Summary
Plasmodium parasites infect hepatocytes of their mammalian hosts and within undergo obligate liver stage development. The specific host cell attributes that are important for liver infection remain largely unknown. Several host signaling pathways are perturbed in infected hepatocytes, some of which are important in the generation of hepatocyte polyploidy. To test the functional consequence of polyploidy in liver infection, we infected hepatocytes with the rodent malaria parasite Plasmodium yoelii both in vitro and in vivo and examined the ploidy of infected and uninfected hepatocytes by flow cytometry. In both hepatoma cell lines and in the mouse liver, the fraction of polyploid cells was higher in the infected cell population than in the uninfected cell population. When the data were reanalyzed by comparing the extent of Plasmodium infection within each ploidy subset, we found that infection rates were elevated in more highly polyploid cells and lower in diploid cells. Furthermore, we found that the parasite’s preference for host cells with high ploidy is conserved among rodent malaria species and the human malaria parasite Plasmodium falciparum. This parasite preference for host cells of high ploidy cannot be explained by differences in hepatocyte size or DNA replication. We conclude that Plasmodium preferentially infects and develops in polyploid hepatocytes.
Keywords: Plasmodium, polyploidy, hepatocyte, malaria, liver stage
Introduction
Parasites of the genus Plasmodium are the causative agents of malaria, which remains one of the deadliest infectious diseases worldwide (WHO, 2013). Infection is transmitted to the mammalian host by the bite of a female Anopheles mosquito, which injects the infectious form of the parasite, the sporozoite, into the dermis. Sporozoites then traverse through the skin, wounding cell membranes, until they reach a blood vessel that facilitates their transport to the liver. Here, the sporozoite invades a hepatocyte where it develops for 2–10 days (Vaughan et al., 2008). Once inside the hepatocyte the parasite divides rapidly, eventually differentiating into tens of thousands of merozoites, which leave the liver, invade red blood cells and cause symptomatic malaria. Because the liver stage is clinically silent and involves orders of magnitude fewer parasites than the later blood stages of infection, it is a critical target for intervention.
The sporozoites invade host hepatocytes by invagination of the host cell membrane to form a vacuole that ensconces them within the cells. During this process the parasite releases proteins from its apical organelles, remodeling the membrane to facilitate invasion of the host cell. Unlike traversal, which can occur in multiple cell types, productive invasion in the liver is possible only in the hepatocyte. The host factors that contribute to parasite invasion remain largely uncharacterized (Lindner et al., 2012).
Recently, we reported that P. yoelii liver stage infection perturbs hepatocyte signaling pathways, including those involved in cell proliferation and replication (Kaushansky et al., 2013b). Yet, proliferation of hepatocytes is only found at low levels in the normal liver and only increases when the liver has been highly damaged. Thus, these trends raise intriguing questions regarding the perturbation of cellular proliferation pathways in infected cells. Interestingly, cell proliferation pathways are linked to the phenomenon of polyploidy, the presence of more than two homologous sets of chromosomes in a cell, which is widespread in hepatocytes (Duncan, 2013). Since polyploidy is an unusual but common feature of hepatocytes, and Plasmodium liver stage development is restricted to hepatocyte host cells, we asked whether this common feature of hepatocytes might affect the process of liver infection. Here, we show that Plasmodium sporozoite infection displays preference for hepatocytes with elevated ploidy.
Results
Plasmodium parasites preferentially infect polyploid cells in vitro
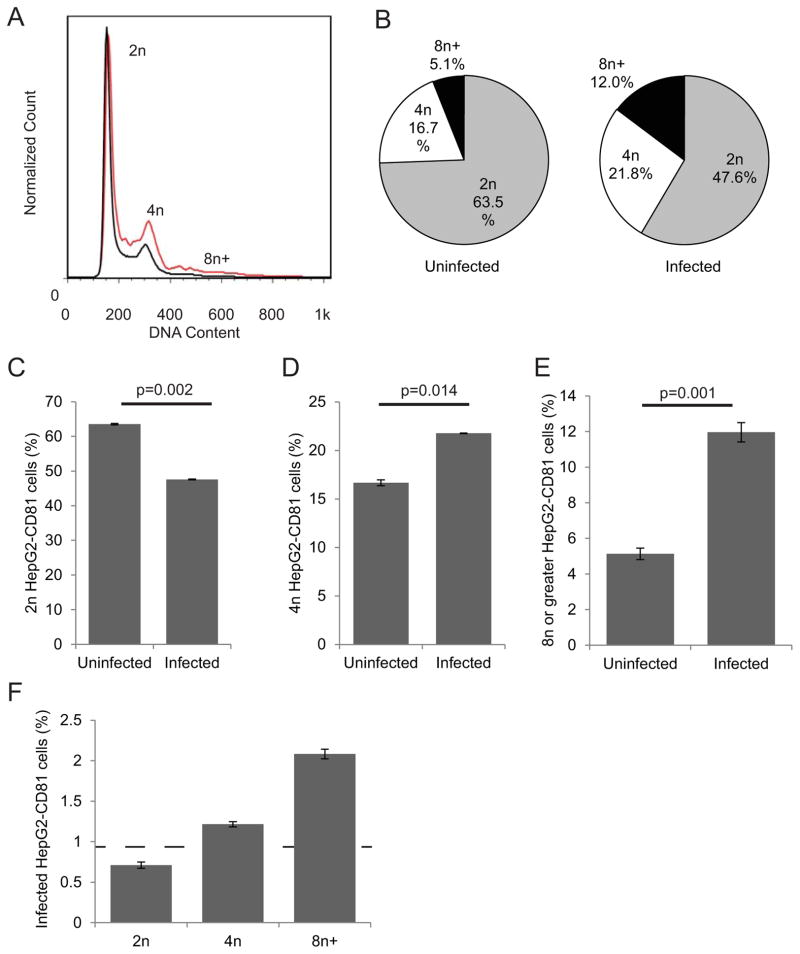
While the natural target cell for the malaria sporozoite is the primary hepatocyte, multiple transformed hepatoma cell lines have been developed for in vitro experimentation (Silvie et al., 2006, Mota et al., 2000, Sattabongkot et al., 2006). We first investigated if levels of ploidy varied between infected and uninfected hepatocytes by infecting HepG2-CD81 cells with P. yoelii and using flow cytometry to assess DNA content of infected single cells (Darzynkiewicz et al., 2010, Kaushansky et al., 2012) (Fig. S1). An overlay of the DNA histograms of the infected and uninfected cell populations at 2 hours post infection (hpi) showed a noticeable difference in the relative distribution of DNA content between infected and uninfected cells (Fig. 1A). The relative distribution of the ploidy differed dramatically between infected and uninfected cells (Fig. 1B). We found the percentage of cells with 2n ploidy was significantly lower in the infected cell population than the percentage of 2n cells in the uninfected cell population (Fig. 1C, p=0.002). In contrast, the percentage of 4n cells was significantly higher in the infected cell population (Fig 1D, p=0.014). While only a small percentage of cells exist in a >4n state in hepatoma cells, this population was heavily enriched among infected cells (Fig. 1E, p=0.001). In order to clearly delineate between cells of different ploidy, we used a conservative gating strategy which did not count cells with DNA content between the distinct peaks; however, including those cells in a more relaxed gating strategy did not significantly alter the results (Fig. S2). When data were reanalyzed by comparing the extent of Plasmodium infection within each ploidy subset, we found the rate of infection was lower in 2n cells than the overall infection rate, and was higher in 4n cells and greatly increased in the polyploid cell population (Fig. 1F).
