnitude of contamination from CVL simulation dataset was estimated to be 20.1 % (95 % confidence interval: 9.58–39.1 %) of model-predicted concentrations and would decrease over time at a first-order rate of 0.0962 h−1 (95 % confidence interval: 0.0632–0.158 h−1).
Fig. 5.
Box-and-whisker plots of a clearance (CL) and b central volume of distribution (V1) estimates from simulations of a prospective trial. Model parameters were re-estimated from 1,000 simulations when PK samples were collected from a separate peripheral catheter (PIV) or the dosing central catheter (CVL). In the latter, catheter contamination (CTM) was either accounted for or ignored in the model
Discussion
Blood collection through a peripherally placed venous catheter has been the traditional gold standard for PK studies but is often not feasible in small children and presents a tremendous obstacle for effective enrollment in pediatric PK trials. The present report focuses on the quantitative assessment of AMD catheter contamination from the central dosing catheter in the clinical setting, testing the hypothesis that our previously described catheter clearing procedure (five blood-draw return cycles using patient’s own blood) is efficient in removing adsorbed drug in the infusion catheter tubing. Using a mixed-effect modeling approach, we have developed a population PK model for AMD incorporating information on sampling catheter types and adjustment for catheter contamination. The model quantified the magnitude of contamination when a single intravenous catheter was utilized for both dosing and sampling, but the model was unable to differentiate contamination between uncleared and cleared catheter due to the limited data contributed from the latter. The simulation exercise illustrates that irrespective of whether or not catheter clearing procedure is implemented, contamination should be accounted for in the PK model to ensure accurate reporting of kinetic parameters but the differentiation of catheter clearance efficiency with respect to estimated parameters will have to be revisited.
The final model indicated that AMD PK samples collected via central venous line (CVL) were overestimated by 19.3 % compared with the corresponding PIV samples immediately after drug administration, while the influence of sampling contamination decreased over time at a first-order rate of 0.0932 h−1. The extent of contamination primarily reflected catheter drug adsorption without any form of systematic catheter clearing procedure which constituted 90 % of the pooled CVL data. The estimated contamination factor from the clinical data was consistent with our previously reported in vitro findings that 25.4 % of AMD was recovered from the infusion catheter under no clearing conditions. Much more extensive contamination, up to twofold higher concentration from the infusion catheter compared with the peripheral intravenous line (PIV), has been reported for cyclosporine and thiotepa in the clinical setting [10-12]. In the case of vincristine, although we did not quantify the magnitude of contamination via modeling in this report, our previous data suggested that there is less potential for catheter adsorption. Hence, the inherent adsorption characteristics of individual drugs should be evaluated experimentally to help inform the best modeling approaches for addressing catheter contamination issues.
The inclusion of the three patients from the CHOP pilot study did not greatly influence the AMD PK estimated from applying the established historical model as the base model [4]. Allometric scaling using body weight was applied to account for developmental changes in AMD clearance and volume of distribution in the pediatric population [5]. In addition, variability in V1 was further explained by age as a covariate in the current base model. The typical value for V1 for a 70 kg person (3.17 L) closely resembled plasma volume, suggesting AMD was primarily distributed in the plasma. The additional effect of age on V1 indicated developmental changes in body composition, plasma protein binding, and tissue partitioning [13]. It is unclear whether this age effect on V1 extends to the very young patients, especially infants less than 1-year old, since the current analysis did not include patients in that age category. Retrospective analysis from COG has shown higher AMD toxicity rate in the less than 1-year-old age group after adjusting for the same dose and body size [7]. Thus, the potential influence of age on the central volume which would impact AMD peak or trough levels will be an important factor in establishing AMD toxicity exposure profiles. The baseline contamination component of the final model applied a proportional contamination factor to reflect the concentration-dependent phenomenon of catheter drug binding. Although this approach does not offer a mechanistic explanation of catheter binding and blood-draw clearing procedure, it allows a simple estimation of overall contamination from the total measured concentrations. Our original hypothesis sought to demonstrate the clinical viability of blood-draw return cycles in eliminating catheter contamination. Due to the unbalanced nature of the pooled dataset (31 patients with uncleared catheter vs. 3 patients with cleared catheter), the effects of catheter clearing could not be differentiated from the overall contamination. The pilot study in which this data were to be collected was open for enrollment for over 2 years which attests to the difficulty in attaining this data. In addition, exact catheter types and dimensions were unknown for each study, which could also represent potential covariates contributing to the variability partition.
The impact of catheter contamination on AMD PK was clearly illustrated in the simulation exercise. Irrespective of the potential different extent of contamination with or without catheter clearance, parameter estimates from the final model, assuming PK sampling from PIV (no contamination) or CVL (with contamination) based on simulations of the ongoing prospective COG trial, closely resembled model parameters. However, if contamination from CVL sampling was unaccounted for in the PK model, the kinetic parameters were poorly estimated and did not encompass the “true” population parameters. In the case of AMD which exhibits large intra-subject variability, failure to adjust for known catheter contamination would add another source of variability, inflate the residual error, and result in inaccurate PK estimates. Although the current model was based on a very limited patient sample size which was not uncommon in pediatric research, it represented the first detailed effort for evaluating catheter contamination issues and providing guidance on adjustment in the PK model. Data from the ongoing prospective trial will serve to validate the current model and refine contamination estimates for the catheter clearing procedure. Collectively, these investigations will help to establish rational dosing and labeling guidance for AMD in children with cancer. Hopefully, they will influence the design and analysis of future phase I pediatric oncology trials as well.
