Abstract
Plants are increasingly used as alternative expression hosts for the production of recombinant proteins offering many advantages including higher biomass and the ability to perform post-translational modifications on complex proteins. Key challenges for optimized accumulation of recombinant proteins in a plant system still remain, including endogenous plant proteolytic activity, which may severely compromise recombinant protein stability. Several strategies have recently been applied to improve protein stability by limiting protease action such as recombinant protein production in various sub-cellular compartments or application of protease inhibitors to limit protease action. A short update on the current strategies applied is provided here, with particular focus on sub-cellular sites previously selected for recombinant protein production and the co-expression of protease inhibitors to limit protease activity.
Keywords: protease inhibitors, proteases, protein stability, recombinant protein production in plants
Introduction
The world-wide demand for recombinant therapeutic and diagnostic proteins requires exploring plant-based protein expression platforms supplementing existing prokaryotic production systems.1 A number of valuable human recombinant proteins have already been successfully produced in plant-based systems,2-5 ranging from soil-grown plants to plant cells grown in a bioreactor. Cells may be used to transiently express the protein over a relatively short time period or be genetically engineered to stably express any recombinant protein. Plants offer the general advantage of a high plant biomass, the ability to perform post-translational modifications on complex proteins when passed through the secretory pathway, correctly folding and assembling complex proteins as well as being relatively safe due to the absence of human pathogens.6
Several approaches with various non-food or non-feed plant species, such as tobacco, are currently used for recombinant protein production. In particular, vacuum infiltration as well as infiltration of the tobacco leaf surface with Agrobacterium cells using a syringe were recently introduced as more effortless techniques for protein production. This avoids bio-safety concerns of genetically engineered transgenic plants and lengthy plant transformation and selection procedures. These two transient expression techniques are also better suited to satisfy any short-term demand for a recombinant protein with leaf harvest occurring within days.7,8 With these transient techniques the recombinant protein is expressed in leaves after infection with Agrobacterium cells carrying the protein coding sequence for the recombinant protein. An appropriate signal sequence may direct protein accumulation to a particular cellular compartment, which influences protein post-translational modifications and protein yield, depending on the resident proteases. Proteolysis may occur in planta or during protein extraction and harvesting, often requiring protease inhibitors to be added to the extraction buffer to improve protein stability and yield.9 However, this strategy is expensive and is seldom economically viable with regards to large scale extractions.
The purpose of this short review is to give an overview on the current knowledge of protease action on recombinant proteins produced in plants and to provide an update of some current strategies applied to improve recombinant protein stability in plant-based production systems.
Proteases Act on Recombinant Proteins
Protease abundance in plant tissues represents a severe burden to effective recombinant protein production.10 The degree of proteolysis, either partial or complete, depends on the amino acid sequence of the recombinant protein, susceptibility of sites to proteolytic action and also the number of protease-susceptible sites. Studies on plant proteases have advanced substantially and a more detailed understanding of the role of proteases, particularly in growth, development and pest resistance, is emerging. Hundreds of plant genes encode for proteins involved in proteolysis. In the model plant Arabidopsis, about 1900 genes involved in peptide bond hydrolysis have already been identified, but only a small number of proteases has so far been characterized, with the biological function of only around 40 proteases elucidated.11,12 Plants with larger genomes are likely to also have a higher number of proteases with highly polymorphic activity profiles in different plant species. Protease functions include assembling and disassembling proteins as well as removing damaged, mis-folded or potentially harmful proteins.13,14 Based on their active site residues for catalysis, most proteases can be distinguished as serine, cysteine, aspartic and metallo-types14 with serine proteases consisting of about 200 members, and the cysteine, aspartic and metallo-type proteases about 100 members in each class (http://merops.sanger.ac.uk).15
In Nicotiana species, often used for recombinant protein production, the majority of proteases are of aspartic or cysteine type (papain-like cysteine proteases) and to a lesser extent serine and metallo-type.16-18 When recombinantly expressing proteins in Nicotiana, the leaves of N. benthamiana is considered to contain lower protease activity compared with leaves of N. tabacum, consisting mostly of cathepsin L- and legumain-like cysteine proteases.19Table 1 outlines the type of proteases and their localizations so far identified in the different plant species previously used for recombinant protein production.