Figure 1. Higher ploidy is more prevalent in parasite-infected hepatoma cells in vitro.
HepG2-CD81 cells were infected with P. yoelii sporozoites, harvested at 2 hpi and stained for infection and ploidy. Overlays of histograms of DNA stain for infected (red line) and uninfected (black) show lower peaks for infected 2n cells, and higher peaks for infected 4n and greater cells (A). The relative percentage of cells in each ploidy state greatly differs between uninfected and infected cells (B). A quantitative analysis of the percent of cells in each ploidy state shows significant decrease in cells with 2n chromosomes in infected cell population (C), and a significant increase in cells with 4n (D) or greater (E). Similarly, the rate of infection is lower in 2n cells, and higher in 4n and greater cells (F). Dashed line indicates the overall infection rate. Error bars show S.E.M., biological replicates of n=3.
We next asked if the observed preference for high ploidy cells was due to parasites that entered their host cell by wounding, or alternatively caused by a preference for cycling cells. To determine if the preference for higher ploidy cells was due to traversing parasites being caught in actively dividing cells, we blocked cell division using the small molecule cell cycle inhibitor nocodazole, which arrested a majority of the cells in G2, as well as the inhibitor L-mimosine, which arrests in G1 (Fig. S3A). We found that eliminating cell cycle progression in G2 dramatically increases the rate of infection, while arresting cell cycle before DNA synthesis decreases infection (Fig. S3B). Thus, the preference for higher ploidy is not dependent on cell division. Furthermore, when we excluded infected cells that had been entered by cell wounding, we obtained nearly identical results (Fig. S3C, D). This suggests that cells which harbor parasites arrested during traversal do not substantially contribute to the observed ploidy distribution in infected cells. Finally, we demonstrated that the shift in ploidy distribution of infected cells is independent of cell proliferation in vivo, as actively replicating (Ki-67 positive) cells are no more likely to be infected than quiescent (Ki-67 negative) cells (Fig. S3E).
The differences in the distribution of ploidy were observed only two hours after infection, before liver stage parasites begin to replicate their genomes. Since the amount of parasite-derived DNA at this time is negligible when compared to the DNA of the host cells, this increase in ploidy/DNA content cannot be attributed to parasite DNA. Furthermore, the shift in infected cells toward higher ploidy remained constant after 24 hours of parasite development despite substantial parasite replication between 2 and 24 hours, further indicating that the parasite DNA is not falsely presenting as a higher ploidy state of the infected cell (Fig. S4). Taken together, these data suggest that hepatoma cells with higher ploidy are more likely to be infected with P. yoelii than those with lower ploidy.
Plasmodium prefers infection of high ploidy cells in mice
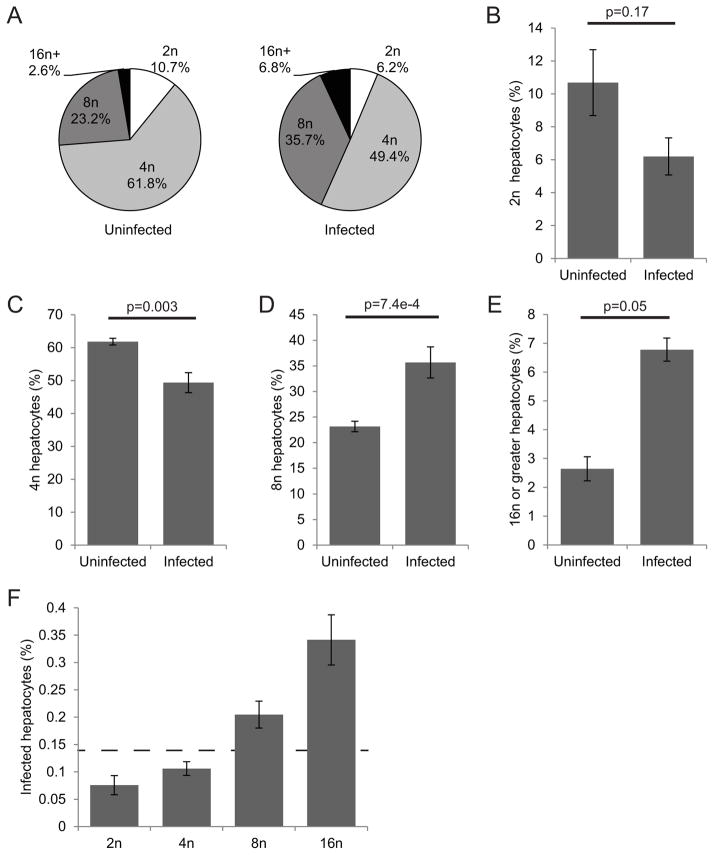
Hepatoma cells rapidly replicate, and most 4n cells are in the G2 stage of the cell cycle. In G2, a number of cellular processes have different activity levels than in the non-replicating hepatocytes. This makes it difficult to uncouple ploidy with changes associated with cell cycle progression. In the healthy adult liver, primary hepatocytes are generally quiescent, with only a fraction of hepatocytes actively going through the cell cycle (Fausto et al., 2003). To determine the role of polyploidy in primary hepatocytes, we investigated the ploidy distribution of infected and uninfected hepatocytes in mice. We injected seven-week-old female BALB/c mice with one million P. yoelii sporozoites intravenously, and analyzed hepatocytes prepared by collagen-mediated perfusion at three or 24 hpi, assessing the infection rates and ploidy using flow cytometry (Fig. S5). As in vitro, we found that parasite-infected cells had an altered distribution of ploidy (Fig. 2A). At three hours post infection, diploid hepatocytes, which comprised 11% of uninfected hepatocytes, were only 6% of infected hepatocytes (Fig. 2B). Tetraploid hepatocytes significantly diminished in the infected population to 49% from 62% of uninfected hepatocytes (Fig. 2C). Conversely, the 8n population increased from 23% in uninfected hepatocytes to 36% of infected hepatocytes (Fig 2D), and the population with 16n or greater jumped from 2.5% of the uninfected hepatocyte population to 6.7% of the infected population (Fig. 2E). Parasite infection rates correlated positively with ploidy level; in fact the infection rate within hepatocytes 16n or greater was nearly 400% the overall infection rate (Fig. 2F). The results were similar at 24 hpi when the genome of the parasite had begun to replicate (Fig. S6). Thus, similar to the in vitro data, higher ploidy hepatocytes are more susceptible to infection in vivo. Since this increased susceptibility is again seen very soon after infection, it is clear that the parasite preference for hepatocytes with elevated ploidy levels is established at the point of infection and is not modified throughout parasite liver stage development.
