Abstract
We established a method to produce a large quantity of myeloid cells from human inducible pluripotent stem cells (iPSCs). When injected intraperitoneally into mice carrying established peritoneal tumors, iPSC-derived myeloid cells (iPS-MCs) efficiently accumulated within neoplastic lesions. The intraperitoneal injection of iPS-MCs expressing interferon β significantly inhibited the growth of human gastric and pancreatic cancers implanted in the peritoneal cavity of immunocompromised mice.
Keywords: gastric cancer, iPS cells, interferon β, macrophages, pancreatic cancer, peritoneal dissemination
Introduction
Macrophage infiltration is frequently observed in solid tumors.1 Recent studies indicate that tumor-associated macrophages (TAMs) are significantly involved in tumor progression, accelerating the local invasion and metastatic dissemination of malignant cells.2 Other studies have highlighted the possibility that macrophages may also mediate a tumoricidal effect, leading to the development of macrophage-based anticancer therapies. As a standalone example, the transfer of autologous monocyte-derived macrophages activated with interferon (IFN)γ ex vivo has been tested as a potential intervention for patients with solid tumors.3 However, no clear therapeutic benefit has thus far associated with macrophage-based anticancer therapies. To optimize the efficacy of such an approach, improvements of the method for supplying macrophages are necessary. Indeed, if sufficient amounts of macrophages exerting potent anticancer effects could be repeatedly administered, patients may achieve robust clinical benefit from this cell-based immunotherapeutic regimen.
iPSC-Derived Proliferating Myeloid Cells
Several groups, including ours, have thus far established methods to generate macrophages from mouse or human inducible pluripotent stem cells (iPSCs).4,5 However, the number of macrophages generated from human iPSCs is only 10–20 times higher than the number of undifferentiated iPSCs used as starting material. In addition, the generation of macrophages from iPSCs is time-consuming, laborious, and too expensive to be applied to the clinical practice.
Recently, we established a method to induce proliferation of the iPSC–derived myeloid cells (iPS-MCs) by the lentivirus-mediated transduction of genes that promote cell proliferation or inhibit cell senescence, i.e., v-myc avian myelocytomatosis viral oncogene homolog (MYC) plus BMI1, to generate an iPSC-derived myeloid/macrophage cell line (iPS-ML). Such an iPS-ML can proliferate in a colony stimulating factor 1 (CSF1)-dependent manner for at least several months while retaining the potential to differentiate into dendritic cells (iPS-ML-DCs) with a potent T cell-stimulating capacity.6
Accumulation and Infiltration of Intraperitoneally Injected iPS-ML in Tumor Tissues
We examined whether or not iPS-ML administered intraperitoneally would infiltrate tumor lesions pre-established in the peritoneal cavity of mice.7 To this end, green fluorescent protein (GFP)-expressing NUGC-4 human gastric cancer cells, which have been established from a peritoneal metastatic lesion removed from an individual with diffuse gastric cancer, were intraperitoneally injected into SCID mice. After 15 d, iPS-ML labeled with the red fluorescent dye PKH26 were administered via the same route. Macroscopic fluorescence analysis on the next day revealed that both NUGC-4-derived tumors and iPS-ML localize for the most part to the greater omentum, demonstrating that iPS-ML efficiently accumulate into neoplastic lesions. Of note, such a preferential accumulation of iPS-ML into the greater omentum was not observed when iPS-ML were inoculated into tumor-free mice. Next, we isolated and microscopically examined neoplastic lesions. PKH26-labeled iPS-ML infiltrated nests of GFP-expressing NUGC-4 cells. Higher magnification analysis of tumor sections clearly demonstrated the infiltration of iPS-ML into the neoplastic tissue. These results indicate that iPS-ML efficiently infiltrate malignant tissues when intraperitoneally injected into mice carrying cancers established in the peritoneal cavity.
