Abstract
Forty percent of people worldwide are at risk of malaria infection, and despite control efforts it remains the most deadly parasitic disease. Unfortunately, rapid discovery and development of new interventions for malaria are hindered by the lack of small animal models that support the complex life cycles of the main parasite species infecting humans. Such tools must accommodate human parasite tropism for human tissue. Mouse models with human tissue developed to date have already enhanced our knowledge of human parasites, and are useful tools for assessing anti-parasitic interventions. Although these systems are imperfect, their continued refinement will likely broaden their utility. Some of the malaria parasite’s interactions with human hepatocytes and human erythrocytes can already be modeled with available humanized mouse systems. However, interactions with other relevant human tissues such as the skin and immune system, as well as most transitions between life cycle stages in vivo will require refinement of existing humanized mouse models. Here, we review the recent successes achieved in modeling human malaria parasite biology in humanized mice, and discuss how these models have potential to become an valuable part of the toolbox used for understanding the biology of, and development of interventions to, malaria.
Introduction
Malaria presents a significant global health burden, with 300–500 million clinical cases and approximately 800,000 deaths caused by Plasmodium falciparum and Plasmodium vivax (Kappe et al., 2010). Due to limitations in the current laboratory models of human malaria parasites, much of what is known about Plasmodium biology has been extrapolated from rodent-infecting malaria parasites such as Plasmodium berghei and Plasmodium yoelii (Lindner et al., 2012). After transmission by an infected Anopheles mosquito, the sporozoite stage traverses skin cells invades skin capillaries, is taken up by the blood stream and transported to the liver. Once there, it traverses the sinusoidal endothelium (Mota et al., 2001, Ishino et al., 2004, Tavares et al., 2013), and each parasite establishes itself in a single hepatocyte. In most Plasmodium species, all parasites then rapidly replicate as liver schizonts and ultimately spawn tens of thousands of exo-erythrocytic merozoites. Upon release from hepatocytes, parasites enter the bloodstream, invade red blood cells, and initiate intra-erythrocytic replication, which causes disease. However, in Plasmodium vivax infection, a subset of parasites form dormant liver stages, called ‘hypnozoites’. These parasites are thought to reactivate at a later time, and again cause blood stage infections. These clinical episodes associated with recurrent blood stage infection are known as “relapse”. During blood stage infection, some parasites develop into sexual forms called gametocytes, which are transmitted to the mosquito vector and complete the life cycle (Figure 1).
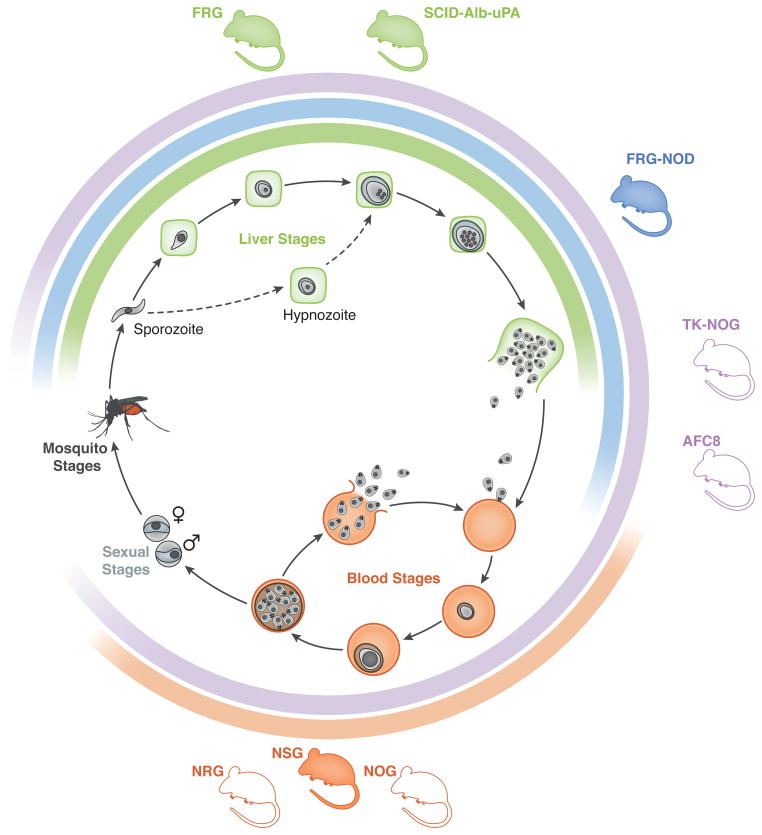
Fig 1. Schematic of humanized mice and their utility to model human malaria parasite life cycle stages.
The different stages of the life cycle of the parasites in the human host are illustrated in the center. The lines surrounding it indicate the extent of the cycle covered by each humanized mouse model, color coded to represent the parts of the cycle covered as follows: from mosquito delivery of sporozoites into the skin to release of exo-erythrocytic merozoites from liver (green, FRG and SCID-Alb-uPA); from mosquito delivery of sporozoites into the skin to erythrocyte invasion (blue, FRG-NOD); from mosquito delivery of sporozoites into the skin to sexual stages (purple, TKG-NOG and ACF8); and from erythrocyte invasion to sexual stages (orange, NSG, NRG and NOG). Filled mice indicate models with available data for that part of the cycle, whereas outlined mice indicate models with proposed roles in the corresponding part of the cycle. Mice shown in Green, Blue and purple have been demonstrated to support repopulation with human hepatocytes. Models depicted in purple and orange have been shown to support long-term maintenance of human erythrocytes. The FRG-NOD mouse can support short term repopulation with human erythrocytes, although to our knowledge long term studies have not been attempted.
The complex life cycle of human malaria parasites and the specificity towards human cell infection have long constituted barriers to study many aspects of these parasite’s biology. Here we review humanized mouse models that have already facilitated the study of P. falciparum liver stages and blood stages in vivo. We aim to highlight where previous models have been unable to fully capture the complex biology of human-infecting malaria parasites and how humanized mouse models will provide opportunities for physiologically relevant studies in the future.
