Abstract
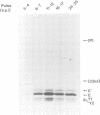
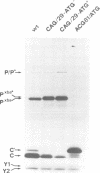
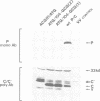
The Sendai virus P/C mRNA contains five ribosomal initiation sites between positions 81 and 201 from the 5' end. One of these sites initiates in the P open reading frame (ORF) (ATG/104), whereas four initiate in the C ORF (ACG/81 and ATGs/114, 183, 201), to give a nested set of C proteins (C', C, Y1, Y2). Introduction of new ATGs or physically breaking the mRNA upstream of these natural sites was used in vitro to prevent ribosomal scanning downstream. The results suggest that a minority of the ribosomes which initiate C (ATG/114) and all of those which initiate Y1 and Y2 (ATGs/183 and 201) do so by a scanning independent mechanism. When the leaky ACG/81 site is changed to a non-leaky ATG site in in vivo experiments, ribosomal initiation at Y is again not diminished, whereas that at C as well as at P becomes undetectable. Ribosomal initiation at Y appears to be scanning independent in vitro and in vivo. That at C is partly independent in vitro, but completely dependent in vivo. These results are discussed in terms of a model of internal initiation at Y.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramson R. D., Dever T. E., Merrick W. C. Biochemical evidence supporting a mechanism for cap-independent and internal initiation of eukaryotic mRNA. J Biol Chem. 1988 May 5;263(13):6016–6019. [PubMed] [Google Scholar]
- Becerra S. P., Rose J. A., Hardy M., Baroudy B. M., Anderson C. W. Direct mapping of adeno-associated virus capsid proteins B and C: a possible ACG initiation codon. Proc Natl Acad Sci U S A. 1985 Dec;82(23):7919–7923. doi: 10.1073/pnas.82.23.7919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienkowska-Szewczyk K., Ehrenfeld E. An internal 5'-noncoding region required for translation of poliovirus RNA in vitro. J Virol. 1988 Aug;62(8):3068–3072. doi: 10.1128/jvi.62.8.3068-3072.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos J. L., Polder L. J., Bernards R., Schrier P. I., van den Elsen P. J., van der Eb A. J., van Ormondt H. The 2.2 kb E1b mRNA of human Ad12 and Ad5 codes for two tumor antigens starting at different AUG triplets. Cell. 1981 Nov;27(1 Pt 2):121–131. doi: 10.1016/0092-8674(81)90366-4. [DOI] [PubMed] [Google Scholar]
- Curran J. A., Richardson C., Kolakofsky D. Ribosomal initiation at alternate AUGs on the Sendai virus P/C mRNA. J Virol. 1986 Feb;57(2):684–687. doi: 10.1128/jvi.57.2.684-687.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran J., Kolakofsky D. Ribosomal initiation from an ACG codon in the Sendai virus P/C mRNA. EMBO J. 1988 Jan;7(1):245–251. doi: 10.1002/j.1460-2075.1988.tb02806.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran J., Kolakofsky D. Scanning independent ribosomal initiation of the Sendai virus X protein. EMBO J. 1988 Sep;7(9):2869–2874. doi: 10.1002/j.1460-2075.1988.tb03143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diano M., Le Bivic A., Hirn M. A method for the production of highly specific polyclonal antibodies. Anal Biochem. 1987 Oct;166(1):224–229. doi: 10.1016/0003-2697(87)90568-9. [DOI] [PubMed] [Google Scholar]
- Dunigan D. D., Dietzgen R. G., Schoelz J. E., Zaitlin M. Tobacco mosaic virus particles contain ubiquitinated coat protein subunits. Virology. 1988 Jul;165(1):310–312. doi: 10.1016/0042-6822(88)90691-5. [DOI] [PubMed] [Google Scholar]
- Esposito J., Condit R., Obijeski J. The preparation of orthopoxvirus DNA. J Virol Methods. 1981 Feb;2(3):175–179. doi: 10.1016/0166-0934(81)90036-7. [DOI] [PubMed] [Google Scholar]
- Giorgi C., Blumberg B. M., Kolakofsky D. Sendai virus contains overlapping genes expressed from a single mRNA. Cell. 1983 Dec;35(3 Pt 2):829–836. doi: 10.1016/0092-8674(83)90115-0. [DOI] [PubMed] [Google Scholar]
- Gupta K. C., Kingsbury D. W. Translational modulation in vitro of a eukaryotic viral mRNA encoding overlapping genes: ribosome scanning and potential roles of conformational changes in the P/C mRNA of Sendai virus. Biochem Biophys Res Commun. 1985 Aug 30;131(1):91–97. doi: 10.1016/0006-291x(85)91774-7. [DOI] [PubMed] [Google Scholar]
- Gupta K. C., Patwardhan S. ACG, the initiator codon for a Sendai virus protein. J Biol Chem. 1988 Jun 25;263(18):8553–8556. [PubMed] [Google Scholar]
- Haarr L., Marsden H. S., Preston C. M., Smiley J. R., Summers W. C., Summers W. P. Utilization of internal AUG codons for initiation of protein synthesis directed by mRNAs from normal and mutant genes encoding herpes simplex virus-specified thymidine kinase. J Virol. 1985 Nov;56(2):512–519. doi: 10.1128/jvi.56.2.512-519.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassin D., Korn R., Horwitz M. S. A major internal initiation site for the in vitro translation of the adenovirus DNA polymerase. Virology. 1986 Nov;155(1):214–224. doi: 10.1016/0042-6822(86)90181-9. [DOI] [PubMed] [Google Scholar]
