Abstract
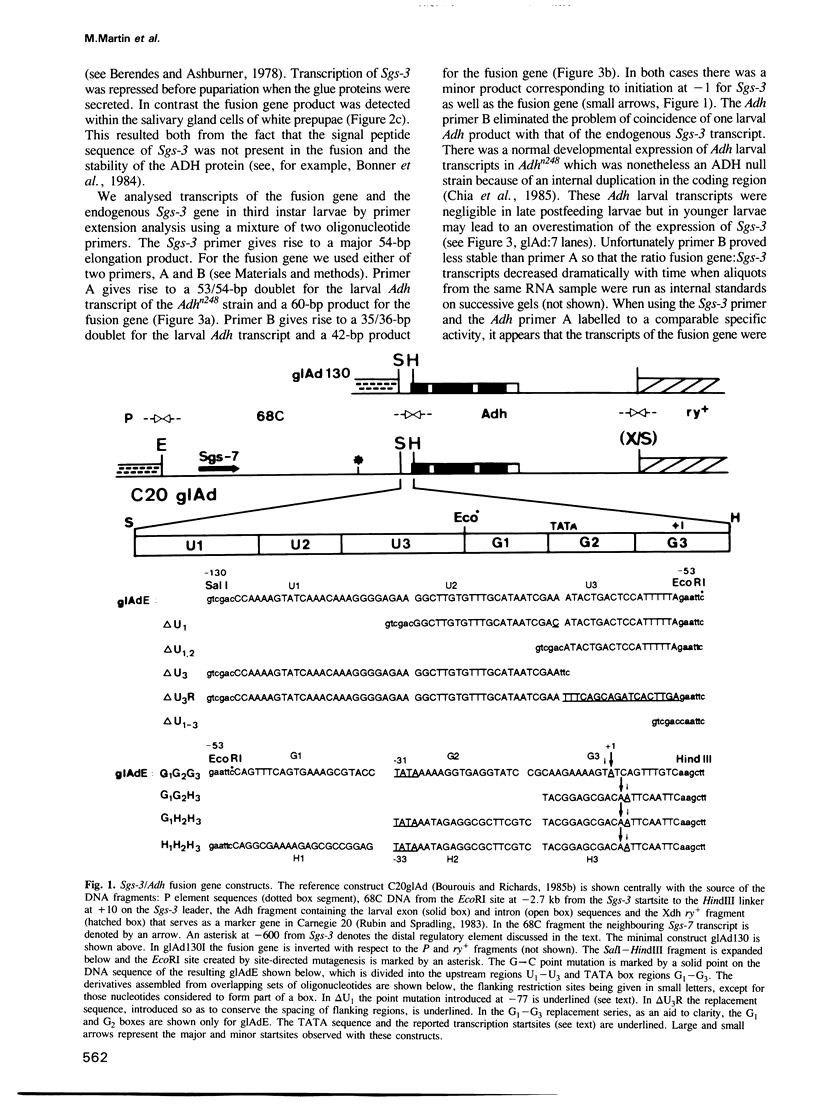
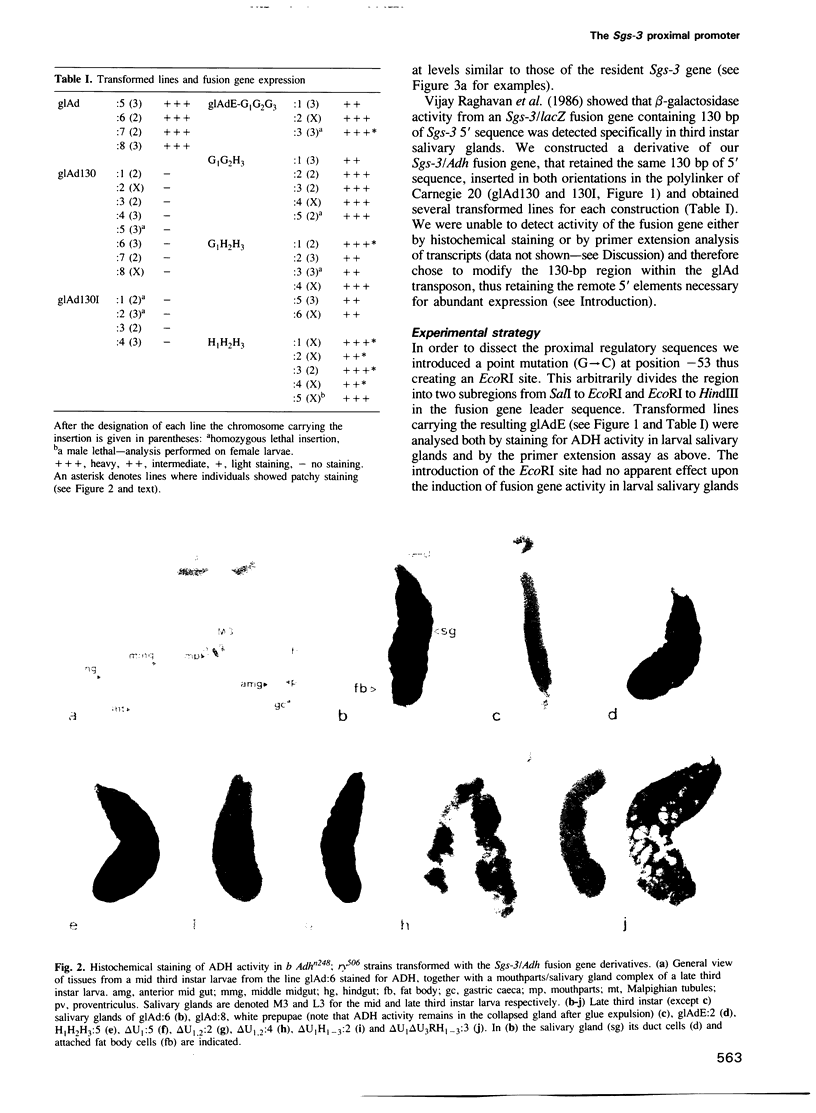
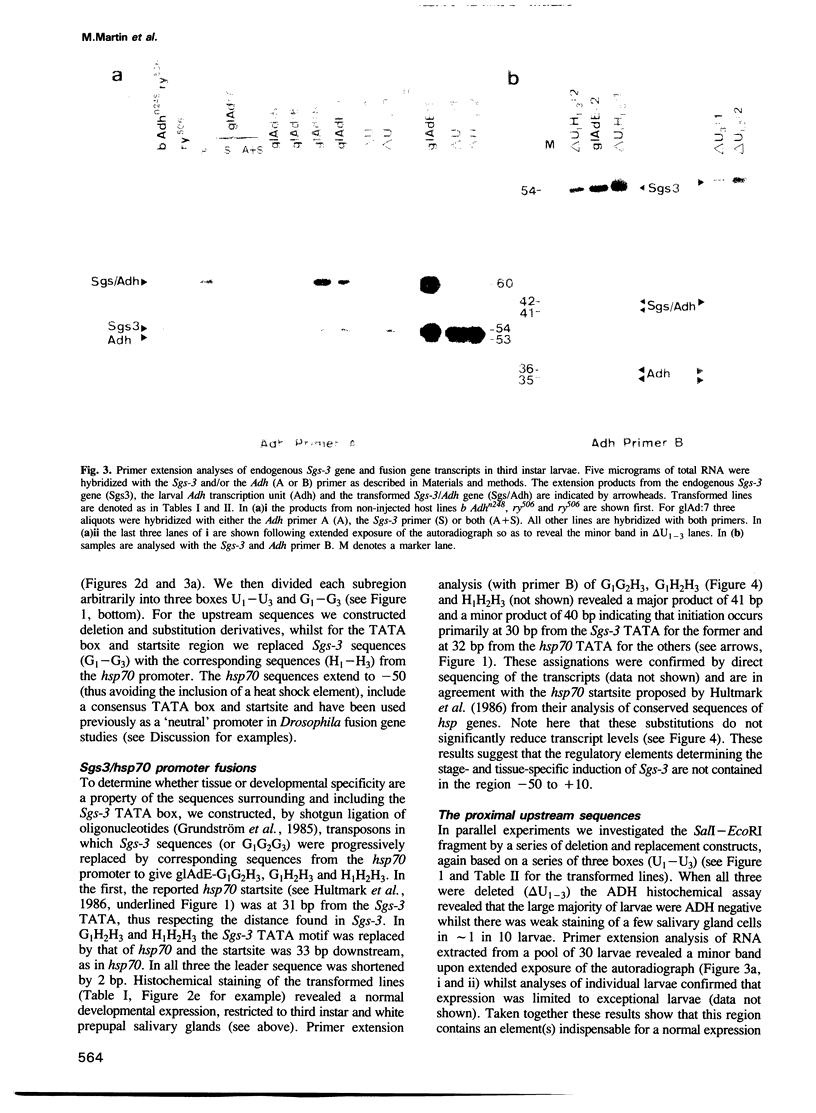
The normal developmental expression of the Drosophila salivary gland secretion protein gene Sgs-3 requires the interaction of a distal and proximal regulatory element. A deletion/replacement analysis of the proximal promoter in stably transformed lines shows that induction of an Sgs-3/Adh fusion gene is normal if sequences from +10 to -50 are replaced by those of the hsp70 gene. Sequences between -98 and -50 are necessary for this expression but there is internal redundancy within this region as two distinct upstream sequences of 18 and 22 bp respectively are sufficient for stage- and tissue-specific expression, albeit at reduced levels. A point mutation at -53 eliminates the ecdysone-mediated repression of the Sgs-3 promoter at pupariation. We report mosaicisms of expression within the salivary gland for a number of stably transformed lines.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akerblom I. E., Slater E. P., Beato M., Baxter J. D., Mellon P. L. Negative regulation by glucocorticoids through interference with a cAMP responsive enhancer. Science. 1988 Jul 15;241(4863):350–353. doi: 10.1126/science.2838908. [DOI] [PubMed] [Google Scholar]
- Bienz M., Saari G., Tremml G., Müller J., Züst B., Lawrence P. A. Differential regulation of Ultrabithorax in two germ layers of Drosophila. Cell. 1988 May 20;53(4):567–576. doi: 10.1016/0092-8674(88)90573-9. [DOI] [PubMed] [Google Scholar]
- Bonner J. J., Parks C., Parker-Thornburg J., Mortin M. A., Pelham H. R. The use of promoter fusions in Drosophila genetics: isolation of mutations affecting the heat shock response. Cell. 1984 Jul;37(3):979–991. doi: 10.1016/0092-8674(84)90432-x. [DOI] [PubMed] [Google Scholar]
- Bourouis M., Richards G. Hybrid genes in the study of glue gene regulation in Drosophila. Cold Spring Harb Symp Quant Biol. 1985;50:355–360. doi: 10.1101/sqb.1985.050.01.045. [DOI] [PubMed] [Google Scholar]