Table 1. Cellular locations for recombinant protein production in various plant host and types of proteases identified at these locations.
| Host | Protein | Compartment | Protease | References | |||
|---|---|---|---|---|---|---|---|
| Solanum tuberosum cv Desireé | Sea anemone equistatin | Secretory pathway, lytic vacuole, ER | Arginine/lysine-specific, legumain-type Asn-specific cysteine | 1 | |||
| Nicotiana tabacum cv Samsun NN | Monoclonal mouse IgG1 | Apoplast | Cysteine, aspartic | 2 | |||
| Nicotiana tabacum L. Cv. Samsun | Glutathione reductase | Cytosol | Cysteine | 3 | |||
| Solanum tuberosum plantlets, cv Kennebec | human α1-antichymotrypsin | Cytosol | Aspartic, serine | 4 | |||
| Oryza sativa L. cv Dongin | Human granulocyte–macrophage colony stimulating factor | Secretory pathway | Cysteine | 5 | |||
| Oryza sativa L. cv Dongin | Synthetic serine proteinase inhibitor II gene | Extracellular | Serine | 6 | |||
| Oryza sativa L. cv Dongin | Human granulocyte–macrophage colony stimulating factor | Extracellular | Serine | 7 | |||
| Solanum tuberosum L., cv Kennebec | Bovine aprotinin | Cytosol, ER, apoplast | Serine | 8 | |||
| Nicotiana tabacum cv BY-2 | Human α1-antichymotrypsin | ER, Golgi, apoplast, extracellular | Serine | 9 | |||
| Nicotiana tabacum L. | Oryzacystatin-1 | ER, chloroplast | Cysteine | 10 | |||
| Nicotiana tabacum (var. xanthi) | Human IgG1κ antibody | Apoplast | Cysteine, aspartic, serine | 11 , 12 | |||
| Solanum tuberosum L., cv Kennebec | Tomato cathepsin D inhibitor (CDI), Bovine aprotinin | Cytosol, ER | Serine | 13 | |||
| Nicotiana tabacum cv “81V9” | Spider dragline silk | ER | Serine | 14 | |||
| Solanum tuberosum L., cv Kennebec | Tomato cathepsin D inhibitor (SlCDI) | Cytosol | Aspartic, serine | 15 | |||
| Nicotiana tabacum | Monoclonal antibodies | Secretory pathway | Serine | 16 | |||
| Solanum lycopersium var PED | Human α1-proteinase inhibitor | ER, apoplast, vacuole, cytosol | Serine | 17 | |||
Selecting the Cellular Compartment for Recombinant Production
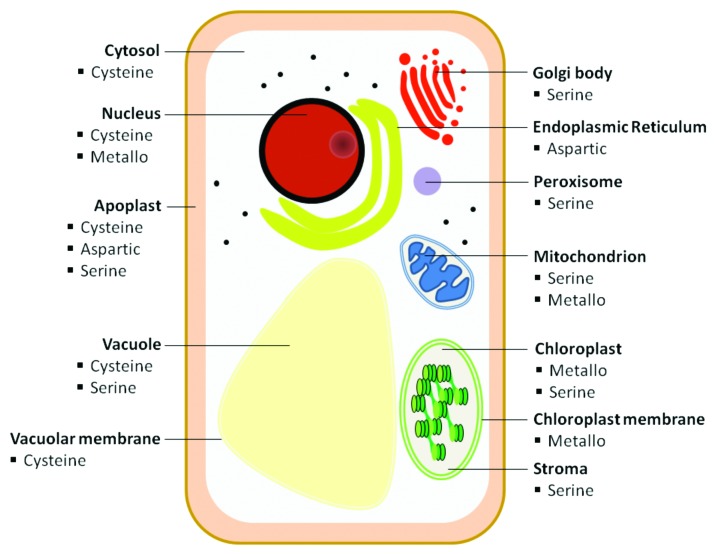
A cellular localization with limited proteolytic activity may be interesting for recombinant protein stability and ultimately yield. Protease activity is pH dependent and proteases therefore reside in different cellular compartments favorable for their respective activities. These enzymes are found in various cellular compartments including the cytosol, the vacuole, the chloroplast, the mitochondria and the lysosome.19-22Figure 1 provides an overview of the different classes of proteases active in the different compartments of a plant cell. A number of proteases are extracellular, residing in the apoplast to which recombinant proteins can be secreted. For secretion, proteins travel from the endoplasmic reticulum (ER) through the Golgi apparatus to the cell surface.
Figure 1. Protease locations within plant cell subcellular compartments.
The cytosol and the vacuoles
Undesired protein modifications changing protein structure folding may occur in the cytosol.23 The cytosolic ubiquitin-proteasome proteolytic pathway further degrades any improperly folded protein.24 Recombinant proteins are generally poorly accumulated when expressed in the cytosol,25 and this compartment is often regarded as unsuitable for effective recombinant protein production.