Shared features of humanized mouse models
The humanized mouse models discussed here utilize a common set of mutations and genetically deficient backgrounds for immuno-compromised status that facilitate engraftment with human cells and tissues. The Severe Combined Immune Deficiency (SCID) mouse (Bosma et al., 1983) carries a point mutation in the PRKDC kinase that eliminates B and T cells (Blunt et al., 1995, Kirchgessner et al., 1995). The Non-Obese Diabetic (NOD) mouse dramatically reduces clearance of human hematopoetic cells (Takenaka et al., 2007), in part because of a polymorphism in the signal regulator protein alpha (SIRPα) gene that enhances binding to human CD47 and diminishes macrophage phagocytosis (O’Brien et al., 2002). Natural killer cells also have limited activity in NOD mice because of a defect in the NKG2D receptor (Ogasawara et al., 2003). These properties have made the NOD background preferred for the development of models that utilize xenotransplantation of hematopoetic cells.
Targeted genes deletions have also contributed to the immuno-compromised status that is required for the development of humanized mouse models. The Recombination activating gene 2 (Rag2) is responsible for VD(J) recombination (Oettinger et al., 1990), and thus its elimination in mice leads to lack of B and T cells (Shinkai et al., 1992). Similarly, deleting the IL-2 receptor gamma chain (IL2Rγ) depletes the common receptor of IL-2, IL-4, IL-7, IL-9, IL-15 and IL-21, eliminating functional T cells and NK cells. Crosses between these mice have resulted in the NOD/SCID mouse, the NSG mouse (NOD/SCID/IL2Rγnull), the NOG mouse (NOD/SCID/IL2Rγtruncated)(Hasegawa et al., 2011)), and the NRG (NOD/Rag2/IL2Rγ) mouse, each of which will be discussed in the sections that follow (Figure 2).
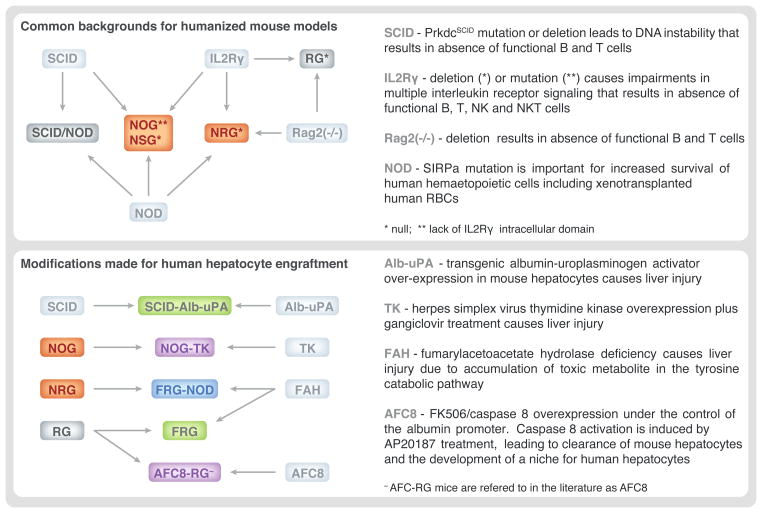
Fig 2. Genealogy of available humanized mice.
Backgrounds common to strains of mice that humanized mice models described in this review are depicted, including crosses that resulted in the different strains. Mice shown in Green, Blue and purple have been demonstrated to support repopulation with human hepatocytes. Models depicted in purple and orange have been shown to support long-term maintenance of human erythrocytes. The top panel describes mice with immune compromised status which supports human tissue engraftment. The bottom panel describes the use of transgenes which provide suitable liver damage to establish niche that facilitates human hepatocyte engraftment.
Modeling human malaria transmission from mosquito vector to host
During Plasmodium journey from the salivary glands of the mosquito to the mammalian hepatocyte within the liver parenchyma, sporozoites encounter a diverse landscape of cells types and tissues. Gliding motility allows Plasmodium spp. to travel through the skin (Menard et al., 2013), and cell traversal allows them to pass through cells of any type (Mota et al., 2001). Initially, it was assumed that the parasites’ biology in the skin is not species-specific. More recently it has been demonstrated that P. berghei can undergo pre-erythrocytic development within skin cells and form exoerythrocytic merozoites (Gueirard et al., 2010) but this complete development does not occur in P. yoelii (Voza et al., 2012). These data prompts consideration of whether this so-called ‘skin-stage’ exists for human Plasmodium parasites. If relevant to human infection, the skin stage could impact the drug development strategies for human malaria, as pharmacological interventions developed towards pre-erythrocytic stages in the liver might not affect skin stages. Novel approaches such as humanized mice with bioengineered skin (reviewed in (Carretero et al., 2011)) could be used to understand what role, if any, the skin plays in pre-erythrocytic development of human malaria parasites. The common SCID/NOD background of human-skin-engrafted mouse models, liver and blood models (see next sections) suggests that the ‘skin stage’ could be incorporated into a comprehensive humanized mouse model of human malaria parasite life cycles.
Modeling human malaria parasite liver stages
The liver stage (LS) infection begins when a Plasmodium sporozoite enters a hepatocyte and surrounds itself with the Parasitophorous Vacuole Membrane (PVM). Since liver stage infection is asymptomatic and lasts seven to ten or more days for human Plasmodium species, it constitutes an attractive target to prevent progression to disease-causing blood stage infection and further transmission of the parasite.
