Abstract
Consequential to its essential role as a mechanical support and affinity regulator in extracellular matrices, collagen constitutes a highly sought after scaffolding material for regeneration and healing applications. However, substantiated concerns have been raised with regard to quality and safety of animal tissue-extracted collagen, particularly in relation to its immunogenicity, risk of disease transmission and overall quality and consistency. In parallel, contamination with undesirable cellular factors can significantly impair its bioactivity, vis-a-vis its impact on cell recruitment, proliferation and differentiation. High-scale production of recombinant human collagen Type I (rhCOL1) in the tobacco plant provides a source of an homogenic, heterotrimeric, thermally stable “virgin” collagen which self assembles to fine homogenous fibrils displaying intact binding sites and has been applied to form numerous functional scaffolds for tissue engineering and regenerative medicine. In addition, rhCOL1 can form liquid crystal structures, yielding a well-organized and mechanically strong membrane, two properties indispensable to extracellular matrix (ECM) mimicry. Overall, the shortcomings of animal- and cadaver-derived collagens arising from their source diversity and recycled nature are fully overcome in the plant setting, constituting a collagen source ideal for tissue engineering and regenerative medicine applications.
Keywords: bovine, collagen, immunogenicity, rhCOL1, safety, scaffold, tobacco
Collagen, the most abundant macromolecule of the extracellular matrix (ECM) and connective tissue, is intimately involved in tissue development, remodeling and repair, by providing essential architectural support and affinity-driven regulation of key signaling cascades. A deficient or defective collagen supply gives rise to a variety of severe hereditary ECM disorders, affecting the vasculature, bone, tendons, gums, hair and skin. Type I collagen is a heterotrimeric protein, composed of two α1 and one α2 polypeptide chains, encoded by the COL1A1 and COL1A2 genes, which are regulated by cytokines and signal transducers and activators of transcription (STATs). The collagen protein undergoes extensive post-translational modifications, before reaching its fully mature and functional state, which require the orchestrated activities of a variety of enzymes, including collagen-specific hydroxylases, glycosyl-transferases, proteinases and one oxidase. The activity of the prolyl 4-hydroxylase (P4H) complex directs the helical conformation of collagen, which dictates the protein's final thermal stability and viability. In parallel, lysine hydroxylation, driven by the lysyl hydroxylases differentially expressed in tissues (LH1–3), plays a central role in collagen fibrillogenesis, fibril stabilization,1 supramolecular aggregation2 and ECM mineralization.3
For centuries, collagen has served as the central element of a myriad of medical and cosmetic products, which harness its biological role in tissue structure and repair processes. Its benefits have been exploited in the design of biomaterials such as wound dressings, dermal patches and fillers, bone and tendon substitutes, and engineered tissues, which can be impregnated with exogenous growth factors, drugs, and/or cells. Its function as an integral scaffolding component of the ECM has made it a prime source material in tissue fabrication, which has brought to marked functional restoration to injured tissues.4 Such tissue-specific patches have proven particularly advantageous in support of the embedded cells, during both pre- and postimplantation stages, and release cues that promote cell infiltration and vascularization in vivo.5 Its close mimicry of natural environs and dynamic crosstalk with its surroundings enables generation of niches tailored to highly specific applications, such as regeneration of cardiac or bone tissue.4,6 More specifically, the clinical prospects of processes, such as autologous stem cell transplantations6 or cartilage repair,4 promise to be significantly advanced by the integration of collagen-based biomaterials. Moreover, as a fibroblast attractant, introduction of collagen scaffolds elicits further collagen deposition at the site of treatment,7 triggering a positive feedback loop accelerating regeneration and repair. Due to its considerable biocompatibility, collagen-based products are generally viewed as safe for application, injection, implantation and oral ingestion, while its biodegradability obviates the need for device extraction.