Lytic vacuoles are also unsuitable for recombinant protein deposition due to their high protease content. Protein storage vacuoles, abundant in seeds, are more suitable for protein accumulation. Targeting proteins to the storage vacuoles is achieved by a specific amino acids sequence, or sorting signal, within the primary sequence of the protein.26 An example of vacuolar accumulation of recombinant proteins achieved through vacuolar-targeting can be found in the case of dog gastric lipase produced in transgenic tobacco plants.27
The ER and the Golgi
The ER has been the production site for several recombinant proteins of industrial and pharmaceutical value. Directing a protein toward the ER results in greater protein yield and lower proteolysis when compared with the cytosol.10 The value of ER retention has been previously demonstrated where a ER retention signal increased human anti-HIV 2G12 levels in N. benthamiana plants.28 Higher expression of ER-retained proteins has also been demonstrated for a structural poly-protein, P1–2A, as well as for a 3C protease from FMDV serotype O when stably expressed in foliar tomato extract.29 In contrast, when a signal peptide such as the CTB signal peptide is absent, expression of the viral protein is not detectable possibly due to degradation within the cytoplasm during, or immediately after, synthesis.30 Proteins that are retained in the ER may also reside in protein bodies enhancing post-translational stability.31 Greater yield in the ER is very likely due to the action of chaperone proteins supporting proper protein folding.32 Folding and/or post-translational modification of recombinant proteins can however differ if post-translational processing occurs in the Golgi apparatus downstream of the ER.30 Recombinant proteins may be directed to this organelle via KDEL or HDEL signal peptides, however this may also result in undesired, structurally distinct, proteins due to non-native amino acid additions or non-authentic protein glycosylation patterns.32,33
The disadvantage of ER retention is the existence of ER proteolytic pathways acting on misfolded proteins. Misfolded proteins are in some cases re-translocated into the cytosol by the ER machinery for proteasomal degradation.34 Several classes of proteases act in the secretory pathway with pepsin-like (A1), papain-like (C1), trypsin chymotrypsin-like (S1), subtilisin-like (S8) and serine carboxypeptidase-like (S10) the most represented protease families.19 Unintended processing of recombinant proteins along this pathway by resident proteases has been reported by several research groups.35-37 Some examples include the systematic processing of mammalian antibodies and the partial trimming of the anti-inflammatory bovine aprotinin protein at the C-and N-termini when retained in the ER.37,38 Cleavage of the C-terminal region of the human α1-anti-chymotrypsin by intracellular and apoplastic proteases when targeted to the secretory pathway of BY-2 tobacco cells and subsequently detected in the culture medium is another example of unintended processing that may occur.36
The apoplast and the chloroplast
A number of recombinant proteins have been successfully expressed in the apoplast with expression of the human interleukin 6 in N. benthamiana recently reported as an excellent example.39 However, abundance and poor specificity of proteolytic enzymes in the apoplast is still a major obstacle.10,17,32,35 Intact bovine aprotinin was for instance detected in the apoplast of transgenic potato leaves, but final yields in planta were much lower when compared with retaining the protein in the ER. In the apoplast proteins are generally exposed to a large number of proteases. The apoplast of N. tabacum leaves primarily contains aspartic-, cysteine- and serine-type proteases17 while the apoplast in N. benthamiana leaves show preferential activity of aspartic- and serine-type proteases.19
A fairly new strategy to improve recombinant protein stability and achieve of higher yields is production in the chloroplast compartment of genetically engineered plants.40 Chloroplast engineering has several advantages including uniform protein expression rates, multiple copies of an integrated transgene and low gene silencing. High plastid number per cell and maternal inheritance of chloroplast DNA leading to minimal transgene escape are also among the advantages.40 Examples of chloroplast-based production of a recombinant protein include production of a cholera toxin B—pro-insulin fusion in transgenic lettuce and tobacco41 and production of the VP1 structural protein from the foot-and-mouth disease virus in tobacco chloroplasts.42 It was also recently reported that a chloroplast-derived vaccine candidate was stable at room temperature for 20 months.43 Despite this success, the inability to perform more complex post-translational modifications, such as glycosylation, or to perform protein subunit assembly and proper protein folding, are disadvantages in a chloroplast-based production system. In addition, endogenous proteases are present in the chloroplast that may compromise recombinant protein accumulation. For instance, high protein accumulation of the rotavirus VP6 protein was found in young tobacco leaves whereas in older leaves the amount of VP6 protein decreased possibly due to proteolytic degradation.44
Preventing Protease Action
Different strategies have been proposed to minimize unintended proteolysis in planta.10,32 Some strategies involve the targeting of recombinant proteins to specific cellular locations using peptide sorting signals45 or the addition of a stabilizing fusion partner to the protein of interest. Elastin-like peptide (ELP) fusions for instance improved the stability of a recombinant antibody and affinity-purified antibodies had kinetic binding parameters identical to an ELP-free antibody produced in Chinese hamster ovary cells.46
