Abstract
We have recently identified that the ligand of natural cytotoxicity triggering receptor 2 (NCR2, best known as NKp44) is expressed on a large panel of malignant cells. This ligand provides a new tool to investigate how stressed cells are recognized and eliminated by natural killer (NK) cells, and to develop novel immunotherapeutic paradigms against cancer.
Keywords: natural killer cells, MLL5, natural cytotoxicity receptors, immunotherapy, tumor cells, NKp44, NKp44L
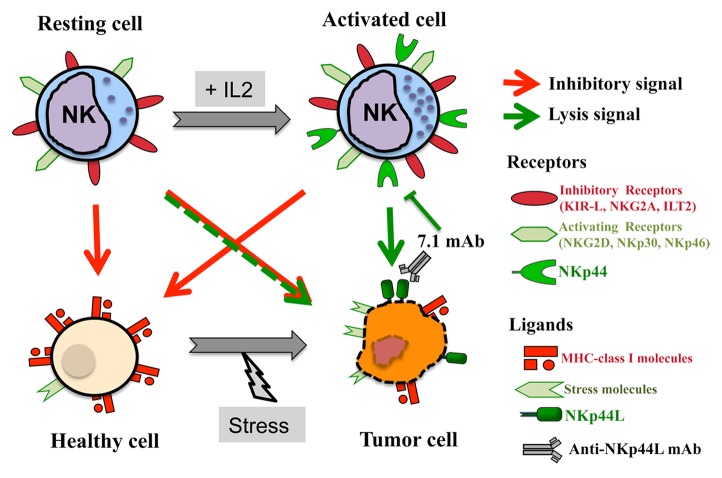
Natural killer (NK) cells play a key role in cancer immunosurveillance. In line with this notion, a robust cytotoxic activity in circulating NK cells has been associated with a reduced risk to develop cancer in subjects monitored over prolonged periods of time.1,2 A delicate balance between inhibitory and activating signals regulates the functions of NK cells. Inhibitory signals arise from the interaction between MCH class I molecules and various NK-cell receptors, including various members of the killer immunoglobulin-like receptor (KIR) family, killer cell lectin-like receptor subfamily C, member 1 (KLRC1, best known as NKG2A), leukocyte immunoglobulin-like receptor, subfamily B (with TM and ITIM domains), member 1 (LILRB1, best known as ILT-2). NK cells are also equipped with activatory receptors, including killer cell lectin-like receptor subfamily K, member 1 (KLRK1, best known as NKG2D), and several natural cytotoxicity receptors (i.e., NCR1, NCR2, and NCR3, best known as NKp46, NKp44, and NKp30, respectively), which are directly involved in the killing of transformed cells.2 Unlike NKp30 and NKp46, NKp44 it is not expressed by resting NK cells but only by their activated counterparts, which may partially explain the increased cytotoxicity of NK cells exposed to interleukin-2 (IL-2) (Fig. 1).3
Figure 1. NKp44-dependent NK cell-mediated cytotoxicity. The natural cytotoxicity receptor NKp44, which is only expressed by activated natural killer (NK) cells, recognizes NKp44L on the surface of tumor cells. The monoclonal antibody 7.1, which is specific for NKp44L, blocks the killing of NKp44L+ tumor cells by NKp44+ NK cells.
Whereas multiple NKG2D ligands were rapidly identified, including members of the MCH class I chain–related (MIC) and UL16-binding protein (ULBP) protein families, all of which are upregulated in response to cellular stress,4 the identity of NCR ligands remain poorly defined. In particular, NCRs have been shown to recognize a broad spectrum of microbe-derived molecules, but their endogenous ligands remain elusive. To date, NKp46 is an orphan receptor, whereas, 2 cellular ligands for NKp30 have been characterized, i.e., BCL2-associated athanogene 6 (BAG6, best known BAT3) and NCR3 ligand 1 (NCR3LG1, best known as B7-H6). B7-H6 is not expressed in steady-state conditions by normal tissues found on the surface of a broad panel of cancer cells.5 Recently, we identified a previously unknown gene that encodes a cellular ligand for NKp44, which we name NKp44L.6
NKp44L was initially cloned by the yeast 2-hybrid system, a powerful method to screen for unknown molecules based on protein-to-protein interactions. Sequencing data strongly supported that NKp44L is a new isoform of lysine-specific methyltransferase 2E (KMT2E, best known as MLL5). MLL5 was first discovered during the search for oncosuppressor genes that are relevant for myeloid leukemia from a commonly deleted 2.5-Mb fragment at chromosome 7q22.7 High expression levels of MLL5 have recently been associated with favorable outcome in acute myeloid leukemia patients.8 MLL5 shares with other members of the MLL/Trithorax gene family distinct SET (Su(var)3–9, Enhancer of Zeste, Trithorax) and PHD (Plant Homeo Domain) domains, which exert histone methyltransferase activity and mediate protein-to-protein interactions, respectively. MLL5 has been described as a nuclear protein involved in the regulation of the hematopoietic differentiation and stem cell self-renewal.8 Several MLL5 isoforms have been identified to date besides NKp44, including a short N-terminal variant specifically produced in human papillomavirus (HPV)16/18-associated cervical cancers.9
Strikingly, NKP44L is composed of 21 exons that encode an mRNA molecules with a very long 5′-UTR as compared with MLL5.7 Such characteristic is shared by many protooncogenes, suggesting that MLL5 and NKP44L use 2 different regulatory processes. This is also highlighted by their differential expression pattern in physiological conditions. Indeed, while MLL5 is ubiquitously expressed,7 NKp44L is virtually undetectable in healthy tissues,6 hence resembling many other ligands for activating NK receptors.4 The only exception to this trend is represent by the lung tissues, which appears to express limited amounts of NKp44L. This suggests that NKp44L is upregulated on stressed cells of the airway epithelium, perhaps representing a component of the pulmonary defense system, which is continually exposed to danger signals. Further studies on the expression and regulation of NKp44L in healthy tissues will be critical for a complete understanding of NKp44 functions in physiological settings.