Limitations of rodent malaria parasite models
Plasmodium species infecting rodents and humans are highly divergent. While some critical factors for pre-erythrocytic infection are known in rodent-infecting P. berghei and P. yoelii, it remains largely unknown how relevant they are for P. falciparum and P. vivax pre-erythrocytic infection. For example, the hepatocyte surface protein CD81, which is required for hepatocyte infection by one rodent malaria parasite species appears also necessary for P. falciparum infection of hepatocytes (Silvie et al., 2003). However, the receptor tyrosine kinase c-Met appears to be involved in early liver infection by P. berghei (Carrolo et al., 2003), but not is not at all important for P. yoelii or P. falciparum (Kaushansky et al., 2011). Binding to heparan sulfate proteoglycans on the hepatocyte surface facilitates rodent malaria sporozoite invasion (Frevert et al., 1993, Coppi et al., 2007) but whether they facilitate human parasite invasion remains unclear. Interestingly, while rodent malaria parasites infect (Hollingdale et al., 1983, Silvie et al., 2003) and sometimes complete LS development in human hepatocytes (reviewed in (Prudencio et al., 2011)), mouse hepatocytes are unsuitable for both P. falciparum and P. vivax LS development. Thus, experimentation that directly assesses factors that impact human malaria parasite LS development is critical.
A better understanding of the receptors required for sporozoite invasion of host cells would uncover a remaining mystery about the malaria parasite and could facilitate the development of novel, immuno-competent, humanized mouse models. A parallel approach has been successful in developing a partially humanized model that supports hepatitis C virus (HCV) infection. More specifically, knock-in mice that express human CD81 and occludin (OCLN) have been used for modeling in vivo HCV infection in fully immuno-competent mice (Ploss et al., 2009). The possibility that some of the HCV receptors (i.e. CD81) overlap with those utilized by Plasmodium spp. suggests that mice developed for supporting infection with other pathogens could be useful for studying human parasite LS.
Current humanized liver mouse models for human Plasmodium pre-erythrocytic infection
The establishment of mouse models that allow for repopulation of mouse livers with human hepatocytes requires two major components: (1) an immuno-compromised recipient mouse to prevent the rejection of human tissue (discussed above), and (2) the initiation of liver injury that depletes mouse hepatocytes and thus creates a niche to allow human hepatocytes to colonize the mouse liver. To date, there are four major approaches that have proven successful in generating liver injury suitable for the establishment of human-hepatocyte repopulated mouse livers: (1) the expression of urokinase Plasminogen Activator (uPA) toxin under the albumin promoter (Rhim et al., 1994); (2) the Fumarylacetoacetate Hydrolase (FAH) knockout that leads to the toxic build-up of fumarylacetoacetate, an intermediate of tyrosine metabolism (Azuma et al., 2007); (3) the expression of the herpes simplex virus type 1 thymidine kinase (HSVtk) transgene (Hasegawa et al., 2011); and (4) the inducible activation of apoptosis via Caspase 8 oligomerization (Washburn et al., 2011). In each of these models the basic premise is similar in that liver injury caused by the death of mouse hepatocytes creates a niche that can be colonized by injected primary human (adult or fetal) hepatocytes. While the uPA model is most established, the advantage of other models is the inducible nature of the liver injury.
The first success in assessing P. falciparum liver stage development in liver-humanized mice used Alb-uPA on a SCID background (Mercer et al., 2001). The liver of these mice can be robustly repopulated with human hepatocytes soon after birth and these hepatocytes are susceptible to P. falciparum infection (Sacci et al., 2006, Mikolajczak et al., 2011). To our knowledge the model has yet to be used to model P. vivax liver stages.
More recently, it has been demonstrated that P. falciparum can infect other humanized mouse models. Specifically, FRG KO mice that lack FAH, Rag2, and IL2rγ can be efficiently transplanted with human hepatocytes (FRG KO huHep) (Azuma et al., 2007). We have recently shown that this model supports robust P. falciparum LS infection and that it is able to support complete maturation of LS parasites (Vaughan et al., 2012) (Figure 3). When these mice are back-crossed on the NOD background (FRG-NOD-HuHep), the resulting new model can support the transition of exo-erythrocytic merozoites stage to blood stage infection (Vaughan et al., 2012). Encouragingly, atypical small arrested LS that are observed during in vitro hepatocyte infection with P. falciparum sporozoites (March et al., 2013) are never observed in this model (Vaughan et al., 2012). This fact will be useful moving forward to the study of P. vivax liver stage infection, which unlike P. falciparum harbors small dormant hypnozoite forms. Thus, models which do not harbor artificially small forms will be critical for understanding P. vivax liver stage dormancy (Vaughan et al., 2012).
Fig 3. FRG huHep mice support development of P. falciparum liver stages.
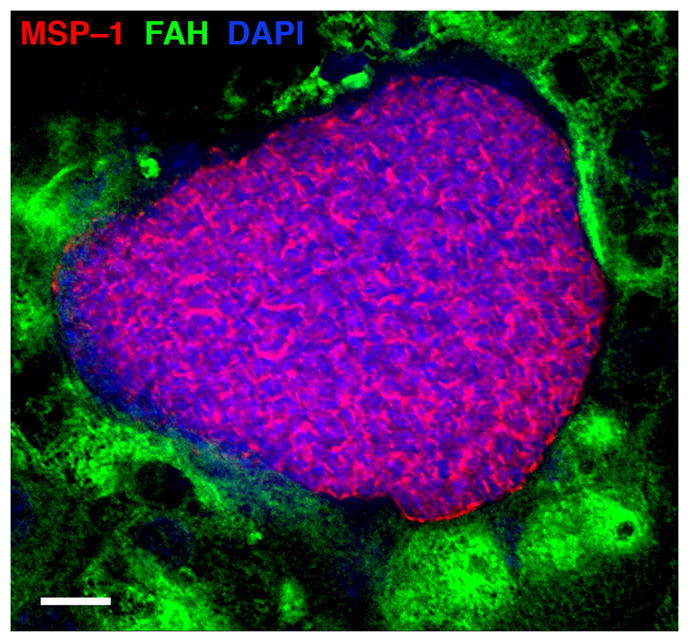
Liver sections of FRG huHep mice at day 7 after P. falciparum sporozoite infection were stained with antibodies specific to P. falciparum merozoite surface protein 1 (MSP-1; in red) and to human Fumarylacetoacetate Hydrolase (FAH; in green) and DAPI (blue) to visualize DNA. A large liver stage schizont is shown before maturation and differentiation into exo-erythrocytic merozoites. Scale bar – 10 μm.