Traditionally, commercial supplies of collagen are extracted from scarce cadaver sources, or from animal sources, where the latter has been reported to evoke both cellular and humoral immune responses in 3–10% of treated patients.8 While these self-limiting reactions generally resolve within a few months, premature resorption and impaired implant function have been observed in association with these adverse responses, which were generally treated with immunosuppressants.9,10 Thus, collagen-based products are explicitly contraindicated in patients demonstrating hypersensitivity to either collagen or to other bovine products or meat. The prevailing pretreatment protocol requires a skin test, where repeat testing, 2–4 weeks after initial testing, is advocated by many practitioners, in efforts to identify the 1–2% of the population which acquires allergy upon subsequent exposure to collagen,11 limiting its use in emergency treatments. In addition to its immunogenicity, animal-derived collagen-based biomaterials bear a risk of prion transmission to human cells.12 In efforts to sidestep these threats, regulatory authorities have limited bovine extraction to either closed herds or herds raised in countries where bovine spongiform encephalopathy (BSE) has never been reported.
While use of human-derived collagens overcomes a number of the drawbacks associated with bovine or porcine-derived collagen, the age, environmental setting, ethnicity and genotypes of the source tissue, dictate the biophysical profile of the derived collagen, yielding marked variability in sample quality. With age, collagen networks undergo intermolecular crosslinking, which has been thought to underlie loss of its osmotic swelling capacities, manifested by reduced elasticity13 and acid solubility,14 and increased resistance to collagenase. In addition, extraction processes introduce undesirable inter- and intramolecular crosslinks within the collagen, where close to 30% of the total collagen weight can be comprised of molecular structures other than the α and β subunits.23 The resulting material demonstrates suboptimal solubility and capacity to assemble into highly structured scaffolds.
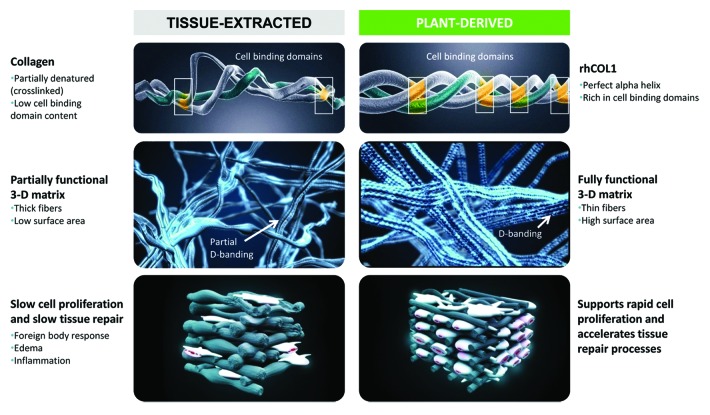
These limitations, alongside the expanding need for collagen-based therapeutics, have spawned the development of tobacco plant-based expression of recombinant human collagen (rhCOL1).24,25 The platform drives simultaneous vacuole-targeted expression of procollagen, P4H-α, P4H-β and LH3, empowering post-translational modifications critical to collagen maturation, formation of a triple helix structure, and self-assembly of thermally stable, densely packed fibrils. The extracted protein is a pure, heterotrimeric atellocollagen, free of high molecular weight contaminants and decorated with a degree of hydroxylated proline and lysine residues similar to that of human collagen.23 The resulting “virgin” collagen assembles into a 3D structure with a preserved native α-helical structure, sharply contrasting that of the tissue-extracted collagen structure, a high surface area, integrin binding sites and close-to-perfect “shape memory.” Moreover, rhCOL1 fibrils exhibit D-band striations, characteristic of natural collagen fibers (Fig. 1), which play a critical role in the mechanical properties and biofunctionality of collagen. Furthermore, the resulting protein is highly hydrophilic (contact angle of absorption = 0; time of absorption: microseconds), with profound water retaining capacities. This versatile expression system produces considerable yields of highly uniform collagen batches, with physicochemical properties identical to those of its natural “virgin” counterpart, while fully abrogating concerns of antigenicity, immunogenicity and disease transmission.
Figure 1. A schematic presentation of the key differences between tissue-extracted vs. plant-derived human collagen. Plant-derived human collagen Type I (rhCOL1) exhibits a natural α-helical structure, with natural cell binding domains, while tissue-extracted collagen is stripped of many of its clinically relevant binding domains, alongside introduction of unwarranted crosslinks, which impact the protein structure and function. Plant-derived human collagen forms functional 3D matrices, surpassing the capacities of their tissue-extracted counterparts. rhCOL1 provides an optimal medium for cell proliferation and accelerated wound healing, with no concerns of antigenicity and immunogenicity.
