Abstract
Depending on tumor type, stage and immunological contexture, the inhibition of chemokines or their receptors may yield positive or deleterious effects on disease progression. We have recently demonstrated in several murine models of anthracycline-based chemotherapy that the inhibition of chemokine (C-C motif) ligand 2 (CCL2) or chemokine (C-C motif) receptor 2 (CCR2) may impair the elicitation of anticancer immune responses that contribute to therapeutic success.
Keywords: ATP, autophagy, cancer stem cells, immunogenic cell death, immunosurveillance, γδ T lymphocytes
Multiple members of the chemokine (chemotactic cytokine) family critically regulate cell migration in physiological and pathological settings, including (post-)embryonic development, immunosurveillance and inflammation. Chemokines bind to 7 transmembrane domain G protein-coupled receptors that are predominantly expressed by leukocytes. Some chemokines are constitutively expressed and guide the homing of leukocytes to lymphoid organs in physiological conditions, hence regulating immune homeostasis. In contrast, the expression of other chemokines is induced in response to infection or tissue damage, resulting in the recruitment of circulating leukocytes to sites that have been exposed to an inflammatory insult.
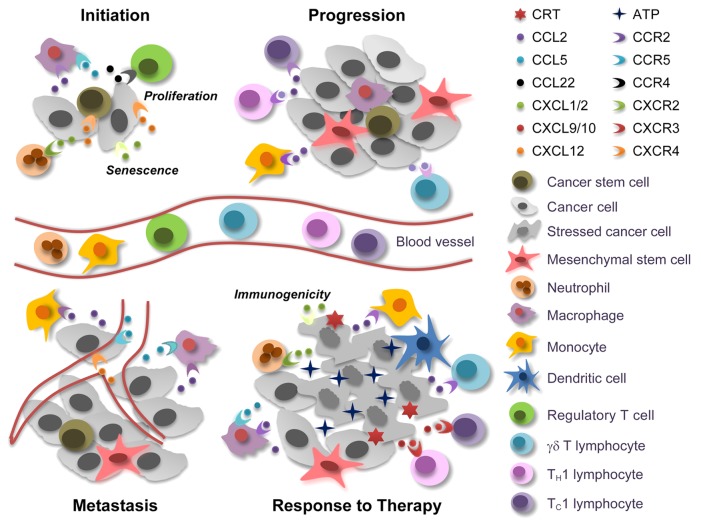
Chemokines are involved in all stages of oncogenesis and tumor progression, including malignant transformation, tumor growth, angiogenesis and metastatic dissemination. In addition, chemokines participate both in the induction of anticancer immune responses and in the evasion thereof, in a Janus-faced fashion that can be explained by at least 3 mechanisms (Fig. 1). First, distinct leukocyte subsets bear specific chemokine receptors. Thus, perhaps due to dynamic changes in the chemokines produced within neoplastic lesions, the composition of the immune infiltrate evolves with disease progression.1 Second, the chemokine network exhibits an elevated degree of redundancy, meaning that 1.)various chemokines share the same receptor; 2.)some chemokines bind to multiple receptors with different affinity; and 3.)the expression levels of chemokine and chemokine receptors can vary to a significant extent in response to microenvironmental cues. Third, besides regulating the motility and activation state of immune cells, chemokines can act on malignant cells, including cancer stem cells, as well as on stromal cells, including mesenchymal stem cells (MSCs), to control chemotaxis, proliferation, angiogenesis and metastatic dissemination.
Figure 1. Janus-faced effects of chemokine and chemokine receptors in cancer. At the tumor initiation stage, cancer stem cells (CSCs) can be recruited to favorable niches by chemokine (C-X-C motif) ligand 12 (CXCL12), which signals via chemokine (C-X-C motif) receptor 4 (CXCR4). Macrophages and regulatory T cells are also attracted to these sites by chemokine (C-C motif) ligand 2 (CCL2), CCL5, and CCL22, contribute to the establishment of a microenvironment that supports tumor initiation. Conversely, neutrophils, which are attracted to developing neoplastic lesions by CXCL1 or CXCL2 (signaling via CXCR2), can exert tumor-supporting or tumor-suppressing effects, depending on their (N1 or N2) phenotype. CXCL1 and CXCL2 can also promote cell senescence, hence exerting direct antineoplastic effects, while CXCL12 generally accelerate tumor growth. When neoplastic lesions are established, CCR2+ tumor-infiltrating monocytes and tumor-associated macrophages cooperatively support disease progression, driving the abortive activation of immune effector cells and promoting the metastatic dissemination of malignant cells the CCL5/CCR5 and CXCL12/CXCR4 signaling axes. In response to chemo- or radiotherapy, neoplastic cells die to massive extents. This results in the release of various danger signals including ATP, which is critical for the recruitment and differentiation of antigen-presenting cells. The immune cells that infiltrate neoplastic lesions in response to chemo- or radiotherapy produce high amounts of CCR2 ligands, hence amplifying their own accumulation. Therapy can also trigger the secretion of CXCL1 or CXCL2 from dying tumor cells, resulting in an optimal exposure of the immunogenic factor calreticulin (CRT). Finally, CCL2 can favor the recruitment of interleukin (IL)-17-producing γδ T cells, and the IL-17-dependent release of CXCL9 or CXCL10 promotes the accumulation of interferon γ-secreting CD8+ T cells that mediate tumor clearance.