Thus, to date, only SCID-Alb-uPA and FRG-HuHep mice have been shown to be suitable for the development of P. falciparum liver stages. However, other currently existing models have the potential to provide a purely in vivo system to study Plasmodium biology and pathogenesis. One of these models is created when the HSVtk transgene is expressed within the liver NOG (NOD/SCID/IL2γtruncated) background mice (TK-NOG), inducing death in mouse hepatocytes and thus allowing for repopulation with human hepatocytes. Because of the NOD and immuno-compromised background, it is possible these mice will be suitable for a combined model of liver and blood stage infection. This model has been demonstrated to be suitable for HCV infection, raising the possibility that it may also be useful for modeling Plasmodium liver stage (Kosaka et al., 2013).
Transition from humanized liver infection to human red blood cell infection
At the end of LS growth and replication, exo-erythrocytic merozoites form and exit the host hepatocyte as merosomes, or ‘packages’ of merozoites surrounded by host plasma membrane (Sturm et al., 2006). Initially this was demonstrated using rodent malaria parasites, but recent work using FRG huHep has shown that P. falciparum has a similar exit strategy (Vaughan et al., 2012). Importantly, exo-erythrocytic merozoites released form the liver in FRG-NOD HuHep mice are infectious to human red blood cells (hRBCs) injected 6–7 days after sporozoite infection (Vaughan et al., 2012).
Asexual blood stage replication of human parasites in mice with human RBCs
Blood stage parasites are responsible for all symptoms of malaria infection. A small animal model for P. falciparum blood stages would enhance understanding of disease pathophysiology, and is critical for modeling the complete life cycle in small animal models. Complex interactions between infected red blood cells (iRBCs) and other cell types (for example, through the phenomenon of cytoadhesion) can be studied only in a limited capacity through in vitro models (Tripathi et al., 2006, Avril et al., 2012). Differences in the cytoadhesion properties of rodent malaria parasites and human malaria parasites have so far prevented the development of accurate in vivo model for severe, cerebral (Nacer et al., 2012) and placental malaria (reviewed in (Held et al., 2013)).
Currently available models: advantages and challenges
Several immuno-deficient mouse strains with a humanized RBC compartment have been evaluated as human malaria blood stage models with variable degrees of success (reviewed in (Khan et al., 2012)). In these models, severe immune deficiency has been a prerequisite to support blood stage infection, in large part due to the requirement for hRBC xenotransplatation. The most successful model of P. falciparum blood stage infection to date has been the NSG mouse model that lacks functional T, B, NKT and NK cells (Arnold et al., 2011). In this model, remaining elements of the immune system (i.e. macrophages) can be depleted by injection of liposomal-clodronate formulations (Arnold et al., 2010, Arnold et al., 2011). Human red blood cells are then injected at regular intervals to replenish cleared cells. Reproducible, high parasitemia can be achieved in this model (Arnold et al., 2010, Arnold et al., 2011).
The alternative approach of adding human cytokines to immuno-deficient mouse models has enhanced their capacity to support engraftment of human hematopoetic lineage cells. Human cytokines can be expressed either as knock-in constructs, administered as purified protein, or expressed as transgenes in the liver by hydrodynamic injection of DNA. Expression of human thrombopoietin (TPO) enhances hematopoetic stem cell (HSC) engraftment as well as the differentiation of the common erythroid/megakaryocytic progenitor cells (Rongvaux et al., 2011). Treatment with erythropoietin (EPO) and IL-3 after macrophage depletion and CD34+ cell transplantation in the NSG model (Hu et al., 2011) allows increased hRBC repopulation in the bloodstream. Similarly, phagocytosis of human cells can be minimized by expression of human SIRPα (Strowig et al., 2011). In addition to enhancing maintenance of P. falciparum blood stages, models that include immature hRBC by differentiating them from hHSCs (CD34+ cells) might be more suitable for the study of P. vivax, as these models develop reticulocytes, which are required for P. vivax blood stage infection.
Sexual stages and blood-to-mosquito transmission
High P. falciparum parasitemia and relatively stable levels of hRBC chimerism in NSG mice allows for the transition to sexual stages, although mature gametocytes are rarely detected (Arnold et al., 2011), perhaps partially due to the fact that it takes two weeks for P. falciparum gametocytes to mature. Interestingly, while asexual stages of P. vivax are more challenging to culture, sexual stages might be more straightforward to develop in vivo since gametocytes appear to develop more quickly (reviewed in (Galinski et al., 2013). FRG-NOD HuHep mice infected with P. falciparum sporozoites allow transition to blood stages, which retain their capacity to become fertile gametocytes and transition to mosquito-infectious forms in vitro (Vaughan et al., 2012), paving the way for following the human parasite through multiple transmission cycles in vivo in the laboratory. Transmission blocking interventions are currently only assessed in vitro, which does not account for factors such as drug metabolism or gametocyte sequestration that might complicate intervention efforts.
Future Directions for the use of humanized mouse models
Genetic crosses
An immediate application of humanized liver mouse models that allow transition of the liver stage infection to blood stage infection is that such models could support forward genetics research with P. falciparum. Genetic crosses have been the workhorse of the geneticist attempting to map genetic determinants of phenotypic variation in animals and plants. Unfortunately human malaria parasite genetic crosses to map determinants encoding phenotypes of importance such as antimalarial drug resistance have only been possible by conducting experimental infections of chimpanzees (Su et al., 2007) with sporozoites derived from mated gametocytes of two distinct parasite strains (e.g. chloroquine resistant versus chloroquine sensitive strains (Su et al., 1997)).