Figure 2. Higher ploidy is more prevalent in parasite-infected hepatocytes in vivo.
Balb/cAnN mice were infected intravenously with 106 P. yoelii sporozoites. Hepatocytes were isolated at 3 hpi and stained for parasite protein and DNA, then analyzed by flow cytometry. Infected and uninfected cells show greatly different ploidy distributions (A). The percentage of cells with 2n (B) and 4n ploidy (C) is lower in infected populations, while 8n (D) and 16n or higher ploidy cells (E) are significantly higher in infected populations. The rate of Plasmodium infection positively correlates with ploidy levels (F). Dashed line indicates the overall infection rate. Error bars show S.E.M., biological replicates of n=3.
Preference for polyploid host cells is conserved across Plasmodium species
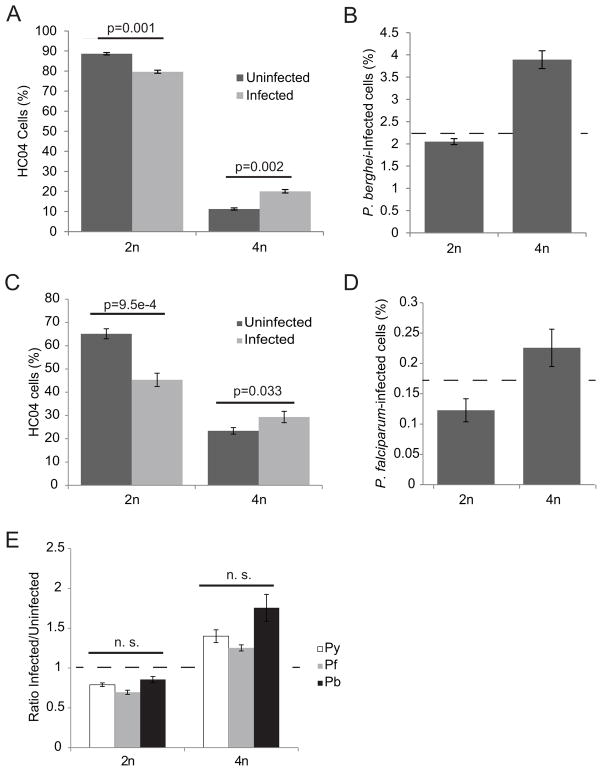
Plasmodium species are genetically and phenotypically diverse and this diversity extends to their dependence on particular host factors for infection. It has been previously demonstrated that sporozoites of different Plasmodium species differ in their preference for host cell invasion factors. For example, P. yoelii and P. falciparum sporozoites both require hepatocyte CD81 expression for infection but P. berghei does not (Silvie et al., 2003). To address whether sporozoite preference for hepatocytes with higher ploidy is restricted to P. yoelii, we infected HC04 hepatoma cells (Sattabongkot et al., 2006) with P. berghei sporozoites, and analyzed ploidy levels at 2 hours post-infection. Like HepG2-CD81 cells, HC04 cells are primarily diploid, but are actively cycling, creating a robust 4n population. When we analyzed the percentage of cells with 2n and 4n ploidy in infected versus uninfected cell populations, we found that like P. yoelii, P. berghei infected cells had a decreased percentage of 2n and an increased percentage of 4n cells compared to the uninfected cells (Figs. 3A and 3B). To further explore the breadth of our findings in the context of human malaria, we infected HC04 cells with the human parasite P. falciparum and found a similar ploidy distribution (Figs. 3C and 3D). Thus, the difference in infection between 2n versus 4n cells remained consistent between Plasmodium species (Fig. 3E), indicating that the mechanism of preferential infection of host cells with higher ploidy is well conserved across parasite species. This is particularly important given that most known host factors involved in parasite infection are not conserved across species (Silvie et al., 2003, Carrolo et al., 2003, Kaushansky et al., 2011), and that the susceptibility of different cell types to infection varies between Plasmodium species (Prudencio et al., 2011).
Figure 3. Host cell ploidy preference is conserved among Plasmodium species.
HC04 cells infected with the human parasite P. falciparum have a decreased percentage 2n cells and an increased percentage 4n cells at 2 hpi (A). The infection rate with P. falciparum increases correlating to ploidy (B). A similar pattern of decrease in 2n cells in infected population holds true for the rodent parasite P. berghei (C). Similarly, 4n cells had an increased rate of infection (D). The amount of preference away from 2n cells holds very steady in all three parasites. No significant difference in the ratio of infected to uninfected cells is found between species (E). Dashed lines in (B) and (D) indicate overall infection rate.