A large body of evidence suggests that some chemokines, including chemokine (C-C motif) ligand 5 (CCL5) and chemokine (C-X-C motif) ligand 12 (CXCL12), which signal through chemokine (C-C motif) receptor 5 (CCR5) and chemokine (C-X-C motif) receptor 4 (CXCR4), respectively, support oncogenesis and tumor progression. Thus, the CCL5/CCR5 and CXCL12/CXCR4 signaling axes may constitute targets for the development of novel antineoplastic agents. CXCR2 also appears to favor the recruitment of disease-promoting leukocytes in both spontaneous and inflammation-driven tumor models,2 yet it may as well limit the growth of early neoplastic lesions by stimulating cell senescence.3 In addition, the pro-inflammatory CXCR2 ligands CXCL2 and CXCL8 have been shown promote the recruitment of innate immune effectors that mediate the clearance of cancer cells or increase their immunogenic properties.4 Thus, the biological activity of the CXCR2 signaling axis exhibits a significant degree of context dependency. Similarly, the CCL2/CCR2 signal transduction cascade enhances immunosurveillance by triggering a TH1 response and recruiting CD8+ and γδ effector T cells to neoplastic lesions, but may also stimulate the progression of established malignancies. High levels of CCL2 reportedly attract inflammatory monocytes to human breast carcinomas, resulting in the differentiation of F4/80+CD11b+Gr1− macrophages that support the metastatic dissemination of malignant cells to the lungs.5 MSCs may also secrete high levels of CCR2 ligands, hence attracting macrophages that support tumor progression.6
We have recently discovered that the intratumoral accumulation of immune cells in response to (anthracycline-based) immunogenic chemotherapy occurs in 3 waves. In a first wave, 24–72 h post-chemotherapy, CD11c+CD11b+Ly6ChighLy6G-MHCII+ cells are recruited. Such cells share features with inflammatory dendritic cells, include granulocyte-monocyte precursors and operate locally as antigen-presenting cells. The recruitment of CD11c+CD11b+Ly6ChighLy6G-MHCII+ cells into the tumor bed relies on various chemoattractants, including the “find-me” signal ATP,7 which is released by stressed/dying cancer cells in an autophagy dependent manner, as well as on CCL2. We observed indeed that immunogenic chemotherapy triggers the release of multiple chemokines within neoplastic lesions, including CCL2, which is produced by both CD45+ leukocytes and CD45− tumor cells, and CCL7, another CCR2 ligand that is predominantly secreted by CD45+ cells. Interestingly, CD11b+Ly6Chigh cells are the major source of CCL2 and CCL7 in the tumor microenvironment, hence establishing a positive feedback loop for the optimal recruitment of such cells to neoplastic lesions.8 The second wave of anthracycline-elicited tumor infiltration by immune cells, which peaks 4–6 d post-chemotherapy, is characterized by the accumulation of interleukin (IL)-17A-producing γδ T cells (harboring either a Vγ4 or a Vγ6 T-cell receptor chain in our setting). As Vγ5Vδ1 dendritic epidermal T cells (DETCs) largely predominate over other γδ T cells in the skin, the Vγ4+ or Vγ6+ γδ T cells that infiltrate subcutaneous tumors are most probably recruited from the circulation. Finally, neoplastic lesions are infiltrated by highly proliferative interferon γ (IFNγ)-secreting CD8+ αβ T cells, a process that peaks approximately 8 d post-chemotherapy, presumably as a result of the IL-17-depdnent secretion of CXCL9 and CXCL10.9 In our models, the depletion or neutralization of all the relevant soluble factors (namely, ATP, CCL2, IL-17A, CXCL9, CXCL10, and IFNγ) as well as of specific immune cells (including myeloid cells, Vγ4 or Vγ6-expressing γδ T cells, and CD8+ αβ T cells) compromises the capacity of chemotherapy to inhibit tumor growth.