Completing the life cycle
A combined humanized mouse model that can harbor liver stages, allows transitions to blood stages and continuously supports blood stage infection would constitute a major advance. The AFC8-hu HSC/Hep model provides the first step towards such a combined model of hepatocyte and hematopoetic humanization. In this model, AFC8 mice are treated with FK506 binding protein (FKBP), which induces caspase-8 activation and elicits apoptosis in hepatocytes (Washburn et al., 2011). Mice are then transplanted with hepatic progenitor cells and hematopoetic stem cells resulting in hepatocyte (15%–25%) and hematopoietic-derived cell repopulation (>90%) (Bility et al., 2012). It remains unclear if human Plasmodium parasites can infect this model, but it can be successfully infected by HCV (Washburn et al., 2011). In a separate model, TK-NOG mice can be transplanted with human HSCs (Hasegawa et al., 2011), and repopulated with human hepatocytes (discussed above), which might also support a combined liver stage-blood stage model.
Repopulating animals with induced pluripotent stem cell-derived cells (iPS cells) could also contribute to a combined liver stage/blood stage model. In this approach, human somatic cells are de-differentiated into stem cells (Takahashi et al., 2006), and then re-differentiated into HSCs (Lengerke et al., 2009) and hepatocytes (Chen et al., 2012). Although this approach has not yet been used in the development of humanized mouse models, the possibility of creating personalized, humanized mice would facilitate the testing of important biological and clinically relevant hypotheses concerning the genetic basis of susceptibility to malaria infection and disease.
Blood stage cytoadherence
One of the most disease-relevant features of P. falciparum iRBCs is their ability to cytoadhere to the vascular endothelium. The specific interactions involved are not conserved in rodent malaria species/mouse infections, making them of little use to study this phenomenon. Several receptors on human endothelial cells have been shown to support interactions with hRBCs infected with P. falciparum, including thrombospondin (Li et al., 2011), CD36 (Ockenhouse et al., 1988), ICAM-1 (Ockenhouse et al., 1991), and EPCR (Turner et al., 2013). A mouse model that supports human blood stage parasite replication and that expresses humanized receptors on endothelial cells would provide a powerful in vivo model of infected hRBC sequestration and its role in malaria pathogenesis.
Models of human immunity
Studies in mice and humans have revealed that both humoral and cellular responses play a role in the development of protective immunity against malaria. It has recently been demonstrated that the SCID-Alb-uPA and FRG mouse models can assess antibody-mediated protection against P. falciparum sporozoite challenge by passive transfer of antibodies to mice prior to challenge (Foquet et al., 2013, Sack et al., 2013). To our knowledge, cellular responses have yet to be assessed in any of these models.
Developing a platform that models human immune responses to malaria infection and vaccination remains a challenge. Towards this goal, the expression of human cytokines such as IL-3 and GM-CSF have enhanced human alveolar macrophage production (Willinger et al., 2011), and mice that express M-CSF have enhanced monocyte and macrophage production after HSC transplantation. Human T cells can be generated by repopulating NSG mice with both HSCs and human fetal thymus and liver tissue (Jaiswal et al., 2012) or alternatively through the expression of HLA class I heavy chain and light chain (Shultz et al., 2010). Finally, mice which produce human antibodies through the expression of human immunoglobulins have been developed (reviewed in (Seung et al., 2013)). Mice with these human immune components can be combined with human liver and blood models and would be invaluable in assessing the protective efficacy of immune responses generated by vaccination with candidate P. falciparum antigens or to assess the immune responses generated by Plasmodium infection.
Use of humanized mouse models to study Plasmodium vivax
P. vivax is the most widespread cause of malaria worldwide (Guerra et al., 2010). No long-term propagation systems for blood stages of P. vivax exist, in large part because P. vivax infection is restricted to reticulocytes (Galinski et al., 2013). A humanized mouse model that supports reticulocyte production and maintenance could provide an alternative method to study P. vivax biology and pathogenesis. Furthermore, for decades it has been assumed that P. vivax relapse is caused by dormant liver stages called hypnozoites, although no formal proof exists for hypnozoites as the source of relapsing blood stage infection (Galinski et al., 2013), and in vivo, P. vivax hypnozoites have only been visualized in chimpanzees (Krotoski et al., 1982). Humanized mouse models which support humanized liver tissue could help unravel hypnozoite biology and the phenomenon of relapse in P. vivax infections.
Conclusions
Studying the complex life cycle and unique aspects of the biology of human malaria parasites requires the development of specialized tools. In recent years development of humanized mouse models have allowed us to broaden our biological knowledge of specific stages of the P. falciparum life cycle. They also start to enable an effective platform for the assessment of pharmacologic and immunological interventional strategies during both pre-erythrocytic and blood stage infection. Further development of these models could provide a combined model of distinct phases of the malaria life cycle from initial delivery by mosquito bite to transmission of sexual stages to the next mosquito. Moving forward, these animal models could well constitute a critical component in the toolbox that will allow for an improved understanding of Plasmodium biology relevant to human infection, leading to more effective approaches to combat malaria.
Acknowledgments
A.K. is a recipient of a NRSA Ruth L. Kirschstein National Research Service Award (F32 AI091129), which has partially funded this work. Additionally, this work has been funded by grants from the Foundation for the National Institutes of Health through the Grand Challenges in Global Health Initiative (ID 1481) and the Bill and Melinda Gates Foundation grant OPP1021571 as well as a contract from the Department of Defense (W81XWH-11-2-0184) to S.H.I.K. We thank Dr. Ashley Vaughan for providing the image in Figure 3.
Footnotes
The Authors declare no conflict of interest.