An interesting, relatively new strategy is to minimize proteolysis by co-expression of a recombinant “companion” protease inhibitor in a transgenic plant or in plants transiently expressing recombinant proteins.18 Recombinant protease inhibitors have been previously applied as anti-digestive compounds for crop protection against insect herbivory or pathogenic infection.47 Pleiotropic effects for these proteins have also been reported in planta causing altered growth characteristics and protection against abiotic stresses.47,48 Co-expression of a “companion” might also be an economically viable option to replace the costly addition of protease inhibitors during recombinant protein recovery and the protein purification process. Knowledge of individual characteristics of each recombinant protein and its sensitivity to proteases is, however, required to decide which inhibitor, or combination of inhibitors, might work best. Since serine protease activity is a major protease activity in plant cells, work has in the past been predominantly focused on serine protease inhibitors active against chymotrypsin and trypsin-like proteases. Co-expression and co-secretion of a soybean Bowman–Birk trypsin inhibitor stabilized recombinant antibodies secreted by transgenic tobacco roots.49 The introduction of a synthetic serine protease inhibitor II gene further decreased protease activity in transgenic rice callus, indicating its potential as a “companion” inhibitor for higher accumulation of a recombinant human granulocyte–macrophage colony stimulating factor.50 Human interleukin 2, a pharmaceutically important cytokine, was also found to be protected against proteolytic action when a trypsin inhibitor I and silk protease inhibitors were co-expressed in transgenic tobacco plants.51 The potential of a tomato cathepsin D inhibitor as an “in-built” stabilizing agent for recombinant proteins in situ and during the recovery process was further reported for transgenic potato plants.9,18 In addition, the in vivo expression of a tomato cathepsin D inhibitor (SlCDI) resulted in an increase in leaf protein content with transient expression of human AACT (α1-anti-chymotrypsin) significantly higher in transgenic lines expressing the SlCDI inhibitor.9 Co-expression of an aspartic/serine “companion” inhibitor also greatly increased leaf apoplast protein content with more murine diagnostic antibody (C5–1) co-secreted in the apoplast.19
There is still very limited knowledge on cysteine protease inhibitors as “companions” possibly due to observations that less activity has been found in plant systems for cysteine proteases when compared with serine proteases. In a first attempt to demonstrate potential of such strategy, co-expression of the rice cystatin OC-I decreased cysteine protease activity resulting in a stabilizing effect on isolated Rubisco.9 SlCYS9, an inhibitor of papain- and legumain-like cysteine proteases, had no impact on apoplast-based production, but stabilized the C5–1 antibody in planta, presumably upstream in the secretory pathway.19 In our group, we investigated the use of an “in-built” protein stabilizing agent in genetically engineered tobacco plants expressing OC-I in the cytosol.52 Constitutively expressing the rice cystatin in tobacco leaves lowered overall cysteine protease activity and increased the amounts produced of enzymatically active recombinant glutathione reductase, which was used as a model enzyme, when the enzyme was transiently produced in transgenic tobacco leaves after agroinfiltration.
Challenges Ahead
Proteolysis caused by plant endogenous proteases is still a key challenge severely compromising recombinant protein yield. However, there is a constant search for new production systems not only to ease purification but also to limit proteolysis. Targeting recombinant proteins either to oil bodies or roots for rhizo-secretion are recent attractive new strategies for easier purification as well as to limit proteolysis.53,54 The advantage of rhizo-secretion to leaf based-production is that after secretion the hydroponic culture medium has lower and less complex levels of proteolytic enzymes when compared with leaf extracts. Engineered carnivorous plants have also recently been suggested as production platform systems.55 Carnivorous plants express and transport digestive enzymes into the traps, where any enzymes would be directly accessible for purification in a viscous and sticky liquid without plant destruction allowing continuous harvest. The limitation of this system is the presence of proteases in the juice which might affect recombinant protein stability. However, all these recent new technologies, although interesting, have so far resulted in insufficient protein yields to be considered commercially viable and the search for new innovative production systems for stable recombinant protein production should therefore be an ongoing activity.
Searching for plant species with low proteolytic activity providing better recombinant protein stability should also be relevant in future activities. In comparison to prokaryotic systems, protease-deficient mutant plants do not exist for plant species currently used for recombinant production. More intense screening of plant species useful for recombinant protein production for low protease activity is therefore urgently required. This is in addition to detailed studies on identifying plant endogenous proteases and investigating their expression profiles in various cellular locations. Better knowledge of protease involvement in senescence processes might be particularly helpful to improve protein stability in any transient expression system, as almost all protease families have been associated with some aspects of plant senescence.56 There is evidence that leaf infiltration with Agrobacterium cells causes leaf senescence resulting in the expression of senescence-related proteases including cysteine proteases.52 Senescence, the final developmental stage of every plant organ, leads to cell death and senescence-associated proteolysis naturally enables the remobilization of nutrients but might also degrade recombinant proteins
Strategies for protein stabilization might also include application of inducible promoters for induced synthesis of inhibitors or induced downregulation of proteases. In this regard, antisense or RNA silencing approaches could be of interest to contain proteolysis in the plant host. Indeed, first evidence that such a strategy might be successful has been recently demonstrated with rice cells where application of the RNA interference technology using a gene to express ihpRNA of α-amylase and cysteine protease resulted in a 2.4-fold increase in the production of human granulocyte-macrophage colony-stimulating factor (hGM-CSF) after downregulation of cysteine protease expression.57 However, identification of particular proteases involved in recombinant protein degradation is required to avoid any protease involved in vital cellular processes required for growth and development from being targeted.