Nuclearity and zonation do not influence ploidy preference
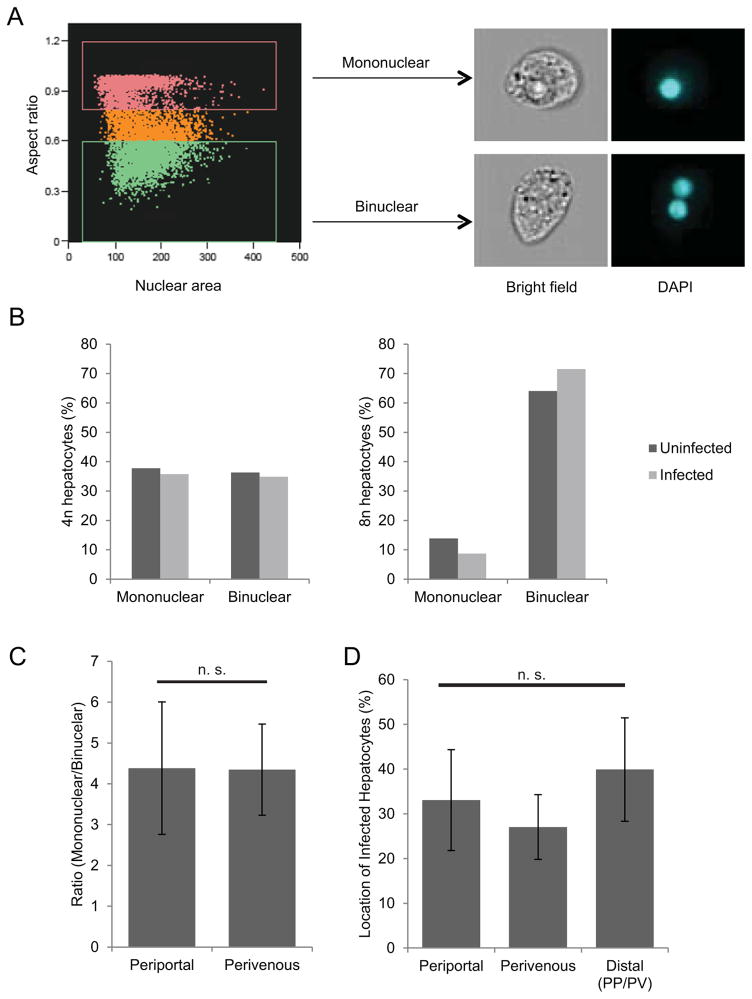
Polyploid hepatocytes can be mononuclear (e.g. one tetraploid nucleus which is 4n) or binuclear (e.g. two diploid nuclei, each 2n). Thus, we asked if the number of hepatocyte nuclei dictated the observed differences in infection. It is difficult, however, to simultaneously measure DNA content and nuclearity in low-frequency events such as Plasmodium hepatocyte infection. We therefore turned to Imagestream technology (Basiji et al., 2007), which combines flow cytometry with fluorescence and light microscopy. By measuring the aspect ratio of the DNA signal—that is, the ratio between the width of the signal and its length—we were able to distinguish mono- and binuclear cell populations (Fig. 4A). We then stratified cells based on ploidy to correct for the increased binuclearity found in cells of higher DNA content, and compared the populations of mononuclear and binuclear cells in infected and uninfected cells in each ploidy group. 2n cells were not analyzed because they are exclusively mononuclear, and 16n cells and above were excluded due to low cell number. When we analyzed the nuclearity of cells of 4n or 8n ploidy, we found that within populations of similar ploidy, no difference in nuclearity existed between infected and uninfected cells (Fig. 4B).
Figure 4. Nuclearity and zonation do not influence hot cell ploidy preference of parasites.
Using Imagestream flow cytometry, infected and uninfected mouse hepatocytes were analyzed by aspect ratio to determine the number of nuclei. Cells were gated into subsets of 2n, 4n, 8n, and 16n or greater. Cells within the 4n and 8n ploidy subsets were analyzed for nuclear number. Nuclearity was confirmed via representative cell images taken from each gate (A). In both 4n and 8n ploidy subsets, the distribution of mononuclear and binuclear cells did not differ between infected and uninfected cells (B). Nuclearity and infection were also analyzed by liver zonation in H&E stained liver slices. Mouse livers show an equal ratio of mononuclear hepatocytes to binuclear hepatocytes between periportal (PP) and perivenous (PV) regions (C). Infected hepatocytes are likewise evenly distributed between periportal, perivenous, and distal (neither PP nor PV) zones of the liver (D).
In addition to their ploidy, hepatocytes in the liver can be classified based on lobule zonation, depending on whether they are closer to the portal vein (periportal), the central vein (perivenous), or distal to both. Some studies have suggested that polyploidy is differentially distributed within these zones (Asahina et al., 2006, Gandillet et al., 2003) while other reports suggest there are similar distributions of binuclear and tetraploid hepatocytes between zones (Margall-Ducos et al., 2007). To address whether hepatocyte zonation might contribute to the increased Plasmodium infection of polyploid hepatocytes, we analyzed tissue sections of infected mouse livers for liver stage parasite distribution in addition to hepatocyte nuclearity. While polyploidy per se is difficult to determine purely by microscopy, binuclearity is increased with increased ploidy so it was used as a marker for polyploid hepatocytes. We found no significant difference in the ratio of mononuclear to binuclear cells between periportal and perivenous zones of the mouse liver (Fig. 4C), in agreement with some previous reports (Margall-Ducos et al., 2007). Moreover, Plasmodium parasites showed no preference for infection of hepatocytes in either zone (Fig. 4D). Therefore, we conclude that zonation of the liver does not impact the elevated infection rates of polyploid hepatocytes.
Cell size does not account for increased infection of polyploid hepatocytes
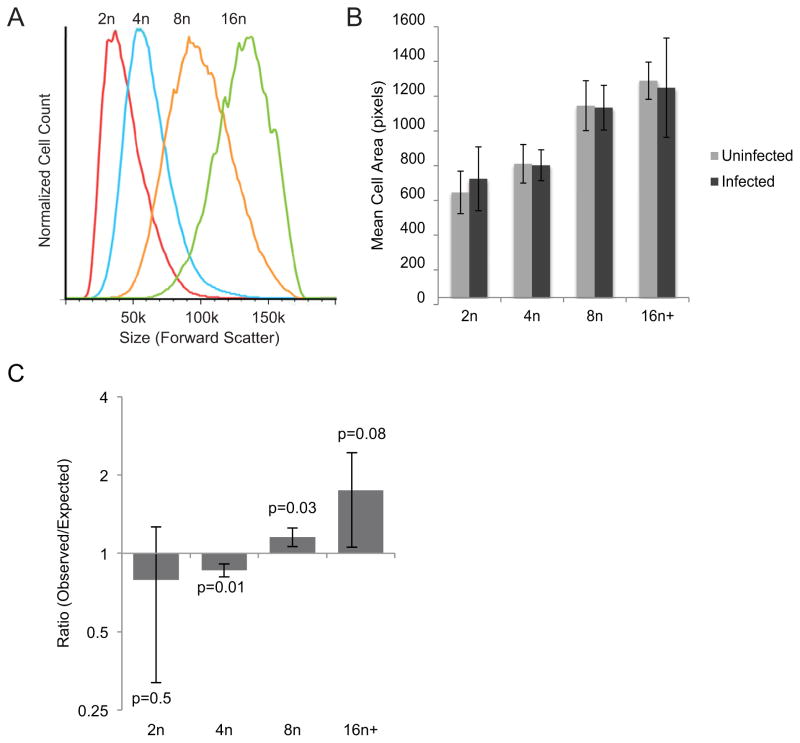
Size is also a possible factor in the susceptibility of polyploid cells as increased ploidy positively correlates with cell volume. If cell size fully explained the preference for infection of polyploidy cells, it would suggest that the parasite is more likely to interact with large cells due to their increased surface area rather than a specific molecular property of these cells. To assess cell size, we first used the forward scatter measurement of flow cytometry. As expected (Epstein, 1966), higher ploidy was associated with larger cell size (Fig. 5A). We then analyzed the difference in size between infected and uninfected cells. Unsurprisingly, infected cells were on average larger than uninfected cells (data not shown). To deconvolute this data, we stratified the cells by ploidy subset and analyzed the average size of cells within each subset. Cell size was measured by Imagestream microscopy using the average pixel area of each cell image. We reasoned that if increased parasite infection were due primarily to larger host cell size, then within each ploidy level parasites would preferentially invade the larger cells within that subgroup. We found that this was not the case; when stratified by ploidy, infected cells and uninfected cells did not significantly differ in size (Fig. 5B). Moreover, when cells were stratified by size using forward scatter, the infected populations still demonstrated altered ploidy distributions with a preference for more polyploid cells (Fig. S7). To further assess whether or not the observed preference for high ploidy populations is due to increased surface area of polyploidy cells, we calculated the relative percentage of cell surface area in the liver represented by each ploidy subset (see Methods). We then used those percentages to predict invasion rates in each subset if infection was determined stochastically based on exposed surface area. We found that the observed values of infected 2n and 4n cells were less than the expected values, and 8n, 16n and higher polyploid cells were higher than expected (Fig. 5C). These data demonstrate that sporozoites preferentially infect hepatocytes with higher DNA content beyond what would be expected based on surface area exposed alone. Thus, the increased susceptibility of polyploid cells can not be explained simply as a function of cell size.