Therapeutic Effects of IFNβ-Secreting iPS-ML on Peritoneally Disseminated NUGC-4 Gastric Cancer Cells in Xenograft Models
IFNβ exerts anti-proliferative and/or pro-apoptotic effects on various types of cancer cells. We examined the effects of iPS-ML genetically modified to secrete IFNβ (iPS-ML/IFNβ) against NUGC-4 cells in vivo.7 We generated NUGC-4 cells expressing the firefly luciferase, NUGC4/Luc cells, which can be easily monitored in vivo by bioluminescence analysis. SCID mice were then inoculated with NUGC-4/Luc cells (day 0), and 4 d later they were divided into a treated and a control group. Mice belonging to the treated group were injected i.p. with iPS-ML/IFNβ from day 4 (2 × 107 cells/injection/mouse, 3 injections per week). Tumor growth was significantly inhibited by the inoculation of iPS-ML/IFNβ cells. Of note, the administration of iPS-ML/IFNβ also inhibited the growth of MIAPaCa-2 pancreatic cancer cells in a similar xenograft model. In summary, iPS-MCs expressing IFNβ potently inhibit the growth of human gastric and pancreatic cancers growing in immunocompromised SCID mice.
Toward Clinical Applications
Gastric cancer is one of most frequent malignancies worldwide and the second most frequent cause of cancer-related mortality. Peritoneal dissemination is the most difficult type of metastasis to treat of those associated with gastric cancer. Pancreatic cancer has a poor prognosis, with overall 5-y survival rate being < 10%. Thus, efficient therapies for these intractable cancers are urgently needed. The present study suggests that iPS-MCs secreting antineoplastic factors may be used to treat cancers for which no standard therapy has been established yet.
For macrophage-based immunotherapeutic regimens to achieve robust clinical effects, cancer patients may need to receive repetitive administrations of large numbers of cells. Since our iPS-ML proliferates for at least several months, sufficient amounts of iPS-MCs may be readily available by this approach. However, the proliferative capacity of our iPS-ML may constitute a concern, as this line could drive leukemogenesis, at least theoretically, in a completely autologous setting.
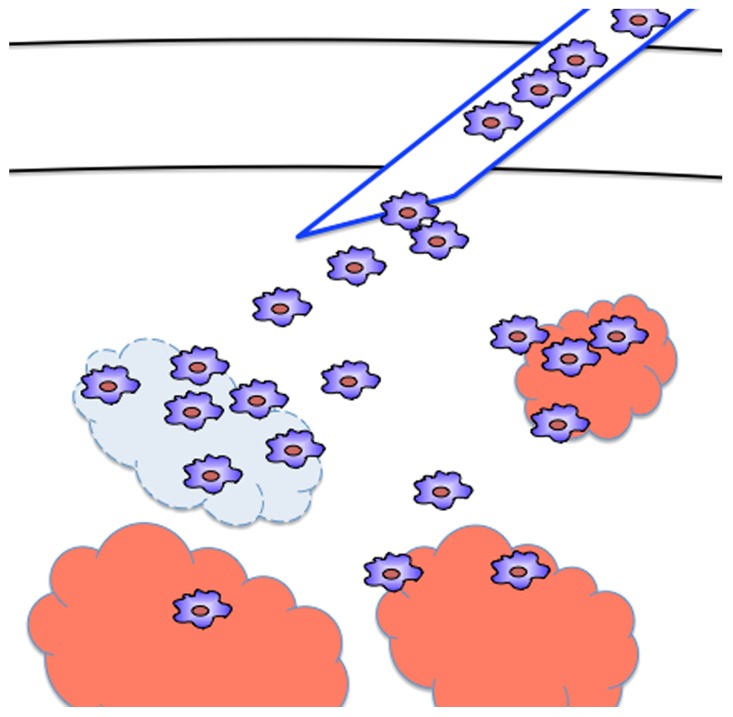
To circumvent such risk, we plan to use allogeneic iPS-ML lacking transporter associated with antigen presentation (TAP) for future clinical applications. TAP plays a key role in antigen-presentation by MHC class I molecules. In TAP-deficient cells, the expression levels of MHC class I molecules on the cell surface are very low. More importantly, the lack of TAP greatly reduces the complexity of peptides presented on MHC class I molecules. In line with these notions, we previously demonstrated that TAP-deficient cells evade recognition by most alloreactive CD8+ T cells (the major immune effector cells mediating acute rejection) upon transfer into allogeneic recipients.8 Nevertheless, alloreactive CD8+ T cells recognizing MHC class I-bound peptides presented via the TAP-independent pathway (mainly derived from signal peptides) may eventually eliminate the allogeneic TAP-deficient iPS-MCs. Based on these premises, we predict that TAP-deficient iPS-MCs would survive in the recipient for several days, allowing them to exert anticancer effects, but would then be eliminated by the recipient’s immune system. Thus, the administration of allogeneic, TAP-deficient iPS-MCs to cancer patients should be effective and safe. (Fig. 1)