Future research might also focus on identifying the specific inhibitors of proteases involved in recombinant protein degradation. A better understanding of the exact nature of these inhibitors would allow the design of more active inhibitors. Design by amino acid mutagenesis would optimize their inhibitory activity for application as a “companion” protease inhibitor in either transient or stable expression of a recombinant protein via genetically engineered plants where proteins and the inhibitor are targeted to cellular compartments high in protein production.19 Application of transgenic plant material expressing a recombinant protein in addition to co-expressing a “companion” protease inhibitor is, however, rather complex due to pleiotropic effects caused by inhibitor expression affecting plant growth and development.48 There is so far only limited knowledge about these protease inhibitor actions. Such a strategy might also be problematic when inhibition involves targeting proteases in the secretory pathway.38 Although such an inhibitor approach might be a major obstacle in a transgenic plant/seed approach, this might possibly be less problematic in short-term transient expression of a recombinant protein, which lasts only a few days.
Recent genomic and proteomic approaches have allowed the large-scale identification of proteases and the elucidation of their particular roles in cellular metabolism. This expanding knowledge will certainly help, in forthcoming years, to develop new techniques for high-throughput analysis of protease activity and identification of target proteins. This will also advance our knowledge on recombinant protein stability and application of this knowledge in the future will be critical to significantly improving plant-based recombinant protein production.
Disclosure of Potential Conflicts of Interest
No potential conflict of interest was disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/bioe/article/25158
References
- 1.Yin J, Li G, Ren X, Herrler G. Select what you need: a comparative evaluation of the advantages and limitations of frequently used expression systems for foreign genes. J Biotechnol. 2007;127:335–47. doi: 10.1016/j.jbiotec.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 2.Ma JK-C, Barros E, Bock R, Christou P, Dale PJ, Dix PJ, et al. European Union Framework 6 Pharma-Planta Consortium Molecular farming for new drugs and vaccines. Current perspectives on the production of pharmaceuticals in transgenic plants. EMBO Rep. 2005;6:593–9. doi: 10.1038/sj.embor.7400470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Daniell H, Singh ND, Mason H, Streatfield SJ. Plant-made vaccine antigens and biopharmaceuticals. Trends Plant Sci. 2009;14:669–79. doi: 10.1016/j.tplants.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Muynck B, Navarre C, Boutry M. Production of antibodies in plants: status after twenty years. Plant Biotechnol J. 2010;8:529–63. doi: 10.1111/j.1467-7652.2009.00494.x. [DOI] [PubMed] [Google Scholar]
- 5.Rybicki EP. Plant-made vaccines for humans and animals. Plant Biotechnol J. 2010;8:620–37. doi: 10.1111/j.1467-7652.2010.00507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karg SR, Kallio PT. The production of biopharmaceuticals in plant systems. Biotechnol Adv. 2009;27:879–94. doi: 10.1016/j.biotechadv.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 7.Simmons CW, VanderGheynst JS, Upadhyaya SK. A model of Agrobacterium tumefaciens vacuum infiltration into harvested leaf tissue and subsequent in planta transgene transient expression. Biotechnol Bioeng. 2009;102:965–70. doi: 10.1002/bit.22118. [DOI] [PubMed] [Google Scholar]
- 8.D'Aoust M-A, Lavoie P-O, Belles-Isles J, Bechtold N, Martel M, Vézina L-P. Transient expression of antibodies in plants using syringe agroinfiltration. Recombinant Proteins From Plants, 2009:41-50. [DOI] [PubMed] [Google Scholar]
- 9.Rivard D, Anguenot R, Brunelle F, Le VQ, Vézina L-P, Trépanier S, et al. An in-built proteinase inhibitor system for the protection of recombinant proteins recovered from transgenic plants. Plant Biotechnol J. 2006;4:359–68. doi: 10.1111/j.1467-7652.2006.00187.x. [DOI] [PubMed] [Google Scholar]