References
- Arnold L, Tyagi RK, Meija P, Swetman C, Gleeson J, Perignon JL, Druilhe P. Further improvements of the P. falciparum humanized mouse model. PloS one. 2011;6:e18045. doi: 10.1371/journal.pone.0018045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold L, Tyagi RK, Mejia P, Van Rooijen N, Perignon JL, Druilhe P. Analysis of innate defences against Plasmodium falciparum in immunodeficient mice. Malaria journal. 2010;9:197. doi: 10.1186/1475-2875-9-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avril M, Tripathi AK, Brazier AJ, Andisi C, Janes JH, Soma VL, et al. A restricted subset of var genes mediates adherence of Plasmodium falciparum-infected erythrocytes to brain endothelial cells. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:E1782–1790. doi: 10.1073/pnas.1120534109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azuma H, Paulk N, Ranade A, Dorrell C, Al-Dhalimy M, Ellis E, et al. Robust expansion of human hepatocytes in Fah−/−/Rag2−/−/Il2rg−/− mice. Nature biotechnology. 2007;25:903–910. doi: 10.1038/nbt1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bility MT, Zhang L, Washburn ML, Curtis TA, Kovalev GI, Su L. Generation of a humanized mouse model with both human immune system and liver cells to model hepatitis C virus infection and liver immunopathogenesis. Nature protocols. 2012;7:1608–1617. doi: 10.1038/nprot.2012.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blunt T, Finnie NJ, Taccioli GE, Smith GC, Demengeot J, Gottlieb TM, et al. Defective DNA-dependent protein kinase activity is linked to V(D)J recombination and DNA repair defects associated with the murine scid mutation. Cell. 1995;80:813–823. doi: 10.1016/0092-8674(95)90360-7. [DOI] [PubMed] [Google Scholar]
- Bosma GC, Custer RP, Bosma MJ. A severe combined immunodeficiency mutation in the mouse. Nature. 1983;301:527–530. doi: 10.1038/301527a0. [DOI] [PubMed] [Google Scholar]
- Carretero M, Guerrero-Aspizua S, Del Rio M. Applicability of bioengineered human skin: from preclinical skin humanized mouse models to clinical regenerative therapies. Bioengineered bugs. 2011;2:203–207. doi: 10.4161/bbug.2.4.16112. [DOI] [PubMed] [Google Scholar]
- Carrolo M, Giordano S, Cabrita-Santos L, Corso S, Vigario AM, Silva S, et al. Hepatocyte growth factor and its receptor are required for malaria infection. Nature medicine. 2003;9:1363–1369. doi: 10.1038/nm947. [DOI] [PubMed] [Google Scholar]
- Chen YF, Tseng CY, Wang HW, Kuo HC, Yang VW, Lee OK. Rapid generation of mature hepatocyte-like cells from human induced pluripotent stem cells by an efficient three-step protocol. Hepatology. 2012;55:1193–1203. doi: 10.1002/hep.24790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppi A, Tewari R, Bishop JR, Bennett BL, Lawrence R, Esko JD, et al. Heparan sulfate proteoglycans provide a signal to Plasmodium sporozoites to stop migrating and productively invade host cells. Cell host & microbe. 2007;2:316–327. doi: 10.1016/j.chom.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foquet L, Hermsen CC, van Gemert GJ, Van Braeckel E, Weening KE, Sauerwein R, et al. Vaccine-induced monoclonal antibodies targeting circumsporozoite protein prevent Plasmodium falciparum infection. The Journal of clinical investigation. 2013 doi: 10.1172/JCI70349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frevert U, Sinnis P, Cerami C, Shreffler W, Takacs B, Nussenzweig V. Malaria circumsporozoite protein binds to heparan sulfate proteoglycans associated with the surface membrane of hepatocytes. The Journal of experimental medicine. 1993;177:1287–1298. doi: 10.1084/jem.177.5.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galinski MR, Meyer EV, Barnwell JW. Plasmodium vivax: modern strategies to study a persistent parasite’s life cycle. Advances in parasitology. 2013;81:1–26. doi: 10.1016/B978-0-12-407826-0.00001-1. [DOI] [PubMed] [Google Scholar]
- Gueirard P, Tavares J, Thiberge S, Bernex F, Ishino T, Milon G, et al. Development of the malaria parasite in the skin of the mammalian host. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:18640–18645. doi: 10.1073/pnas.1009346107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra CA, Howes RE, Patil AP, Gething PW, Van Boeckel TP, Temperley WH, et al. The international limits and population at risk of Plasmodium vivax transmission in 2009. PLoS neglected tropical diseases. 2010;4:e774. doi: 10.1371/journal.pntd.0000774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa M, Kawai K, Mitsui T, Taniguchi K, Monnai M, Wakui M, et al. The reconstituted ‘humanized liver’ in TK-NOG mice is mature and functional. Biochemical and biophysical research communications. 2011;405:405–410. doi: 10.1016/j.bbrc.2011.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Held J, Kreidenweiss A, Mordmuller B. Novel approaches in antimalarial drug discovery. Expert opinion on drug discovery. 2013;8:1325–1337. doi: 10.1517/17460441.2013.843522. [DOI] [PubMed] [Google Scholar]
- Hollingdale MR, Leland P, Schwartz AL. In vitro cultivation of the exoerythrocytic stage of Plasmodium berghei in a hepatoma cell line. The American journal of tropical medicine and hygiene. 1983;32:682–684. doi: 10.4269/ajtmh.1983.32.682. [DOI] [PubMed] [Google Scholar]
- Hu Z, Van Rooijen N, Yang YG. Macrophages prevent human red blood cell reconstitution in immunodeficient mice. Blood. 2011;118:5938–5946. doi: 10.1182/blood-2010-11-321414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishino T, Yano K, Chinzei Y, Yuda M. Cell-passage activity is required for the malarial parasite to cross the liver sinusoidal cell layer. PLoS biology. 2004;2:E4. doi: 10.1371/journal.pbio.0020004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaiswal S, Pazoles P, Woda M, Shultz LD, Greiner DL, Brehm MA, Mathew A. Enhanced humoral and HLA-A2-restricted dengue virus-specific T-cell responses in humanized BLT NSG mice. Immunology. 2012;136:334–343. doi: 10.1111/j.1365-2567.2012.03585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappe SH, Vaughan AM, Boddey JA, Cowman AF. That was then but this is now: malaria research in the time of an eradication agenda. Science. 2010;328:862–866. doi: 10.1126/science.1184785. [DOI] [PubMed] [Google Scholar]