Figure 5. Preferential parasite infection of higher ploidy cells is not dependent on their increased size.
The size of hepatocytes of different ploidy from infected mice was analyzed using forward scatter during flow cytometry. Cell size increases with increased polyploidy (A). Using Imagestream analysis, cells were gated by ploidy, and the pixel size of infected and uninfected cell images were measured within each ploidy subset. When stratified by ploidy level no difference was found in the average sizes of infected and uninfected cells (B). Expected rates for hepatocyte infection were calculated based on surface area of each ploidy subset as described in equation (2) and compared to observed infection rates. The observed infection rates were lower than expected rates for 2n and 4n cells, and higher in 8n and 16n and greater cells (C). This shows that preferential infection of polyploid cells is not dependent on the relative surface area of each population.
Discussion
Here, we demonstrate that polyploidy of hepatocytes plays a substantial role in the host cell preference of Plasmodium parasites. Since the parasites’ selection of hepatocytes with higher ploidy can be seen almost immediately after infection, it is likely that this preference is due to an intrinsic hepatocyte factor that plays a role during the early stages of infection, or the process of invasion itself. The host factors supporting Plasmodium liver infection remain largely uncharacterized and the few that have been identified vary in their importance between different Plasmodium species (Silvie et al., 2006, Kaushansky et al., 2011). We have previously demonstrated that Plasmodium liver stages perturb signaling pathways in their host hepatocytes, including those pathways involved in cell proliferation and replication (Kaushansky et al., 2013b). However, this analysis was not designed to distinguish between hepatocyte factors that the parasite selects for during its invasion process and factors that the parasite actively engages and changes throughout infection.
Interestingly, several cell proliferation proteins, including p53 (Kurinna et al., 2013) and cell cycle transcription factors of the E2F family (Pandit et al., 2012, Chen et al., 2012) have been implicated in the generation of polyploidy in hepatocytes. p53 in particular might be playing a role in the relationship between polyploidy and parasite infection. We have previously shown that infected hepatocytes exhibit reduced levels of p53. In turn, elevated p53 levels greatly reduce hepatocyte infection. In contrast, mice with a p53 knockout genotype were significantly more susceptible to liver stage infection (Kaushansky et al., 2013b). In its role as a cell cycle regulator p53 acts as a transcription factor controlling many factors involved in the cell cycle and polyploidization of hepatocytes. Strikingly, p53−/− mice exhibit lower levels of diploid and tetraploid hepatocytes (Kurinna et al., 2013). Thus, it will be of interest to examine the relationship between parasite infection levels, host cell p53 levels and host cell ploidy.
Polyploidy is a ubiquitous feature of mammalian hepatocytes. While the development of hepatocyte polyploidy has been extensively characterized (Gupta, 2000, Celton-Morizur et al., 2010, Gentric et al., 2012), the functional consequences of polyploidy are still largely unknown. Different species have variable hepatocyte ploidy distributions within a narrow range in healthy adults. While these percentages change over the lifespan of an individual, the distribution of hepatocyte ploidy among healthy animals of the same age are comparatively uniform (Duncan, 2013). It has been described that hepatocytes exist within a “ploidy conveyor”, meaning that ploidy is not entirely fixed: diploid cells can become polyploid, or vice versa (Duncan et al., 2010). Additionally, polyploidy can be altered in response to liver injury via partial hepatectomy (Sigal et al., 1999), oxidative (Gorla et al., 2001) or chemical (Madra et al., 1995) stress. Interestingly, increased polyploidy due to partial hepatectomy returns to its standard levels within weeks after the injury (Sigal et al., 1999), suggesting that there is a mechanism of polyploid homeostasis in the mammalian liver. The conservation of hepatocyte polyploidy both within and among species strongly implies that this phenomenon is of critical importance in liver function.
Hepatocytes are constantly subjected to the stress of toxins and oxidation involved in liver metabolism, and polyploidy might play a role in protection against DNA damage or other environmental stresses (Gentric et al., 2012, Uryvaeva, 1981). If so, this could be an attractive host cell feature for the intracellular parasite because it depends on survival of its host cell until completion of development and egress. Apoptosis of infected cells prevents parasite development, and Plasmodium has developed ways to block host cell apoptosis. Infected hepatocytes are partially resistant to induced apoptosis (van de Sand et al., 2005), and several pro-apoptotic pathways are disregulated in infected hepatocytes (Kaushansky et al., 2013b, Albuquerque et al., 2009, Kaushansky et al., 2013a). However, this protection against apoptosis is incomplete, and a proportion of parasite infected cells still undergo apoptosis (Kaushansky et al., 2013a). The persistence of parasites within the liver, then, is proposed to depend heavily on the ability of the parasite to suppress host cell stress responses. The preferential infection of polyploid cells inherently more resistant to stress might thus be an important selection mechanism for that increases the likelihood of successful intracellular development.
The skewing of Plasmodium infection towards polyploid cells suggests that these cells display an altered molecular state that increases sporozoite infection. The phenomenon is highly consistent throughout experimental cell lines, mouse models, and parasite species. This preference, however, is not complete as parasites are able to infect cells of low ploidy at high numbers. Most of the infected hepatocytes are only 4n cells, which comprise the majority of total mouse hepatocytes. These data suggest that the factor that mediates elevated infection in polyploidy hepatocytes is present at some level in hepatocytes of lower ploidy. Thus, further investigation into quantitative differences between hepatocytes of low and high ploidy might elucidate novel factors that mediate sporozoite invasion or early development.