- 10.Benchabane M, Goulet C, Rivard D, Faye L, Gomord V, Michaud D. Preventing unintended proteolysis in plant protein biofactories. Plant Biotechnol J. 2008;6:633–48. doi: 10.1111/j.1467-7652.2008.00344.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schaller A. A cut above the rest: the regulatory function of plant proteases. Planta. 2004;220:183–97. doi: 10.1007/s00425-004-1407-2. [DOI] [PubMed] [Google Scholar]
- 12.van der Hoorn RAL. Plant proteases: from phenotypes to molecular mechanisms. Annu Rev Plant Biol. 2008;59:191–223. doi: 10.1146/annurev.arplant.59.032607.092835. [DOI] [PubMed] [Google Scholar]
- 13.Vierstra RD. Proteolysis in plants: mechanisms and functions. Plant Mol Biol. 1996;32:275–302. doi: 10.1007/BF00039386. [DOI] [PubMed] [Google Scholar]
- 14.Powers JC, Asgian JL, Ekici OD, James KE. Irreversible inhibitors of serine, cysteine, and threonine proteases. Chem Rev. 2002;102:4639–750. doi: 10.1021/cr010182v. [DOI] [PubMed] [Google Scholar]
- 15.Rawlings ND, Barrett AJ, Bateman A. MEROPS: the database of proteolytic enzymes, their substrates and inhibitors. Nucleic Acids Res. 2012;40(Database issue):D343–50. doi: 10.1093/nar/gkr987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beyene G, Foyer CH, Kunert KJ. Two new cysteine proteinases with specific expression patterns in mature and senescent tobacco (Nicotiana tabacum L.) leaves. J Exp Bot. 2006;57:1431–43. doi: 10.1093/jxb/erj123. [DOI] [PubMed] [Google Scholar]
- 17.Delannoy M, Alves G, Vertommen D, Ma J, Boutry M, Navarre C. Identification of peptidases in Nicotiana tabacum leaf intercellular fluid. Proteomics. 2008;8:2285–98. doi: 10.1002/pmic.200700507. [DOI] [PubMed] [Google Scholar]
- 18.Goulet C, Goulet C, Goulet M-C, Michaud D. 2-DE proteome maps for the leaf apoplast of Nicotiana benthamiana. Proteomics. 2010;10:2536–44. doi: 10.1002/pmic.200900382. [DOI] [PubMed] [Google Scholar]
- 19.Goulet C, Khalf M, Sainsbury F, D’Aoust MA, Michaud D. A protease activity-depleted environment for heterologous proteins migrating towards the leaf cell apoplast. Plant Biotechnol J. 2012;10:83–94. doi: 10.1111/j.1467-7652.2011.00643.x. [DOI] [PubMed] [Google Scholar]
- 20.Callis J. Regulation of Protein Degradation. Plant Cell. 1995;7:845–57. doi: 10.1105/tpc.7.7.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adam Z, Adamska I, Nakabayashi K, Ostersetzer O, Haussuhl K, Manuell A, et al. Chloroplast and mitochondrial proteases in Arabidopsis. A proposed nomenclature. Plant Physiol. 2001;125:1912–8. doi: 10.1104/pp.125.4.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chi W, Sun X, Zhang L. The roles of chloroplast proteases in the biogenesis and maintenance of photosystem II. Biochim Biophys Acta. 2012;1817:239–46. doi: 10.1016/j.bbabio.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 23.Faye L, Boulaflous A, Benchabane M, Gomord V, Michaud D. Protein modifications in the plant secretory pathway: current status and practical implications in molecular pharming. Vaccine. 2005;23:1770–8. doi: 10.1016/j.vaccine.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 24.Smalle J, Vierstra RD. The ubiquitin 26S proteasome proteolytic pathway. Annu Rev Plant Biol. 2004;55:555–90. doi: 10.1146/annurev.arplant.55.031903.141801. [DOI] [PubMed] [Google Scholar]
- 25.Conrad U, Fiedler U. Compartment-specific accumulation of recombinant immunoglobulins in plant cells: an essential tool for antibody production and immunomodulation of physiological functions and pathogen activity. Plant Mol Biol. 1998;38:101–9. doi: 10.1023/A:1006029617949. [DOI] [PubMed] [Google Scholar]
- 26.Stoger E, Ma JKC, Fischer R, Christou P. Sowing the seeds of success: pharmaceutical proteins from plants. Curr Opin Biotechnol. 2005;16:167–73. doi: 10.1016/j.copbio.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 27.Gruber V, Berna PP, Arnaud T, Bournat P, Clément C, Mison D, et al. Large-scale production of a therapeutic protein in transgenic tobacco plants: effect of subcellular targeting on quality of a recombinant dog gastric lipase. Mol Breed. 2001;7:329–40. doi: 10.1023/A:1011653220724. [DOI] [Google Scholar]