We have previously developed an immunotherapeutic cocktail comprising a vaccine, chemotherapy and a Toll-like receptor 3 agonist (VCT) specific for hardly curable tumors, such as melanoma and glioma. The administration of VCT to tumor-bearing mice induced copious amounts of CCL5 and CXCL10 with distinct kinetics. CXCL10 was shown to contribute to the therapeutic success of VCT by promoting the recruitment of CD8+CXCR3+ T cells to neoplastic lesions. In contrast, the secretion of CCL5 by neoplastic cells appeared to support the accumulation of tumor-supporting CCR5+ immune cells. In line with this notion, the blockade of CCL5 improve the therapeutic efficacy of VCT.10
The aforementioned findings underscore the ambiguous role of chemokines in the crosstalk between cancer and the host. Hence, the therapeutic application of chemokine agonists and antagonist should be explored with great care. Indeed, depending on tumor type, stage and immunological contexture, chemokine-targeted therapeutic intervention may yield a positive or deleterious clinical outcome.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Citation: Ma Y, Adjemian S, Zitvogel L, Kroemer G, Galluzzi L. Chemokines and chemokine receptors required for optimal responses to anticancer chemotherapy. OncoImmunology 2014; 3:e27663; 10.4161/onci.27663
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/27663
References
- 1.Bindea G, Mlecnik B, Tosolini M, Kirilovsky A, Waldner M, Obenauf AC, Angell H, Fredriksen T, Lafontaine L, Berger A, et al. Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer. Immunity. 2013;39:782–95. doi: 10.1016/j.immuni.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 2.Jamieson T, Clarke M, Steele CW, Samuel MS, Neumann J, Jung A, Huels D, Olson MF, Das S, Nibbs RJ, et al. Inhibition of CXCR2 profoundly suppresses inflammation-driven and spontaneous tumorigenesis. J Clin Invest. 2012;122:3127–44. doi: 10.1172/JCI61067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Acosta JC, O’Loghlen A, Banito A, Guijarro MV, Augert A, Raguz S, Fumagalli M, Da Costa M, Brown C, Popov N, et al. Chemokine signaling via the CXCR2 receptor reinforces senescence. Cell. 2008;133:1006–18. doi: 10.1016/j.cell.2008.03.038. [DOI] [PubMed] [Google Scholar]
- 4.Sukkurwala AQ, Martins I, Wang Y, Schlemmer F, Ruckenstuhl C, Durchschlag M, Michaud M, Senovilla L, Sistigu A, Ma Y, et al. Immunogenic calreticulin exposure occurs through a phylogenetically conserved stress pathway involving the chemokine CXCL8. Cell Death Differ. 2014;21:59–68. doi: 10.1038/cdd.2013.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qian BZ, Li J, Zhang H, Kitamura T, Zhang J, Campion LR, Kaiser EA, Snyder LA, Pollard JW. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature. 2011;475:222–5. doi: 10.1038/nature10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ren G, Zhao X, Wang Y, Zhang X, Chen X, Xu C, Yuan ZR, Roberts AI, Zhang L, Zheng B, et al. CCR2-dependent recruitment of macrophages by tumor-educated mesenchymal stromal cells promotes tumor development and is mimicked by TNFα. Cell Stem Cell. 2012;11:812–24. doi: 10.1016/j.stem.2012.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ma Y, Adjemian S, Mattarollo SR, Yamazaki T, Aymeric L, Yang H, Portela Catani JP, Hannani D, Duret H, Steegh K, et al. Anticancer chemotherapy-induced intratumoral recruitment and differentiation of antigen-presenting cells. Immunity. 2013;38:729–41. doi: 10.1016/j.immuni.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 8.Ma Y, Mattarollo SR, Adjemian S, Yang H, Aymeric L, Hannani D, Portela Catani JP, Duret H, Teng MW, Kepp O, et al. CCL2/CCR2-dependent recruitment of functional antigen-presenting cells into tumors upon chemotherapy. Cancer Res. 2013 doi: 10.1158/0008-5472.CAN-13-1265. Forthcoming. [DOI] [PubMed] [Google Scholar]
- 9.Ma Y, Aymeric L, Locher C, Mattarollo SR, Delahaye NF, Pereira P, Boucontet L, Apetoh L, Ghiringhelli F, Casares N, et al. Contribution of IL-17-producing gamma delta T cells to the efficacy of anticancer chemotherapy. J Exp Med. 2011;208:491–503. doi: 10.1084/jem.20100269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Conforti R, Ma Y, Morel Y, Paturel C, Terme M, Viaud S, Ryffel B, Ferrantini M, Uppaluri R, Schreiber R, et al. Opposing effects of toll-like receptor (TLR3) signaling in tumors can be therapeutically uncoupled to optimize the anticancer efficacy of TLR3 ligands. Cancer Res. 2010;70:490–500. doi: 10.1158/0008-5472.CAN-09-1890. [DOI] [PubMed] [Google Scholar]