- Herman R. C. Internal initiation of translation on the vesicular stomatitis virus phosphoprotein mRNA yields a second protein. J Virol. 1986 Jun;58(3):797–804. doi: 10.1128/jvi.58.3.797-804.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang S. K., Kräusslich H. G., Nicklin M. J., Duke G. M., Palmenberg A. C., Wimmer E. A segment of the 5' nontranslated region of encephalomyocarditis virus RNA directs internal entry of ribosomes during in vitro translation. J Virol. 1988 Aug;62(8):2636–2643. doi: 10.1128/jvi.62.8.2636-2643.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. A profusion of controls. J Cell Biol. 1988 Jul;107(1):1–7. doi: 10.1083/jcb.107.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Comparison of initiation of protein synthesis in procaryotes, eucaryotes, and organelles. Microbiol Rev. 1983 Mar;47(1):1–45. doi: 10.1128/mr.47.1.1-45.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell. 1986 Jan 31;44(2):283–292. doi: 10.1016/0092-8674(86)90762-2. [DOI] [PubMed] [Google Scholar]
- Liu C. C., Simonsen C. C., Levinson A. D. Initiation of translation at internal AUG codons in mammalian cells. Nature. 1984 May 3;309(5963):82–85. doi: 10.1038/309082a0. [DOI] [PubMed] [Google Scholar]
- Mackett M., Smith G. L., Moss B. General method for production and selection of infectious vaccinia virus recombinants expressing foreign genes. J Virol. 1984 Mar;49(3):857–864. doi: 10.1128/jvi.49.3.857-864.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minshull J., Hunt T. The use of single-stranded DNA and RNase H to promote quantitative 'hybrid arrest of translation' of mRNA/DNA hybrids in reticulocyte lysate cell-free translations. Nucleic Acids Res. 1986 Aug 26;14(16):6433–6451. doi: 10.1093/nar/14.16.6433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munemitsu S. M., Samuel C. E. Biosynthesis of reovirus-specified polypeptides: effect of point mutation of the sequences flanking the 5'-proximal AUG initiator codons of the reovirus S1 and S4 genes on the efficiency of mRNA translation. Virology. 1988 Apr;163(2):643–646. doi: 10.1016/0042-6822(88)90309-1. [DOI] [PubMed] [Google Scholar]
- Nagy E., Duncan R., Krell P., Dobos P. Mapping of the large RNA genome segment of infectious pancreatic necrosis virus by hybrid arrested translation. Virology. 1987 May;158(1):211–217. doi: 10.1016/0042-6822(87)90255-8. [DOI] [PubMed] [Google Scholar]
- Orvell C., Grandien M. The effects of monoclonal antibodies on biologic activities of structural proteins of Sendai virus. J Immunol. 1982 Dec;129(6):2779–2787. [PubMed] [Google Scholar]
- Patwardhan S., Gupta K. C. Translation initiation potential of the 5' proximal AUGs of the polycistronic P/C mRNA of Sendai virus. A multipurpose vector for site-specific mutagenesis. J Biol Chem. 1988 Apr 5;263(10):4907–4913. [PubMed] [Google Scholar]
- Peabody D. S. Translation initiation at an ACG triplet in mammalian cells. J Biol Chem. 1987 Aug 25;262(24):11847–11851. [PubMed] [Google Scholar]
- Pelletier J., Kaplan G., Racaniello V. R., Sonenberg N. Cap-independent translation of poliovirus mRNA is conferred by sequence elements within the 5' noncoding region. Mol Cell Biol. 1988 Mar;8(3):1103–1112. doi: 10.1128/mcb.8.3.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier J., Sonenberg N. Internal initiation of translation of eukaryotic mRNA directed by a sequence derived from poliovirus RNA. Nature. 1988 Jul 28;334(6180):320–325. doi: 10.1038/334320a0. [DOI] [PubMed] [Google Scholar]
- Shaw M. W., Choppin P. W., Lamb R. A. A previously unrecognized influenza B virus glycoprotein from a bicistronic mRNA that also encodes the viral neuraminidase. Proc Natl Acad Sci U S A. 1983 Aug;80(16):4879–4883. doi: 10.1073/pnas.80.16.4879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas S. M., Lamb R. A., Paterson R. G. Two mRNAs that differ by two nontemplated nucleotides encode the amino coterminal proteins P and V of the paramyxovirus SV5. Cell. 1988 Sep 9;54(6):891–902. doi: 10.1016/S0092-8674(88)91285-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal S., Curran J., Orvell C., Kolakofsky D. Mapping of monoclonal antibodies to the Sendai virus P protein and the location of its phosphates. J Virol. 1988 Jun;62(6):2200–2203. doi: 10.1128/jvi.62.6.2200-2203.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams M. A., Lamb R. A. Effect of mutations and deletions in a bicistronic mRNA on the synthesis of influenza B virus NB and NA glycoproteins. J Virol. 1989 Jan;63(1):28–35. doi: 10.1128/jvi.63.1.28-35.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]