- 28.Sainsbury F, Lomonossoff GP. Extremely high-level and rapid transient protein production in plants without the use of viral replication. Plant Physiol. 2008;148:1212–8. doi: 10.1104/pp.108.126284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pan L, Zhang Y, Wang Y, Wang B, Wang W, Fang Y, et al. Foliar extracts from transgenic tomato plants expressing the structural polyprotein, P1-2A, and protease, 3C, from foot-and-mouth disease virus elicit a protective response in guinea pigs. Vet Immunol Immunopathol. 2008;121:83–90. doi: 10.1016/j.vetimm.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 30.Mikschofsky H, Schirrmeier H, Keil GM, Lange B, Polowick PL, Keller W, et al. Pea-derived vaccines demonstrate high immunogenicity and protection in rabbits against rabbit haemorrhagic disease virus. Plant Biotechnol J. 2009;7:537–49. doi: 10.1111/j.1467-7652.2009.00422.x. [DOI] [PubMed] [Google Scholar]
- 31.Pueyo JJ, Chrispeels MJ, Herman EM. Degradation of transport-competent destabilized phaseolin with a signal for retention in the endoplasmic reticulum occurs in the vacuole. Planta. 1995;196:586–96. doi: 10.1007/BF00203660. [DOI] [PubMed] [Google Scholar]
- 32.Doran PM. Foreign protein degradation and instability in plants and plant tissue cultures. Trends Biotechnol. 2006;24:426–32. doi: 10.1016/j.tibtech.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 33.Sethuraman N, Stadheim TA. Challenges in therapeutic glycoprotein production. Curr Opin Biotechnol. 2006;17:341–6. doi: 10.1016/j.copbio.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 34.Gomord V, Fitchette A-C, Menu-Bouaouiche L, Saint-Jore-Dupas C, Plasson C, Michaud D, et al. Plant-specific glycosylation patterns in the context of therapeutic protein production. Plant Biotechnol J. 2010;8:564–87. doi: 10.1111/j.1467-7652.2009.00497.x. [DOI] [PubMed] [Google Scholar]
- 35.Schiermeyer A, Schinkel H, Apel S, Fischer R, Schillberg S. Production of Desmodus rotundus salivary plasminogen activator α1 (DSPAalpha1) in tobacco is hampered by proteolysis. Biotechnol Bioeng. 2005;89:848–58. doi: 10.1002/bit.20410. [DOI] [PubMed] [Google Scholar]
- 36.Benchabane M, Saint-Jore-Dupas C, Bardor M, Faye L, Michaud D, Gomord V. Targeting and post-translational processing of human α1-antichymotrypsin in BY-2 tobacco cultured cells. Plant Biotechnol J. 2009;7:146–60. doi: 10.1111/j.1467-7652.2008.00382.x. [DOI] [PubMed] [Google Scholar]
- 37.De Muynck B, Navarre C, Nizet Y, Stadlmann J, Boutry M. Different subcellular localization and glycosylation for a functional antibody expressed in Nicotiana tabacum plants and suspension cells. Transgenic Res. 2009;18:467–82. doi: 10.1007/s11248-008-9240-1. [DOI] [PubMed] [Google Scholar]
- 38.Badri MA, Rivard D, Coenen K, Michaud D. Unintended molecular interactions in transgenic plants expressing clinically useful proteins: the case of bovine aprotinin traveling the potato leaf cell secretory pathway. Proteomics. 2009;9:746–56. doi: 10.1002/pmic.200700234. [DOI] [PubMed] [Google Scholar]
- 39.Nausch H, Mikschofsky H, Koslowski R, Meyer U, Broer I, Huckauf J. High-level transient expression of ER-targeted human interleukin 6 in Nicotiana benthamiana. PLoS One. 2012;7:e48938. doi: 10.1371/journal.pone.0048938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Daniell H. Production of biopharmaceuticals and vaccines in plants via the chloroplast genome. Biotechnol J. 2006;1:1071–9. doi: 10.1002/biot.200600145. [DOI] [PubMed] [Google Scholar]
- 41.Ruhlman T, Ahangari R, Devine A, Samsam M, Daniell H. Expression of cholera toxin B-proinsulin fusion protein in lettuce and tobacco chloroplasts--oral administration protects against development of insulitis in non-obese diabetic mice. Plant Biotechnol J. 2007;5:495–510. doi: 10.1111/j.1467-7652.2007.00259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lentz EM, Segretin ME, Morgenfeld MM, Wirth SA, Dus Santos MJ, Mozgovoj MV, et al. High expression level of a foot and mouth disease virus epitope in tobacco transplastomic plants. Planta. 2010;231:387–95. doi: 10.1007/s00425-009-1058-4. [DOI] [PubMed] [Google Scholar]