- Kaushansky A, Kappe SH. The crucial role of hepatocyte growth factor receptor during liver-stage infection is not conserved among Plasmodium species. Nature medicine. 2011;17:1180–1181. doi: 10.1038/nm.2456. author reply 1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan SM, Janse CJ, Kappe SH, Mikolajczak SA. Genetic engineering of attenuated malaria parasites for vaccination. Current opinion in biotechnology. 2012;23:908–916. doi: 10.1016/j.copbio.2012.04.003. [DOI] [PubMed] [Google Scholar]
- Kirchgessner CU, Patil CK, Evans JW, Cuomo CA, Fried LM, Carter T, et al. DNA-dependent kinase (p350) as a candidate gene for the murine SCID defect. Science. 1995;267:1178–1183. doi: 10.1126/science.7855601. [DOI] [PubMed] [Google Scholar]
- Kosaka K, Hiraga N, Imamura M, Yoshimi S, Murakami E, Nakahara T, et al. A novel TK-NOG based humanized mouse model for the study of HBV and HCV infections. Biochemical and biophysical research communications. 2013;441:230–235. doi: 10.1016/j.bbrc.2013.10.040. [DOI] [PubMed] [Google Scholar]
- Krotoski WA, Collins WE, Bray RS, Garnham PC, Cogswell FB, Gwadz RW, et al. Demonstration of hypnozoites in sporozoite-transmitted Plasmodium vivax infection. The American journal of tropical medicine and hygiene. 1982;31:1291–1293. doi: 10.4269/ajtmh.1982.31.1291. [DOI] [PubMed] [Google Scholar]
- Lengerke C, Grauer M, Niebuhr NI, Riedt T, Kanz L, Park IH, Daley GQ. Hematopoietic development from human induced pluripotent stem cells. Annals of the New York Academy of Sciences. 2009;1176:219–227. doi: 10.1111/j.1749-6632.2009.04606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li A, Lim TS, Shi H, Yin J, Tan SJ, Li Z, et al. Molecular mechanistic insights into the endothelial receptor mediated cytoadherence of Plasmodium falciparum-infected erythrocytes. PloS one. 2011;6:e16929. doi: 10.1371/journal.pone.0016929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindner SE, Miller JL, Kappe SH. Malaria parasite pre-erythrocytic infection: preparation meets opportunity. Cellular microbiology. 2012;14:316–324. doi: 10.1111/j.1462-5822.2011.01734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- March S, Ng S, Velmurugan S, Galstian A, Shan J, Logan DJ, et al. A microscale human liver platform that supports the hepatic stages of Plasmodium falciparum and vivax. Cell host & microbe. 2013;14:104–115. doi: 10.1016/j.chom.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menard R, Tavares J, Cockburn I, Markus M, Zavala F, Amino R. Looking under the skin: the first steps in malarial infection and immunity. Nature reviews. Microbiology. 2013;11:701–712. doi: 10.1038/nrmicro3111. [DOI] [PubMed] [Google Scholar]
- Mercer DF, Schiller DE, Elliott JF, Douglas DN, Hao C, Rinfret A, et al. Hepatitis C virus replication in mice with chimeric human livers. Nature medicine. 2001;7:927–933. doi: 10.1038/90968. [DOI] [PubMed] [Google Scholar]
- Mikolajczak SA, Sacci JB, Jr, De La Vega P, Camargo N, VanBuskirk K, Krzych U, et al. Disruption of the Plasmodium falciparum liver-stage antigen-1 locus causes a differentiation defect in late liver-stage parasites. Cellular microbiology. 2011;13:1250–1260. doi: 10.1111/j.1462-5822.2011.01617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mota MM, Pradel G, Vanderberg JP, Hafalla JC, Frevert U, Nussenzweig RS, et al. Migration of Plasmodium sporozoites through cells before infection. Science. 2001;291:141–144. doi: 10.1126/science.291.5501.141. [DOI] [PubMed] [Google Scholar]
- Nacer A, Movila A, Baer K, Mikolajczak SA, Kappe SH, Frevert U. Neuroimmunological blood brain barrier opening in experimental cerebral malaria. PLoS pathogens. 2012;8:e1002982. doi: 10.1371/journal.ppat.1002982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien BA, Huang Y, Geng X, Dutz JP, Finegood DT. Phagocytosis of apoptotic cells by macrophages from NOD mice is reduced. Diabetes. 2002;51:2481–2488. doi: 10.2337/diabetes.51.8.2481. [DOI] [PubMed] [Google Scholar]
- Ockenhouse CF, Chulay JD. Plasmodium falciparum sequestration: OKM5 antigen (CD36) mediates cytoadherence of parasitized erythrocytes to a myelomonocytic cell line. The Journal of infectious diseases. 1988;157:584–588. doi: 10.1093/infdis/157.3.584. [DOI] [PubMed] [Google Scholar]
- Ockenhouse CF, Ho M, Tandon NN, Van Seventer GA, Shaw S, White NJ, et al. Molecular basis of sequestration in severe and uncomplicated Plasmodium falciparum malaria: differential adhesion of infected erythrocytes to CD36 and ICAM-1. The Journal of infectious diseases. 1991;164:163–169. doi: 10.1093/infdis/164.1.163. [DOI] [PubMed] [Google Scholar]
- Oettinger MA, Schatz DG, Gorka C, Baltimore D. RAG-1 and RAG-2, adjacent genes that synergistically activate V(D)J recombination. Science. 1990;248:1517–1523. doi: 10.1126/science.2360047. [DOI] [PubMed] [Google Scholar]
- Ogasawara K, Hamerman JA, Hsin H, Chikuma S, Bour-Jordan H, Chen T, et al. Impairment of NK cell function by NKG2D modulation in NOD mice. Immunity. 2003;18:41–51. doi: 10.1016/s1074-7613(02)00505-8. [DOI] [PubMed] [Google Scholar]
- Ploss A, Evans MJ, Gaysinskaya VA, Panis M, You H, de Jong YP, Rice CM. Human occludin is a hepatitis C virus entry factor required for infection of mouse cells. Nature. 2009;457:882–886. doi: 10.1038/nature07684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prudencio M, Mota MM, Mendes AM. A toolbox to study liver stage malaria. Trends in parasitology. 2011;27:565–574. doi: 10.1016/j.pt.2011.09.004. [DOI] [PubMed] [Google Scholar]