The demonstrated preference of sporozoites for infection of polyploid hepatocytes occurs very early in the course of infection. This points to potential differences in the host cell plasma membrane and its resident proteins that the parasite is able to exploit for cell entry. One possibility is that polyploid hepatocytes have different complements or organization of surface receptors. Polyploid hepatocytes have been shown to have higher levels of the adhesion molecule ICAM-1 (Martin et al., 2002), although this is unlikely to a factor in hepatocyte infection as mice with deletions of ICAM-1 are able to support P. yoelii liver stage infection (Sultan et al., 1997). Another possibility is that the preference for high ploidy is due to receptor organization, rather than quantity. The known host cell infection factor CD81 acts as a scaffolding protein for organization of lipid microdomains, which can contain a number of different membrane receptors (Levy et al., 2005). The organization of these microdomains may differ in cells with variant ploidy, thus increasing local receptor density, which might favor Plasmodium infection.
Both the functional relevance of hepatocyte polyploidy and the specific mechanisms of Plasmodium infection are still poorly understood. In a healthy liver, only hepatocytes display significant polyploidy, while other cell types are diploid. Likewise, hepatocytes are the only liver cell type that Plasmodium sporozoites will productively infect. While several host factors for infection have been identified, none of them are required for all species of Plasmodium (Kaushansky et al., 2011, Silvie et al., 2006). However, the preference for infection of polyploid cells is conserved in three Plasmodium species we have tested to date, pointing toward a conserved host factor or factors for parasite infection that are differentially expressed in highly polyploid cells. By inspecting the molecular differences found in different states of host cell ploidy, we might identify new host factors important to malaria parasite liver infection. While modulation of hepatocyte polyploidy by viral hepatitis is known, to our knowledge this investigation is the first description of polyploidy playing an important role in the initiation of infection with a eukaryotic parasite. A greater understanding of the role of hepatocyte ploidy in malaria parasite liver infection might provide novel mechanistic insights into the processes of host cell selection and susceptibility to infection and might also provide insights into the physiological relevance of hepatocyte ploidy in the liver.
Methods
Cell lines, cell culture, and experimental animals
In vitro, HepG2-CD81 cells were used for P. yoelii infections, and HC04 cells for P. falciparum and P. berghei. Cells were maintained in Dulbecco’s Modified Eagle Medium (DMEM) complete media (Cellgro), supplemented with 10% FBS (Sigma-Aldrich), 100IU/ml penicillin (Cellgro), 100mg/ml streptomycin (Cellgro), 2.5mg/ml Fungizone (HyClone/Thermo Fisher), and split 1–2 times weekly. Female Balb/cAnN mice (6–8 weeks old) were purchased from Harlan Laboratories and maintained in accordance with protocols approved by Seattle Biomed Institutional Animal Control and Use Committee (IACUC).
Mosquito rearing and sporozoite production
For Plasmodium sporozoite production, female 6–8-week-old Swiss Webster mice (Harlan, Indianapolis, IN, USA) were injected with blood stage P. yoelii (17XNL), P. berghei (ANKA), or P. falciparum (NF54) parasites to begin the growth cycle. Animal handling was conducted according to the Institutional Animal Care and Use Committee-approved protocols. We used infected mice to feed female Anopheles stephensi mosquitoes after gametocyte exflagellation was observed. We isolated salivary gland sporozoites according to the standard procedures at days 14 or 15 post blood meal for P. yoelii, day 20 for P. berghei, and days 14–19 for P. falciparum.
In vitro cell ploidy assay
2x106 HepG2-CD81 cells were seeded into each well of a 6-well plate and allowed to adhere overnight in DMEM. About 24 hours after plating, cells were infected with 1.5x106 P. yoelii sporozoites. One hour post infection (hpi), extracellular parasites were washed off cells and media was replaced. Cells were harvested with 0.25%Trypsin-EDTA at 2 hpi and 24 hpi, and fixed with Cytoperm/Cytofix (BD Biosciences). The cells were blocked with Perm/Wash (BD Biosciences)+2% BSA overnight at 4 C, then stained for one hour at room temperature with antibodies to P. yoelii Circumsporozoite protein (PyCSP) conjugated to Alexa Fluor 488 (Life Technologies). The cells were washed in PBS+5 mM EDTA, then resuspended in PBS+5 mM EDTA+ RNAse A (0.1 mg/mL)+ FxCycle Far Red DNA Dye (Invitrogen). Infection rate and DNA content were measured by flow cytometry on an LSRII (Becton-Dickson) and analyzed by FlowJo (Tree Star).
Traversal analysis of infected cell cultures
5x105 HepG2-CD81 cells were seeded into each well of a 12-well plate and allowed to adhere overnight in DMEM. 24 hours after plating, cells were infected with 1x105 P. yoelii sporozoites in the presence of 1mg/mL FITC-Dextran (Invitrogen). Cells were harvested 2 hpi and analyzed as described above except parasites were stained with PyCSP antibody conjugated to Pacific Blue (Life Technologies).
Cell cycle inhibition of cell cultures
2.5x105 HepG2-CD81 cells were seeded into each well of a 24-well plate and allowed to adhere overnight in DMEM. About 24 hours after plating, cells were treated with either 400 μM L-mimosine (Sigma) or 100 ng/mL nocodazole (Sigma). 24 hours after treatment, cells were washed for one hour with DMEM, then infected with 5x104 P. yoelii sporozoites per well. Cells were harvested 2 hpi and analyzed as described above.
P. falciparum and P. berghei cell ploidy assay
8x105 HC04 cells were seeded into a 12-well plate and infected with 2x105 P. falciparum or 4x105 P. berghei sporozoites. Cells were harvested 2 hpi and fixed as above, and stained with antibody and FxCycle Far Red DNA dye (Invitrogen). P. falciparum-infected cells were stained with anti-PfCSP conjugated with Alexa Fluor 488, and P. berghei-infected cells were stained with unconjugated mouse antibodies to PbCSP, and a secondary anti-mouse conjugated to Alexa Fluor 488.
Hepatocyte isolation and analysis
Female Balb/cAnN mice were inoculated with 106 P. yoelii sporozoites each via tail vein injection. 3 hours and 24 hours post infection, the livers were perfused in situ with HBSS supplemented with HEPES and 5 mM EDTA. When the livers blanched, the solution was changed to HBSS with HEPES and 5 mM CaCl2 and the livers perfused until dissociation. The livers were removed, and hepatocytes dissociated by pushing through a 100 μm cell strainer into DMEM. The liver cell suspension was centrifuged at 50x g, and the remaining pellet washed in DMEM. This step was repeated twice until the supernatant was clear, to isolate hepatocytes from other parenchymal cells. The hepatocytes were fixed, stained, and analyzed by flow cytometry as above.