- 43.Dreesen IA, Charpin-El Hamri G, Fussenegger M. Heat-stable oral alga-based vaccine protects mice from Staphylococcus aureus infection. J Biotechnol. 2010;145:273–80. doi: 10.1016/j.jbiotec.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 44.Birch-Machin I, Newell CA, Hibberd JM, Gray JC. Accumulation of rotavirus VP6 protein in chloroplasts of transplastomic tobacco is limited by protein stability. Plant Biotechnol J. 2004;2:261–70. doi: 10.1111/j.1467-7652.2004.00072.x. [DOI] [PubMed] [Google Scholar]
- 45.Conley AJ, Joensuu JJ, Menassa R, Brandle JE. Induction of protein body formation in plant leaves by elastin-like polypeptide fusions. BMC Biol. 2009;7:48. doi: 10.1186/1741-7007-7-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Floss DM, Sack M, Arcalis E, Stadlmann J, Quendler H, Rademacher T, et al. Influence of elastin-like peptide fusions on the quantity and quality of a tobacco-derived human immunodeficiency virus-neutralizing antibody. Plant Biotechnol J. 2009;7:899–913. doi: 10.1111/j.1467-7652.2009.00452.x. [DOI] [PubMed] [Google Scholar]
- 47.Schlüter U, Benchabane M, Munger A, Kiggundu A, Vorster J, Goulet M-C, et al. Recombinant protease inhibitors for herbivore pest control: a multitrophic perspective. J Exp Bot. 2010;61:4169–83. doi: 10.1093/jxb/erq166. [DOI] [PubMed] [Google Scholar]
- 48.Van der Vyver C, Schneidereit J, Driscoll S, Turner J, Kunert K, Foyer CH. Oryzacystatin I expression in transformed tobacco produces a conditional growth phenotype and enhances chilling tolerance. Plant Biotechnol J. 2003;1:101–12. doi: 10.1046/j.1467-7652.2003.00010.x. [DOI] [PubMed] [Google Scholar]
- 49.Komarnytsky S, Borisjuk N, Yakoby N, Garvey A, Raskin I. Cosecretion of protease inhibitor stabilizes antibodies produced by plant roots. Plant Physiol. 2006;141:1185–93. doi: 10.1104/pp.105.074419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim TG, Lee HJ, Jang YS, Shin YJ, Kwon TH, Yang MS. Co-expression of proteinase inhibitor enhances recombinant human granulocyte-macrophage colony stimulating factor production in transgenic rice cell suspension culture. Protein Expr Purif. 2008;61:117–21. doi: 10.1016/j.pep.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 51.Redkiewicz P, Więsyk A, Góra-Sochacka A, Sirko A. Transgenic tobacco plants as production platform for biologically active human interleukin 2 and its fusion with proteinase inhibitors. Plant Biotechnol J. 2012;10:806–14. doi: 10.1111/j.1467-7652.2012.00698.x. [DOI] [PubMed] [Google Scholar]
- 52.Pillay P, Kibido T, du Plessis M, van der Vyver C, Beyene G, Vorster BJ, et al. Use of transgenic oryzacystatin-I-expressing plants enhances recombinant protein production. Appl Biochem Biotechnol. 2012;168:1608–20. doi: 10.1007/s12010-012-9882-6. [DOI] [PubMed] [Google Scholar]
- 53.Nykiforuk CL, Shen Y, Murray EW, Boothe JG, Busseuil D, Rhéaume E, et al. Expression and recovery of biologically active recombinant Apolipoprotein AI(Milano) from transgenic safflower (Carthamus tinctorius) seeds. Plant Biotechnol J. 2011;9:250–63. doi: 10.1111/j.1467-7652.2010.00546.x. [DOI] [PubMed] [Google Scholar]
- 54.Drake PMW, Barbi T, Sexton A, McGowan E, Stadlmann J, Navarre C, et al. Development of rhizosecretion as a production system for recombinant proteins from hydroponic cultivated tobacco. FASEB J. 2009;23:3581–9. doi: 10.1096/fj.09-131771. [DOI] [PubMed] [Google Scholar]
- 55.Biteau F, Bourgaud F, Gontier E, Fevre J-P. Process for the production of recombinant proteins using carnivorous plants. WO Patent 2,008,040,599, 2008.
- 56.Roberts IN, Caputo C, Criado MV, Funk C. Senescence-associated proteases in plants. Physiol Plant. 2012;145:130–9. doi: 10.1111/j.1399-3054.2012.01574.x. [DOI] [PubMed] [Google Scholar]
- 57.Kim N-S, Jang S-H, Yu H-Y, Chung N-D, Kwon T-H, Yang M-S, et al. Amylase and cysteine proteinase gene knockdown in rice cells using RNA interference for enhancing production of recombinant proteins. Plant Cell Tissue Organ Cult. 2013 doi: 10.1007/s11240-013-0309-z. [DOI] [Google Scholar]