Assessment of rhCOL1 scaffolding properties was performed by fabricating electrospun or freeze-dried rhCOL1-based vs. bovine collagen-based scaffolds and sponges.26 rhCOL1 processing proved simpler and faster (20 min vs. 48 h) and produced rounder fibers of more uniform diameter, when compared with bovine collagen, at concentrations > 12% (w/v). In addition, its biocompatibility surpassed that of bovine collagen scaffolds, as manifested by enhanced proliferation of primary cells and approximately 2-fold lower IL-1 secretion upon macrophage exposure to the scaffold. rhCOL1 scaffolds embedded with primary human dermal fibroblasts and primary human epidermal keratinocytes, supported epidermal differentiation and eventual formation of engineered skin, which reached normal values of human capacitance within 21 d in culture. Its capacity to facilitate wound healing processes was evaluated in a comparative study of the performance of an rhCOL1-based gel (VergenixTMFG) to that of a commercial flowable bovine collagen- or human cadaver-based gel applied to full-thickness cutaneous wounds in rats. VergenixTMFG initiated a more rapid healing process, where wound size contracted within 24 h of treatment and 66% closure was observed within 6 d of VergenixTMFG application, in parallel to enhanced reepitheliazation of the wound site.27 Similar results were reported in a porcine full-thickness wound model, where VergenixTMFG brought to 95% wound closure within 21 d, in comparison to the 68% closure achieved with the reference flowable bovine collagen gel product.27
The homogenous plant-derived rhCOL1 has also proven a reliable source material for generation of highly ordered collagen surfaces, a feature essential to the mechanically supportive role of load-bearing tissues. Optimal organizational hierarchy and collagen fiber alignment is essential when attempting to closely mimic natural microenvironments, in which tensile strength and elasticity provide both mechanical and chemical cues to proximal cells. When prepared beyond a certain concentration threshold and under specific pH conditions, rhCOL1 enters a liquid crystalline phase, which can be easily manipulated to generate highly ordered structures. Shear force-driven alignment of “virgin” rhCOL1 generated long, densely packed, uniform and parallel aligned, D-banded fibers, resembling the structure and morphology of natural tendon.28 Similarly, membranes formed under shear force featured orderly, crimp-like organization of parallel, well separated fibrils. Exploitation of this unique physical phase can considerably advance the clinical relevance of engineered tissue samples, by significantly upgrading their mechanophysical properties to better simulate those of tissues they are designed to replace.
Thus, the tobacco-based biofactory does not merely provide a low-cost and highly scalable source of collagen, but also provides a safer and functional protein, most closely mimicking its natural counterpart (Table 1 and Fig. 1), while capitalizing on the nature of is “virginity.” Judicious exploitation of the unique features of rhCOL1 can set the stage for focused design of tissue-specific specimens applicable in a broad spectrum of medical and pharmaceutical disciplines.
Table 1. Key physical and biological features of rhCOL1.
| Feature | Advantage | Benefit |
|---|---|---|
| Human amino acid sequence | • Non-immunogenic | • Improved biocompatibility |
| Triple helix structure | • Increased stability • Densely packed fibers • D-band striation • Improved cell adhesion and cell proliferation |
• Accelerated healing • Improved scaffold mechanical properties |
| Non-crosslinked collagen molecules | • Higher solubility • Higher fiber organization • Controllable degree of crosslinking • Controlled biodegradation |
• Optimal healing processes • Simplicity of processing (electrospinning) • Improved scaffold mechanical properties • Control of new tissue growth • Improved safety • Improved cell proliferation and adhesion • |