Supplementary Material
Acknowledgments
This work was supported by NIH Grant # CA 098543-02S1.
Footnotes
Present Address: A. Y. Z. Edwards ICON Development Solutions, Marlow, UK
Electronic supplementary material The online version of this article (doi:10.1007/s00280-012-1878-y) contains supplementary material, which is available to authorized users.
Contributor Information
Alena Y. Z. Edwards, Division of Clinical Pharmacology and Therapeutics, The Children’s Hospital of Philadelphia, Colket Translational Research Building, Room 4012, Philadelphia, PA, USA
Jeffrey M. Skolnik, Division of Clinical Pharmacology and Therapeutics, The Children’s Hospital of Philadelphia, Colket Translational Research Building, Room 4012, Philadelphia, PA, USA; Division of Oncology, The Children’s Hospital of Philadelphia, Philadelphia, PA, USA
Erin Dombrowsky, Division of Clinical Pharmacology and Therapeutics, The Children’s Hospital of Philadelphia, Colket Translational Research Building, Room 4012, Philadelphia, PA, USA.
Dimple Patel, Division of Clinical Pharmacology and Therapeutics, The Children’s Hospital of Philadelphia, Colket Translational Research Building, Room 4012, Philadelphia, PA, USA.
Jeffrey S. Barrett, Division of Clinical Pharmacology and Therapeutics, The Children’s Hospital of Philadelphia, Colket Translational Research Building, Room 4012, Philadelphia, PA, USA
References
- 1.Smith MA, Seibel NL, Altekruse SF, et al. Outcomes for children and adolescents with cancer: challenges for the twentyfirst century. J Clin Oncol. 2010;28(15):2625–2634. doi: 10.1200/JCO.2009.27.0421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Skolnik JM, Zhang AY, Barrett JS, et al. Approaches to clear residual chemotherapeutics from indwelling catheters in children with cancer. Ther Drug Monit. 2010;32(6):741–748. doi: 10.1097/FTD.0b013e3181fa3c68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Veal GJ, Cole M, Errington J, et al. Pharmacokinetics of dactinomycin in a pediatric patient population: a United Kingdom children’s cancer study group study. Clin Cancer Res. 2005;11(16):5893–5899. doi: 10.1158/1078-0432.CCR-04-2546. [DOI] [PubMed] [Google Scholar]
- 4.Mondick JT, Gibiansky L, Gastonguay MR, et al. Population pharmacokinetic investigation of actinomycin-D in children and young adults. J Clin Pharmacol. 2008;48(1):35–42. doi: 10.1177/0091270007310383. [DOI] [PubMed] [Google Scholar]
- 5.Holford NH. A size standard for pharmacokinetics. Clin Pharmacokinet. 1996;30(5):329–332. doi: 10.2165/00003088-199630050-00001. [DOI] [PubMed] [Google Scholar]
- 6.Patel D, Dombrowsky E, Barrett JS. A SAS-based solution for the extraction of population demographic priors from the CDC’s NHANES survey database: application in the creation of datasets for modeling and simulation projects. J Clin Pharmacol. 2010;50(9):1085. [Google Scholar]
- 7.Langholz B, Skolnik JM, Barrett JS, et al. Dactinomycin and vincristine toxicity in the treatment of childhood cancer: a retrospective study from the children’s oncology group. Pediatr Blood Cancer. 2011;57(2):252–257. doi: 10.1002/pbc.22882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gurney JG, Young JL, Roffers SD, et al. Soft tissue sarcomas. In: Ries L, Smith M, Gurney J, editors. Cancer incidence and survival among children and adolescents: United States SEER program 1975–1995. National Cancer Institute; Bethesda: 1999. [Google Scholar]
- 9.Gurney JG, Swensen AR, Bulterys M. Malignant bone tumors. In: Ries L, Smith M, Gurney J, editors. Cancer incidence and survival among children and adolescents: United States SEER program 1975–1995. National Cancer Institute; Bethesda: 1999. [Google Scholar]
- 10.Leson C, Bryson S, Giesbrecht E, et al. Therapeutic monitoring of cyclosporine following pediatric bone marrow transplantation: problems with sampling from silicone central venous lines. Ann Pharmacother. 1989;23(4):300–303. doi: 10.1177/106002808902300405. [DOI] [PubMed] [Google Scholar]
- 11.Shulman RJ, Ou C, Reed T, et al. Central venous catheters versus peripheral veins for sampling blood levels of commonly used drugs. J Parent Enter Nutr. 1998;22(4):234–237. doi: 10.1177/0148607198022004234. [DOI] [PubMed] [Google Scholar]
- 12.de Jonge ME, Mathot RAA, van Dam SM, et al. Sorption of thiotepa to polyurethane catheter causes falsely elevated plasma levels. Ther Drug Monit. 2003;25(3):261–263. doi: 10.1097/00007691-200306000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Puig M. Body composition and growth. In: Walker WA, Watkins JB, editors. Nutrition in pediatrics: basic science and clinical applications. B.C. Decker; Hamilton: 1997. pp. 44–62. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.