Figure 1. Anticancer therapy with iPSC-derived macrophages producing interferon β. Inducible pluripotent stem cell (iPSC)-derived myeloid cells (iPS-MCs) infiltrate tumor tissues upon injection into cancer-bearing recipients. Interferon β (IFNβ)-expressing iPS-MCs secrete IFNβ within neoplastic lesions, hence causing disease regression.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Citation: Senju S, Koba C, Haruta M, Matsunaga Y, Matsumura K, Haga E, Sasaki Y, Ikeda T, Takamatsu K, Nishimura Y. Application of iPS cell-derived macrophages to cancer therapy. OncoImmunology 2014; 3:e27927; 10.4161/onci.27927
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/27927
References
- 1.Lewis CE, Pollard JW. Distinct role of macrophages in different tumor microenvironments. Cancer Res. 2006;66:605–12. doi: 10.1158/0008-5472.CAN-05-4005. [DOI] [PubMed] [Google Scholar]
- 2.Mantovani A, Schioppa T, Porta C, Allavena P, Sica A. Role of tumor-associated macrophages in tumor progression and invasion. Cancer Metastasis Rev. 2006;25:315–22. doi: 10.1007/s10555-006-9001-7. [DOI] [PubMed] [Google Scholar]
- 3.Andreesen R, Hennemann B, Krause SW. Adoptive immunotherapy of cancer using monocyte-derived macrophages: rationale, current status, and perspectives. J Leukoc Biol. 1998;64:419–26. doi: 10.1002/jlb.64.4.419. [DOI] [PubMed] [Google Scholar]
- 4.Choi KD, Vodyanik MA, Slukvin II. Generation of mature human myelomonocytic cells through expansion and differentiation of pluripotent stem cell-derived lin-CD34+CD43+CD45+ progenitors. J Clin Invest. 2009;119:2818–29. doi: 10.1172/JCI38591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Senju S, Haruta M, Matsumura K, Matsunaga Y, Fukushima S, Ikeda T, Takamatsu K, Irie A, Nishimura Y. Generation of dendritic cells and macrophages from human induced pluripotent stem cells aiming at cell therapy. Gene Ther. 2011;18:874–83. doi: 10.1038/gt.2011.22. [DOI] [PubMed] [Google Scholar]
- 6.Haruta M, Tomita Y, Yuno A, Matsumura K, Ikeda T, Takamatsu K, Haga E, Koba C, Nishimura Y, Senju S. TAP-deficient human iPS cell-derived myeloid cell lines as unlimited cell source for dendritic cell-like antigen-presenting cells. Gene Ther. 2013;20:504–13. doi: 10.1038/gt.2012.59. [DOI] [PubMed] [Google Scholar]
- 7.Koba C, Haruta M, Matsunaga Y, Matsumura K, Haga E, Sasaki Y, Ikeda T, Takamatsu K, Nishimura Y, Senju S. Therapeutic effect of human iPS-cell-derived myeloid cells expressing IFN-β against peritoneally disseminated cancer in xenograft models. PLoS One. 2013;8:e67567. doi: 10.1371/journal.pone.0067567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matsunaga Y, Fukuma D, Hirata S, Fukushima S, Haruta M, Ikeda T, Negishi I, Nishimura Y, Senju S. Activation of antigen-specific cytotoxic T lymphocytes by beta 2-microglobulin or TAP1 gene disruption and the introduction of recipient-matched MHC class I gene in allogeneic embryonic stem cell-derived dendritic cells. J Immunol. 2008;181:6635–43. doi: 10.4049/jimmunol.181.9.6635. [DOI] [PubMed] [Google Scholar]