| Homogeneity, liquid crystal formation | • Manipulable physical properties (e.g., compression/tensile strength; modulus of elasticity) | • Scaffold optimization for various indications • Product consistency |
| Plant-derived material | • Free of foreign animal tissue contaminants • Virgin non-recycled material • Consistent raw material properties |
• Improved safety • Product consistency |
| Hydrophilicity | • Higher solubility • Higher liquid retention • Increased cell binding • Increased growth factor binding |
• Ideal delivery vehicle for growth factors • Enables dose reduction of growth factors • Scaffold reproducibility • Increased safety |
Glossary
Abbreviations:
- BSE
bovine spongiform encephalopathy
- ECM
extracellular matrix
- LH1-3
lysyl hydroxylase
- P4H
prolyl 4-hydroxylase
- rhCOL1
recombinant human collagen
- STATs
signal transducers and activators of transcription
Disclosure of Potential Conflicts of Interest
No potential conflict of interest was disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/bioe/article/26002
References
- 1.Pornprasertsuk S, Duarte WR, Mochida Y, Yamauchi M. Overexpression of lysyl hydroxylase-2b leads to defective collagen fibrillogenesis and matrix mineralization. J Bone Miner Res. 2005;20:81–7. doi: 10.1359/JBMR.041026. [DOI] [PubMed] [Google Scholar]
- 2.Torre-Blanco A, Adachi E, Hojima Y, Wootton JA, Minor RR, Prockop DJ. Temperature-induced post-translational over-modification of type I procollagen. Effects of over-modification of the protein on the rate of cleavage by procollagen N-proteinase and on self-assembly of collagen into fibrils. J Biol Chem. 1992;267:2650–5. [PubMed] [Google Scholar]
- 3.Yamauchi M, Shiiba M. Lysine hydroxylation and cross-linking of collagen. Methods Mol Biol. 2008;446:95–108. doi: 10.1007/978-1-60327-084-7_7. [DOI] [PubMed] [Google Scholar]
- 4.Benders KE, van Weeren PR, Badylak SF, Saris DB, Dhert WJ, Malda J. Extracellular matrix scaffolds for cartilage and bone regeneration. Trends Biotechnol. 2013;31:169–76. doi: 10.1016/j.tibtech.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 5.He Q, Zhao Y, Chen B, Xiao Z, Zhang J, Chen L, Chen W, Deng F, Dai J. Improved cellularization and angiogenesis using collagen scaffolds chemically conjugated with vascular endothelial growth factor. Acta Biomater. 2011;7:1084–93. doi: 10.1016/j.actbio.2010.10.022. [DOI] [PubMed] [Google Scholar]
- 6.Shi C, Li Q, Zhao Y, Chen W, Chen B, Xiao Z, Lin H, Nie L, Wang D, Dai J. Stem-cell-capturing collagen scaffold promotes cardiac tissue regeneration. Biomaterials. 2011;32:2508–15. doi: 10.1016/j.biomaterials.2010.12.026. [DOI] [PubMed] [Google Scholar]
- 7.Venkataraman L, Ramamurthi A. Induced elastic matrix deposition within three-dimensional collagen scaffolds. Tissue Eng Part A. 2011;17:2879–89. doi: 10.1089/ten.tea.2010.0749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pollack SV. Silicone, fibrel, and collagen implantation for facial lines and wrinkles. J Dermatol Surg Oncol. 1990;16:957–61. doi: 10.1111/j.1524-4725.1990.tb01560.x. [DOI] [PubMed] [Google Scholar]
- 9.Baumann LS, Kerdel F. The treatment of bovine collagen allergy with cyclosporin. Dermatol Surg. 1999;25:247–9. doi: 10.1046/j.1524-4725.1999.8228a.x. [DOI] [PubMed] [Google Scholar]
- 10.Moody BR, Sengelmann RD. Topical tacrolimus in the treatment of bovine collagen hypersensitivity. Dermatol Surg. 2001;27:789–91. doi: 10.1046/j.1524-4725.2001.01075.x. [DOI] [PubMed] [Google Scholar]
- 11.Klein AW. Techniques for soft tissue augmentation: an ‘a to z’. Am J Clin Dermatol. 2006;7:107–20. doi: 10.2165/00128071-200607020-00004. [DOI] [PubMed] [Google Scholar]