- Bourouis M., Richards G. Remote regulatory sequences of the Drosophila glue gene sgs3 as revealed by P-element transformation. Cell. 1985 Feb;40(2):349–357. doi: 10.1016/0092-8674(85)90149-7. [DOI] [PubMed] [Google Scholar]
- Bray S. J., Johnson W. A., Hirsh J., Heberlein U., Tjian R. A cis-acting element and associated binding factor required for CNS expression of the Drosophila melanogaster dopa decarboxylase gene. EMBO J. 1988 Jan;7(1):177–188. doi: 10.1002/j.1460-2075.1988.tb02798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breathnach R., Chambon P. Organization and expression of eucaryotic split genes coding for proteins. Annu Rev Biochem. 1981;50:349–383. doi: 10.1146/annurev.bi.50.070181.002025. [DOI] [PubMed] [Google Scholar]
- Bucher P., Trifonov E. N. Compilation and analysis of eukaryotic POL II promoter sequences. Nucleic Acids Res. 1986 Dec 22;14(24):10009–10026. doi: 10.1093/nar/14.24.10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chia W., Savakis C., Karp R., Pelham H., Ashburner M. Mutation of the Adh gene of Drosophila melanogaster containing an internal tandem duplication. J Mol Biol. 1985 Dec 20;186(4):679–688. doi: 10.1016/0022-2836(85)90388-2. [DOI] [PubMed] [Google Scholar]
- Fischer J. A., Maniatis T. Drosophila Adh: a promoter element expands the tissue specificity of an enhancer. Cell. 1988 May 6;53(3):451–461. doi: 10.1016/0092-8674(88)90165-1. [DOI] [PubMed] [Google Scholar]
- Garabedian M. J., Shepherd B. M., Wensink P. C. A tissue-specific transcription enhancer from the Drosophila yolk protein 1 gene. Cell. 1986 Jun 20;45(6):859–867. doi: 10.1016/0092-8674(86)90560-x. [DOI] [PubMed] [Google Scholar]
- Garfinkel M. D., Pruitt R. E., Meyerowitz E. M. DNA sequences, gene regulation and modular protein evolution in the Drosophila 68C glue gene cluster. J Mol Biol. 1983 Aug 25;168(4):765–789. doi: 10.1016/s0022-2836(83)80074-6. [DOI] [PubMed] [Google Scholar]