Imagestream imaging
One mouse was injected with 107 P. yoelii sporozoites, and the hepatocytes were isolated at 3 hours post infection and processed as described above. The cells were stained with anti-PyCSP conjugated with Alexa Fluor 488 overnight. The nuclei were then stained with DAPI prior to analysis with an Imagestream Mark II Imaging Flow Cytometer (Amnis-EMD Millipore Corporation). The sample was split into multiple aliquots, and a minimum of 30,000 events per aliquot was collected with 10 mW 488 and 10 mW 405 nm laser powers. During data acquisition, cell images of Bright field, Side Scatter, Alexa Fluor 488, and DAPI were simultaneously collected in different detection channels. During the data analysis with IDEAS software, single cells of best focus were first separated from cell aggregates and debris using several features such Bright Field Area and Aspect Ratio. Then the PyCSP-Alexa Fluor 488 and DAPI intensities were plotted. The DAPI+ CSP+ populations of the data files were identified and merged into one file to maximize the number of CSP+ cells in the file.
Histological analysis of mouse livers
Infected (1 million P. yoelii sporozoites per mouse) Balb/c livers (n=3) were harvested 42 hours post infection, then harvested and fixed in formalin. Livers were embedded in paraffin, sectioned, and mounted on glass slides. Slices were then stained with hematoxylin and eosin. Parasites were visually identified as being periportal, perivenous, or distal to both at 10x power magnification, with a minimum of fifty parasites per mouse quantified. Hepatocyte nuclei were counted at 40x power, with a minimum of 10 fields per zone per mouse counted. Hepatocytes without clearly identifiable nuclei were excluded from the analysis.
Size analysis of infected hepatocytes
The average areas of 2n, 4n, 8n or 16n cells were determined by Imagestream analysis (Amnis Corporation). From this, the average radius in pixels was determined, and the average cell surface area (ASA) calculated using standard geometric formulas. The average surface area of each ploidy was multiplied by the percentage of total cells of that ploidy (e.g. 10.7% of cells were 2n) to get the total surface area (TSA) represented by each ploidy subset (Formula 1). Each TSA was divided by the sum of all ploidy TSAs to get the percentage of TSA represented by each ploidy subset (%SA) (Formula 2). If infection were due to surface area alone, the expected ploidy distribution of infected cells would be identical to the ploidy distribution of %SA. The expected %SA was compared to the observed ploidy distribution as shown in Figure 2. The equation to determine the expected percentage of 2n cells in the infected population was as follows:
| (1) |
| (2) |
Supplementary Material
Acknowledgments
We are grateful to William W. Betz, Heather S. Kain, Mark F. Kennedy and Jen C.C. Hume for mosquito and sporozoite production. We thank Hieu Nguyen and Nick Lejarcegui for technical assistance with flow cytometry, Alyse Douglass and Nadia Arang for excellent technical assistance with in vitro experiments, and Isaac Mohar for his assistance with liver slide pathology. We thank Seattle Biomedical Research Institute vivarium staff for their work with mice. We are extremely grateful to Raymond Kong, Ben Alderete, and Amnis-EMD Millipore for the Imagestream analysis. L.S.A. is a recipient of NIH Training Grant T32 AI007509-13, and further supported by the Department of Defense (DoD) through the National Defense Science & Engineering Graduate Fellowship (NDSEG) Program, both of which have partially funded this work. Additional funding was provided by NIH grants F32 AI091129 to A.K. and 1R01GM101183-01A1 to S.H.I.K.
Abbreviations
- LS
liver stage
- CSP
circumsporozoite protein
- hpi
hours post infection
Footnotes
Author Contributions: L.S.A., A.K., and S.H.I.K. designed the research, L.S.A. and A.K. performed experiments, S.H.I.K. supervised the research, and L.S.A., A.K., and S.H.I.K. wrote the paper.
The authors declare that they have no personal or financial conflicts of interest.
References
- Albuquerque SS, Carret C, Grosso AR, Tarun AS, Peng X, Kappe SH, et al. Host cell transcriptional profiling during malaria liver stage infection reveals a coordinated and sequential set of biological events. BMC Genomics. 2009;10:270. doi: 10.1186/1471-2164-10-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basiji DA, Ortyn WE, Liang L, Venkatachalam V, Morrissey P. Cellular image analysis and imaging by flow cytometry. Clin Lab Med. 2007;27:653–670. viii. doi: 10.1016/j.cll.2007.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrolo M, Giordano S, Cabrita-Santos L, Corso S, Vigario AM, Silva S, et al. Hepatocyte growth factor and its receptor are required for malaria infection. Nat Med. 2003;9:1363–1369. doi: 10.1038/nm947. [DOI] [PubMed] [Google Scholar]
- Celton-Morizur S, Merlen G, Couton D, Desdouets C. Polyploidy and liver proliferation: Central role of insulin signaling. Cell Cycle. 2010;9:460–466. doi: 10.4161/cc.9.3.10542. [DOI] [PubMed] [Google Scholar]
- Chen HZ, Ouseph MM, Li J, Pecot T, Chokshi V, Kent L, et al. Canonical and atypical E2Fs regulate the mammalian endocycle. Nat Cell Biol. 2012;14:1192–1202. doi: 10.1038/ncb2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darzynkiewicz Z, Halicka HD, Zhao H. Analysis of cellular DNA content by flow and laser scanning cytometry. Adv Exp Med Biol. 2010;676:137–147. doi: 10.1007/978-1-4419-6199-0_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan AW. Aneuploidy, polyploidy and ploidy reversal in the liver. Semin Cell Dev Biol. 2013;24:347–356. doi: 10.1016/j.semcdb.2013.01.003. [DOI] [PubMed] [Google Scholar]
- Duncan AW, Taylor MH, Hickey RD, Hanlon Newell AE, Lenzi ML, Olson SB, et al. The ploidy conveyor of mature hepatocytes as a source of genetic variation. Nature. 2010;467:707–710. doi: 10.1038/nature09414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein CJ. Cell size, nuclear content, and the development of polyploidy in the mammalian liver. Proc Natl Acad Sci U S A. 1966;57:327–334. doi: 10.1073/pnas.57.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fausto N, Campbell JS. The role of hepatocytes and oval cells in liver regeneration and repopulation. Mech Dev. 2003;120:117–130. doi: 10.1016/s0925-4773(02)00338-6. [DOI] [PubMed] [Google Scholar]