- 12.Pammer J, Weninger W, Tschachler E. Human keratinocytes express cellular prion-related protein in vitro and during inflammatory skin diseases. Am J Pathol. 1998;153:1353–8. doi: 10.1016/S0002-9440(10)65720-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Banfield WG. Age changes in the swelling capacity of the human Achilles tendon. J Gerontol. 1956;11:372. doi: 10.1093/geronj/11.4.372. [DOI] [PubMed] [Google Scholar]
- 14.Kohn RR, Rollerson E. Aging of human collagen in relation to susceptibility to the action of collagenase. J Gerontol. 1960;15:10–4. doi: 10.1093/geronj/15.1.10. [DOI] [PubMed] [Google Scholar]
- 15.Varani J, Dame MK, Rittie L, Fligiel SE, Kang S, Fisher GJ, Voorhees JJ. Decreased collagen production in chronologically aged skin: roles of age-dependent alteration in fibroblast function and defective mechanical stimulation. Am J Pathol. 2006;168:1861–8. doi: 10.2353/ajpath.2006.051302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baroni EdoR, Biondo-Simões MdeL, Auersvald A, Auersvald LA, Montemor Netto MR, Ortolan MC, Kohler JN. Influence of aging on the quality of the skin of white women: the role of collagen. Acta Cir Bras. 2012;27:736–40. doi: 10.1590/S0102-86502012001000012. [DOI] [PubMed] [Google Scholar]
- 17.Talwar HS, Griffiths CE, Fisher GJ, Hamilton TA, Voorhees JJ. Reduced type I and type III procollagens in photodamaged adult human skin. J Invest Dermatol. 1995;105:285–90. doi: 10.1111/1523-1747.ep12318471. [DOI] [PubMed] [Google Scholar]
- 18.Fisher GJ. The pathophysiology of photoaging of the skin. Cutis. 2005;75(Suppl):5–8, discussion 8-9. [PubMed] [Google Scholar]
- 19.Sun WQ, Gouk SS. Aging of a regenerative biologic scaffold (AlloDerm native tissue matrix) during storage at elevated humidity and temperature. Tissue Eng Part C Methods. 2009;15:23–31. doi: 10.1089/ten.tec.2008.0164. [DOI] [PubMed] [Google Scholar]
- 20.Chan TF, Poon A, Basu A, Addleman NR, Chen J, Phong A, Byers PH, Klein TE, Kwok PY. Natural variation in four human collagen genes across an ethnically diverse population. Genomics. 2008;91:307–14. doi: 10.1016/j.ygeno.2007.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giacomoni PU, Mammone T, Teri M. Gender-linked differences in human skin. J Dermatol Sci. 2009;55:144–9. doi: 10.1016/j.jdermsci.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 22.Tamir E, Brenner S. Gender differences in collagen diseases. Skinmed. 2003;2:113–7. doi: 10.1111/j.1540-9740.2003.02189.x. [DOI] [PubMed] [Google Scholar]
- 23.Zeugolis DI, Li B, Lareu RR, Chan CK, Raghunath M. Collagen solubility testing, a quality assurance step for reproducible electro-spun nano-fibre fabrication. A technical note. J Biomater Sci Polym Ed. 2008;19:1307–17. doi: 10.1163/156856208786052344. [DOI] [PubMed] [Google Scholar]
- 24.Stein H, Wilensky M, Tsafrir Y, Rosenthal M, Amir R, Avraham T, Ofir K, Dgany O, Yayon A, Shoseyov O. Production of bioactive, post-translationally modified, heterotrimeric, human recombinant type-I collagen in transgenic tobacco. Biomacromolecules. 2009;10:2640–5. doi: 10.1021/bm900571b. [DOI] [PubMed] [Google Scholar]
- 25.Shoseyov O, Posen Y, Grynspan F. Human recombinant type I collagen produced in plants. Tissue Eng Part A. 2013;19:1527–33. doi: 10.1089/ten.tea.2012.0347. [DOI] [PubMed] [Google Scholar]
- 26.Willard JJ, Drexler JW, Das A, Roy S, Shilo S, Shoseyov O, Powell HM. Plant-derived human collagen scaffolds for skin tissue engineering. Tissue Eng Part A. 2013;19:1507–18. doi: 10.1089/ten.tea.2012.0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shilo S, Roth S, Amzel T, Harel-Adar T, Tamir E, Grynspan F, Shoseyov O. Cutaneous wound healing after treatment with plant-derived human recombinant collagen flowable gel. Tissue Eng Part A. 2013;19:1519–26. doi: 10.1089/ten.tea.2012.0345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yaari A, Posen Y, Shoseyov O. Liquid crystalline human recombinant collagen: the challenge and the opportunity. Tissue Eng Part A. 2013;19:1502–6. doi: 10.1089/ten.tea.2012.0335. [DOI] [PubMed] [Google Scholar]