- Giangrande A., Mettling C., Richards G. Sps-3 transcript levels are determined by multiple remote sequence elements. EMBO J. 1987 Oct;6(10):3079–3084. doi: 10.1002/j.1460-2075.1987.tb02615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundström T., Zenke W. M., Wintzerith M., Matthes H. W., Staub A., Chambon P. Oligonucleotide-directed mutagenesis by microscale 'shot-gun' gene synthesis. Nucleic Acids Res. 1985 May 10;13(9):3305–3316. doi: 10.1093/nar/13.9.3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiromi Y., Gehring W. J. Regulation and function of the Drosophila segmentation gene fushi tarazu. Cell. 1987 Sep 11;50(6):963–974. doi: 10.1016/0092-8674(87)90523-x. [DOI] [PubMed] [Google Scholar]
- Hiromi Y., Kuroiwa A., Gehring W. J. Control elements of the Drosophila segmentation gene fushi tarazu. Cell. 1985 Dec;43(3 Pt 2):603–613. doi: 10.1016/0092-8674(85)90232-6. [DOI] [PubMed] [Google Scholar]
- Hultmark D., Klemenz R., Gehring W. J. Translational and transcriptional control elements in the untranslated leader of the heat-shock gene hsp22. Cell. 1986 Feb 14;44(3):429–438. doi: 10.1016/0092-8674(86)90464-2. [DOI] [PubMed] [Google Scholar]
- Ingolia T. D., Craig E. A., McCarthy B. J. Sequence of three copies of the gene for the major Drosophila heat shock induced protein and their flanking regions. Cell. 1980 Oct;21(3):669–679. doi: 10.1016/0092-8674(80)90430-4. [DOI] [PubMed] [Google Scholar]
- Jongens T. A., Fowler T., Shermoen A. W., Beckendorf S. K. Functional redundancy in the tissue-specific enhancer of the Drosophila Sgs-4 gene. EMBO J. 1988 Aug;7(8):2559–2567. doi: 10.1002/j.1460-2075.1988.tb03105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karess R. E., Rubin G. M. Analysis of P transposable element functions in Drosophila. Cell. 1984 Aug;38(1):135–146. doi: 10.1016/0092-8674(84)90534-8. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Goodbourn S., Fischer J. A. Regulation of inducible and tissue-specific gene expression. Science. 1987 Jun 5;236(4806):1237–1245. doi: 10.1126/science.3296191. [DOI] [PubMed] [Google Scholar]