- Rhim JA, Sandgren EP, Degen JL, Palmiter RD, Brinster RL. Replacement of diseased mouse liver by hepatic cell transplantation. Science. 1994;263:1149–1152. doi: 10.1126/science.8108734. [DOI] [PubMed] [Google Scholar]
- Rongvaux A, Willinger T, Takizawa H, Rathinam C, Auerbach W, Murphy AJ, et al. Human thrombopoietin knockin mice efficiently support human hematopoiesis in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:2378–2383. doi: 10.1073/pnas.1019524108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacci JB, Jr, Alam U, Douglas D, Lewis J, Tyrrell DL, Azad AF, Kneteman NM. Plasmodium falciparum infection and exoerythrocytic development in mice with chimeric human livers. International journal for parasitology. 2006;36:353–360. doi: 10.1016/j.ijpara.2005.10.014. [DOI] [PubMed] [Google Scholar]
- Sack BK, Miller JL, Vaughan AM, Douglass A, Kaushansky A, Mikolajczak S, et al. A model for in vivo assessment of humoral protection against malaria sporozoite challenge by passive transfer of monoclonal antibodies and immune serum. Infection and immunity. 2013 doi: 10.1128/IAI.01249-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seung E, Tager AM. Humoral immunity in humanized mice: a work in progress. The Journal of infectious diseases. 2013;208(Suppl 2):S155–159. doi: 10.1093/infdis/jit448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinkai Y, Rathbun G, Lam KP, Oltz EM, Stewart V, Mendelsohn M, et al. RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell. 1992;68:855–867. doi: 10.1016/0092-8674(92)90029-c. [DOI] [PubMed] [Google Scholar]
- Shultz LD, Saito Y, Najima Y, Tanaka S, Ochi T, Tomizawa M, et al. Generation of functional human T-cell subsets with HLA-restricted immune responses in HLA class I expressing NOD/SCID/IL2r gamma(null) humanized mice. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:13022–13027. doi: 10.1073/pnas.1000475107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvie O, Rubinstein E, Franetich JF, Prenant M, Belnoue E, Renia L, et al. Hepatocyte CD81 is required for Plasmodium falciparum and Plasmodium yoelii sporozoite infectivity. Nature medicine. 2003;9:93–96. doi: 10.1038/nm808. [DOI] [PubMed] [Google Scholar]
- Strowig T, Rongvaux A, Rathinam C, Takizawa H, Borsotti C, Philbrick W, et al. Transgenic expression of human signal regulatory protein alpha in Rag2−/−gamma(c)−/− mice improves engraftment of human hematopoietic cells in humanized mice. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:13218–13223. doi: 10.1073/pnas.1109769108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturm A, Amino R, van de Sand C, Regen T, Retzlaff S, Rennenberg A, et al. Manipulation of host hepatocytes by the malaria parasite for delivery into liver sinusoids. Science. 2006;313:1287–1290. doi: 10.1126/science.1129720. [DOI] [PubMed] [Google Scholar]
- Su X, Hayton K, Wellems TE. Genetic linkage and association analyses for trait mapping in Plasmodium falciparum. Nature reviews. Genetics. 2007;8:497–506. doi: 10.1038/nrg2126. [DOI] [PubMed] [Google Scholar]
- Su X, Kirkman LA, Fujioka H, Wellems TE. Complex polymorphisms in an approximately 330 kDa protein are linked to chloroquine-resistant P. falciparum in Southeast Asia and Africa. Cell. 1997;91:593–603. doi: 10.1016/s0092-8674(00)80447-x. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Takenaka K, Prasolava TK, Wang JC, Mortin-Toth SM, Khalouei S, Gan OI, et al. Polymorphism in Sirpa modulates engraftment of human hematopoietic stem cells. Nature immunology. 2007;8:1313–1323. doi: 10.1038/ni1527. [DOI] [PubMed] [Google Scholar]
- Tavares J, Formaglio P, Thiberge S, Mordelet E, Van Rooijen N, Medvinsky A, et al. Role of host cell traversal by the malaria sporozoite during liver infection. The Journal of experimental medicine. 2013;210:905–915. doi: 10.1084/jem.20121130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi AK, Sullivan DJ, Stins MF. Plasmodium falciparum-infected erythrocytes increase intercellular adhesion molecule 1 expression on brain endothelium through NF-kappaB. Infection and immunity. 2006;74:3262–3270. doi: 10.1128/IAI.01625-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner L, Lavstsen T, Berger SS, Wang CW, Petersen JE, Avril M, et al. Severe malaria is associated with parasite binding to endothelial protein C receptor. Nature. 2013;498:502–505. doi: 10.1038/nature12216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan AM, Mikolajczak SA, Wilson EM, Grompe M, Kaushansky A, Camargo N, et al. Complete Plasmodium falciparum liver-stage development in liver-chimeric mice. The Journal of clinical investigation. 2012;122:3618–3628. doi: 10.1172/JCI62684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voza T, Miller JL, Kappe SH, Sinnis P. Extrahepatic exoerythrocytic forms of rodent malaria parasites at the site of inoculation: clearance after immunization, susceptibility to primaquine, and contribution to blood-stage infection. Infection and immunity. 2012;80:2158–2164. doi: 10.1128/IAI.00246-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washburn ML, Bility MT, Zhang L, Kovalev GI, Buntzman A, Frelinger JA, et al. A humanized mouse model to study hepatitis C virus infection, immune response, and liver disease. Gastroenterology. 2011;140:1334–1344. doi: 10.1053/j.gastro.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willinger T, Rongvaux A, Takizawa H, Yancopoulos GD, Valenzuela DM, Murphy AJ, et al. Human IL-3/GM-CSF knock-in mice support human alveolar macrophage development and human immune responses in the lung. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:2390–2395. doi: 10.1073/pnas.1019682108. [DOI] [PMC free article] [PubMed] [Google Scholar]