The expression of ligands for activating NK receptors is currently considered as an indicator of a pathological scenario. In line with this notion, NKp44L can be detected on the surface of a large panel of transformed cell lines, including lymphoma, leukemia, melanoma, and carcinoma cells, as well as on primary tumor samples from individuals with kidney and bladder malignancies (Fig. 1). The mechanism(s) underlying the exposure of NKp44L on the surface of malignant cells remain(s) to be elucidated, yet our data demonstrate that this requires a specific C-terminal motif.6 Interestingly, like B7-H6 and several NKG2D ligands,5 NKp44L transmits a danger signal to activated NKp44+ NK cells, hence stimulating their cytotoxic activity. Such a cytotoxic response is unrelated to the expression of MHC class I molecules on target cells, but strongly linked to NKp44L levels, as it can be blocked by an anti-NKp44L monoclonal antibody (Fig. 1).6 Further investigation is required to determine NKp44L expression in a vast array of primary tumors at different stages in relationship with their sensitivity to NK cell-mediated cytotoxicity.
Consistent efforts are being dedicated at uncovering the molecular mechanism(s) that underlie(s) the expression of NKp44L. As a possibility, this may linked to cell cycle defects. Indeed, an NKG2D ligand has been involved in the control of the G1/S transition, which plays a central role in cell cycle entry.10 Given the preferential expression of NKp44L by malignant cells, it is worth exploring if this molecule is also implicated in alterations of the cell cycle.
As NKp44L is preferentially expressed by neoplastic cells, it is tempting to speculate that the ability of NK cells to target NKp44L may be exploited for the development of novel anticancer immunotherapeutic regimens. Approaches that are worth testing in this sense include the administration of anti-NKp44L monoclonal antibodies and the infusion of NKp44+ activated NK cells, both of which are expected to selectively target malignant tissues while sparing their healthy counterparts.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Citation: Vieillard V, Baychelier F, Debré P. NKp44L: A new tool for fighting cancer. OncoImmunology 2014; 3:e27988; 10.4161/onci.27988
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/27988
References
- 1.Imai K, Matsuyama S, Miyake S, Suga K, Nakachi K. Natural cytotoxic activity of peripheral-blood lymphocytes and cancer incidence: an 11-year follow-up study of a general population. Lancet. 2000;356:1795–9. doi: 10.1016/S0140-6736(00)03231-1. [DOI] [PubMed] [Google Scholar]
- 2.Vivier E, Ugolini S, Blaise D, Chabannon C, Brossay L. Targeting natural killer cells and natural killer T cells in cancer. Nat Rev Immunol. 2012;12:239–52. doi: 10.1038/nri3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vitale M, Bottino C, Sivori S, Sanseverino L, Castriconi R, Marcenaro E, Augugliaro R, Moretta L, Moretta A. NKp44, a novel triggering surface molecule specifically expressed by activated natural killer cells, is involved in non-major histocompatibility complex-restricted tumor cell lysis. J Exp Med. 1998;187:2065–72. doi: 10.1084/jem.187.12.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raulet DH, Gasser S, Gowen BG, Deng W, Jung H. Regulation of ligands for the NKG2D activating receptor. Annu Rev Immunol. 2013;31:413–41. doi: 10.1146/annurev-immunol-032712-095951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kruse PH, Matta J, Ugolini S, Vivier E. Natural cytotoxicity receptors and their ligands. Immunol Cell Biol. 2013 doi: 10.1038/icb.2013.98. In press. [DOI] [PubMed] [Google Scholar]
- 6.Baychelier F, Sennepin A, Ermonval M, Dorgham K, Debré P, Vieillard V. Identification of a cellular ligand for the natural cytotoxicity receptor NKp44. Blood. 2013;122:2935–42. doi: 10.1182/blood-2013-03-489054. [DOI] [PubMed] [Google Scholar]
- 7.Emerling BM, Bonifas J, Kratz CP, Donovan S, Taylor BR, Green ED, Le Beau MM, Shannon KM. MLL5, a homolog of Drosophila trithorax located within a segment of chromosome band 7q22 implicated in myeloid leukemia. Oncogene. 2002;21:4849–54. doi: 10.1038/sj.onc.1205615. [DOI] [PubMed] [Google Scholar]
- 8.Damm F, Oberacker T, Thol F, Surdziel E, Wagner K, Chaturvedi A, Morgan M, Bomm K, Göhring G, Lübbert M, et al. Prognostic importance of histone methyltransferase MLL5 expression in acute myeloid leukemia. J Clin Oncol. 2011;29:682–9. doi: 10.1200/JCO.2010.31.1118. [DOI] [PubMed] [Google Scholar]
- 9.Yew CW, Lee P, Chan WK, Lim VK, Tay SK, Tan TM, Deng LW. A novel MLL5 isoform that is essential to activate E6 and E7 transcription in HPV16/18-associated cervical cancers. Cancer Res. 2011;71:6696–707. doi: 10.1158/0008-5472.CAN-11-1271. [DOI] [PubMed] [Google Scholar]
- 10.Baychelier F, Vieillard V. The Modulation of the Cell-Cycle: A Sentinel to Alert the NK Cells of Dangers. Front Immunol. 2013;4:325. doi: 10.3389/fimmu.2013.00325. [DOI] [PMC free article] [PubMed] [Google Scholar]