- Gentric G, Desdouets C, Celton-Morizur S. Hepatocytes polyploidization and cell cycle control in liver physiopathology. Int J Hepatol. 2012;2012:282430. doi: 10.1155/2012/282430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorla GR, Malhi H, Gupta S. Polyploidy associated with oxidative injury attenuates proliferative potential of cells. J Cell Science. 2001;114:2943–2951. doi: 10.1242/jcs.114.16.2943. [DOI] [PubMed] [Google Scholar]
- Gupta S. Hepatic polyploidy and liver growth control. Semin Cancer Biol. 2000;10:161–171. doi: 10.1006/scbi.2000.0317. [DOI] [PubMed] [Google Scholar]
- Kaushansky A, Kappe SH. The crucial role of hepatocyte growth factor receptor during liver-stage infection is not conserved among Plasmodium species. Nat Med. 2011;17:1180–1181. doi: 10.1038/nm.2456. author reply 1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushansky A, Metzger PG, Douglass AN, Mikolajczak SA, Lakshmanan V, Kain HS, Kappe SH. Malaria parasite liver stages render host hepatocytes susceptible to mitochondria-initiated apoptosis. Cell Death Dis. 2013a;4:e762. doi: 10.1038/cddis.2013.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushansky A, Rezakhani N, Mann H, Kappe SH. Development of a quantitative flow cytometry-based assay to assess infection by Plasmodium falciparum sporozoites. Mol Biochem Parasitol. 2012;183:100–103. doi: 10.1016/j.molbiopara.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushansky A, Ye AS, Austin LS, Mikolajczak SA, Vaughan AM, Camargo N, et al. Suppression of host p53 is critical for Plasmodium liver-stage infection. Cell Rep. 2013b;3:630–637. doi: 10.1016/j.celrep.2013.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurinna S, Stratton SA, Coban Z, Schumacher JM, Grompe M, Duncan AW, Barton MC. p53 regulates a mitotic transcription program and determines ploidy in normal mouse liver. Hepatology. 2013;57:2004–2013. doi: 10.1002/hep.26233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy S, Shoham T. The tetraspanin web modulates immune-signalling complexes. Nat Rev Immunol. 2005;5:136–148. doi: 10.1038/nri1548. [DOI] [PubMed] [Google Scholar]
- Lindner SE, Miller JL, Kappe SH. Malaria parasite pre-erythrocytic infection: preparation meets opportunity. Cell Microbiol. 2012;14:316–324. doi: 10.1111/j.1462-5822.2011.01734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madra S, Styles J, Smith AG. Perturbation of hepatocyte nuclear populations induced by iron and polychlorinated biphenyls in C57BL/10ScSn mice during carcinogenesis. Carcinogenesis. 1995;16:719–727. doi: 10.1093/carcin/16.4.719. [DOI] [PubMed] [Google Scholar]
- Margall-Ducos G, Celton-Morizur S, Couton D, Bregerie O, Desdouets C. Liver tetraploidization is controlled by a new process of incomplete cytokinesis. J Cell Sci. 2007;120:3633–3639. doi: 10.1242/jcs.016907. [DOI] [PubMed] [Google Scholar]
- Martin NC, McCullough CT, Bush PG, Sharp L, Hall AC, Harrison DJ. Functional analysis of mouse hepatocytes differing in DNA content: Volume, receptor expression, and effect of IFNγ. J Cell Physiol. 2002;191:138–144. doi: 10.1002/jcp.10057. [DOI] [PubMed] [Google Scholar]
- Mota MM, Rodriguez A. Plasmodium yoelii: efficient in vitro invasion and complete development of sporozoites in mouse hepatic cell lines. Exp Parasitol. 2000;96:257–259. doi: 10.1006/expr.2000.4570. [DOI] [PubMed] [Google Scholar]
- Pandit SK, Westendorp B, Nantasanti S, van Liere E, Tooten PC, Cornelissen PW, et al. E2F8 is essential for polyploidization in mammalian cells. Nat Cell Biol. 2012;14:1181–1191. doi: 10.1038/ncb2585. [DOI] [PubMed] [Google Scholar]
- Prudencio M, Mota MM, Mendes AM. A toolbox to study liver stage malaria. Trends Parasitol. 2011;27:565–574. doi: 10.1016/j.pt.2011.09.004. [DOI] [PubMed] [Google Scholar]
- Sattabongkot J, Yimamnuaychoke N, Leelaudomlipi S, Rasameesoraj M, Jenwithisuk R, Coleman RE, et al. Establishment of a human hepatocyte line that supports in vitro development of the exo-erythrocytic stages of the malaria parasites Plasmodium falciparum and P. vivax. Am J Trop Med Hyg. 2006;74:708–715. [PubMed] [Google Scholar]
- Sigal SH, Rajvanshi P, Gorla GR, Sokhi RP, Saxena R, Gebhard DR, et al. Partial hepatectomy-induced polyploidy attenuates hepatocyte replication and activates cell aging events. Am J Physiol Gastrointest Liver Physiol. 1999;276:G1260–G1272. doi: 10.1152/ajpgi.1999.276.5.G1260. [DOI] [PubMed] [Google Scholar]
- Silvie O, Greco C, Franetich JF, Dubart-Kupperschmitt A, Hannoun L, van Gemert GJ, et al. Expression of human CD81 differently affects host cell susceptibility to malaria sporozoites depending on the Plasmodium species. Cell Microbiol. 2006;8:1134–1146. doi: 10.1111/j.1462-5822.2006.00697.x. [DOI] [PubMed] [Google Scholar]
- Silvie O, Rubinstein E, Franetich JF, Prenant M, Belnoue E, Renia L, et al. Hepatocyte CD81 is required for Plasmodium falciparum and Plasmodium yoelii sporozoite infectivity. Nat Med. 2003;9:93–96. doi: 10.1038/nm808. [DOI] [PubMed] [Google Scholar]
- Sultan AA, Briones MRS, Gerwin N, Carroll MC, Nussenzweig V. Sporozoites of Plasmodium yoelii infect mice with targeted deletions in ICAM-1 and ICAM-2 or complement components C3 and C4. Molecular and Biochemical Parasitology. 1997;88:263–266. doi: 10.1016/s0166-6851(97)00075-3. [DOI] [PubMed] [Google Scholar]
- Uryvaeva IV. Biological significance of liver cell polyploidy: an hypothesis. J Theor Biol. 1981;89:557–571. doi: 10.1016/0022-5193(81)90028-x. [DOI] [PubMed] [Google Scholar]
- van de Sand C, Horstmann S, Schmidt A, Sturm A, Bolte S, Krueger A, et al. The liver stage of Plasmodium berghei inhibits host cell apoptosis. Mol Microbiol. 2005;58:731–742. doi: 10.1111/j.1365-2958.2005.04888.x. [DOI] [PubMed] [Google Scholar]
- Vaughan AM, Aly AS, Kappe SH. Malaria parasite pre-erythrocytic stage infection: gliding and hiding. Cell Host Microbe. 2008;4:209–218. doi: 10.1016/j.chom.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. World Malaria Report 2013. Geneva: World Health Organization; 2013. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.