- Martin C. H., Mayeda C. A., Meyerowitz E. M. Evolution and expression of the Sgs-3 glue gene of Drosophila. J Mol Biol. 1988 May 20;201(2):273–287. doi: 10.1016/0022-2836(88)90138-6. [DOI] [PubMed] [Google Scholar]
- Martin P., Martin A., Osmani A., Sofer W. A transient expression assay for tissue-specific gene expression of alcohol dehydrogenase in Drosophila. Dev Biol. 1986 Oct;117(2):574–580. doi: 10.1016/0012-1606(86)90326-x. [DOI] [PubMed] [Google Scholar]
- Nakamaye K. L., Eckstein F. Inhibition of restriction endonuclease Nci I cleavage by phosphorothioate groups and its application to oligonucleotide-directed mutagenesis. Nucleic Acids Res. 1986 Dec 22;14(24):9679–9698. doi: 10.1093/nar/14.24.9679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris K., Norris F., Christiansen L., Fiil N. Efficient site-directed mutagenesis by simultaneous use of two primers. Nucleic Acids Res. 1983 Aug 11;11(15):5103–5112. doi: 10.1093/nar/11.15.5103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavan K. V., Crosby M. A., Mathers P. H., Meyerowitz E. M. Sequences sufficient for correct regulation of Sgs-3 lie close to or within the gene. EMBO J. 1986 Dec 1;5(12):3321–3326. doi: 10.1002/j.1460-2075.1986.tb04646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramain P., Giangrande A., Richards G., Bellard M. Analysis of a DNase I-hypersensitive site in transgenic Drosophila reveals a key regulatory element of Sgs3. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2718–2722. doi: 10.1073/pnas.85.8.2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddihough G., Pelham H. R. An ecdysone response element in the Drosophila hsp27 promoter. EMBO J. 1987 Dec 1;6(12):3729–3734. doi: 10.1002/j.1460-2075.1987.tb02707.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano C. P., Bienz-Tadmor B., Mariani B. D., Kafatos F. C. Both early and late Drosophila chorion gene promoters confer correct temporal, tissue and sex specificity on a reporter Adh gene. EMBO J. 1988 Mar;7(3):783–790. doi: 10.1002/j.1460-2075.1988.tb02876.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin G. M., Spradling A. C. Genetic transformation of Drosophila with transposable element vectors. Science. 1982 Oct 22;218(4570):348–353. doi: 10.1126/science.6289436. [DOI] [PubMed] [Google Scholar]
- Rubin G. M., Spradling A. C. Vectors for P element-mediated gene transfer in Drosophila. Nucleic Acids Res. 1983 Sep 24;11(18):6341–6351. doi: 10.1093/nar/11.18.6341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffner G., Schirm S., Müller-Baden B., Weber F., Schaffner W. Redundancy of information in enhancers as a principle of mammalian transcription control. J Mol Biol. 1988 May 5;201(1):81–90. doi: 10.1016/0022-2836(88)90440-8. [DOI] [PubMed] [Google Scholar]
- Schmitt P., Gattoni R., Keohavong P., Stévenin J. Alternative splicing of E1A transcripts of adenovirus requires appropriate ionic conditions in vitro. Cell. 1987 Jul 3;50(1):31–39. doi: 10.1016/0092-8674(87)90659-3. [DOI] [PubMed] [Google Scholar]
- Shermoen A. W., Jongens J., Barnett S. W., Flynn K., Beckendorf S. K. Developmental regulation by an enhancer from the Sgs-4 gene of Drosophila. EMBO J. 1987 Jan;6(1):207–214. doi: 10.1002/j.1460-2075.1987.tb04740.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon M. C., Fisch T. M., Benecke B. J., Nevins J. R., Heintz N. Definition of multiple, functionally distinct TATA elements, one of which is a target in the hsp70 promoter for E1A regulation. Cell. 1988 Mar 11;52(5):723–729. doi: 10.1016/0092-8674(88)90410-2. [DOI] [PubMed] [Google Scholar]
- Xiao H., Lis J. T. Germline transformation used to define key features of heat-shock response elements. Science. 1988 Mar 4;239(4844):1139–1142. doi: 10.1126/science.3125608. [DOI] [PubMed] [Google Scholar]