Abstract
Eosinophilic meningitis caused by the parasitic nematode Angiostrongylus cantonensis is an emerging infectious disease with recent outbreaks primarily in tropical and subtropical locations around the world, including Hawaii. Humans contract the disease primarily through ingestion of infected gastropods, the intermediate hosts of Angiostrongylus cantonensis. Effective prevention of the disease and control of the spread of the parasite require a thorough understanding of the parasite's hosts, including their distributions, as well as the human and environmental factors that contribute to transmission. The aim of this study was to screen a large cross section of gastropod species throughout the main Hawaiian Islands to determine which act as hosts of Angiostrongylus cantonensis and to assess the parasite loads in these species. Molecular screening of 7 native and 30 non-native gastropod species revealed the presence of the parasite in 16 species (2 native, 14 non-native). Four of the species tested are newly recorded hosts, two species introduced to Hawaii (Oxychilus alliarius, Cyclotropis sp.) and two native species (Philonesia sp., Tornatellides sp.). Those species testing positive were from a wide diversity of heterobranch taxa as well as two distantly related caenogastropod taxa. Review of the global literature showed that many gastropod species from 34 additional families can also act as hosts. There was a wide range of parasite loads among and within species, with an estimated maximum of 2.8 million larvae in one individual of Laevicaulis alte. This knowledge of the intermediate host range of Angiostrongylus cantonensis and the range of parasite loads will permit more focused efforts to detect, monitor and control the most important hosts, thereby improving disease prevention in Hawaii as well as globally.
Introduction
Angiostrongylus cantonensis is a parasitic nematode and one of the major causes of eosinophilic meningitis, a potentially fatal disease in humans and other mammals, as well as birds [1]–[6]. Additional causes of eosinophilic meningitis include other parasitic, bacterial, viral and fungal infections, as well as intracranial malignancies or medical devices and allergic reactions to drugs [7]. Angiostrongylus cantonensis has been recorded on all continents except Europe and Antarctica and over 2,800 human cases of eosinophilic meningitis caused by it have been reported from about 30 countries [8], [9]. Most records of the disease, also known as rat lungworm disease, have been from tropical and subtropical areas in Southeast Asia and the Pacific Basin. However, cases have also been sporadically reported in other regions, including places where A. cantonensis is not present, when people return from regions where it occurs [8]–[13].
Definitive hosts of A. cantonensis include various rat species, mainly in the genus Rattus, which become infected by ingesting intermediate hosts (gastropods) or paratenic hosts (e.g. frogs, crabs, prawns, planarians) containing third stage A. cantonensis larvae [14]–[18]. These larvae mature fully and reproduce in the rat, resulting in eggs that hatch into first stage larvae. The first stage larvae are ultimately released in the rat's feces [9], [19], which may then be ingested by the gastropod intermediate hosts. The ingested first stage larvae go through two molts to become third stage larvae while in the intermediate host, which is then consumed by the definitive host and the cycle repeats [19].
Numerous birds and mammals, including humans, are accidental hosts and are infected in the same manner as rats [3]–[5]. However, in these accidental hosts the larvae die when they reach the central nervous system, primarily in the brain, which can lead to eosinophilic meningitis [20]. In humans, the resulting symptoms include nausea and headache, and in more severe cases, neurologic dysfunction, coma, and death [8], [21]. The severity of the symptoms depends on the parasite load of the infected gastropod ingested, which can vary within and among snail species [15], [21]–[26]. Ingestion of infective larvae can be as a result of either deliberate or accidental ingestion of infected intermediate hosts [27]–[29]. In other mammals, as well as birds, there are also severe neurological manifestations, including mortality [3]–[5].
The spread of A. cantonensis has been driven by human activity, through dispersal of definitive and intermediate hosts. Definitive hosts have long been associated with human travel and trade and if infected provide a source of A. cantonensis in areas where snails occur [8], [30]. Snail intermediate hosts are also easily dispersed by human activities, and are transported around the world both intentionally and accidentally by various pathways, notably the agricultural and horticultural industries [31], [32]. As a result of the increased movement of these hosts around the world, eosinophilic meningitis caused by A. cantonensis is an emerging infectious disease, increasing in incidence and expanding in geographical range [7], [33]. With global climate change, suitable habitat for intermediate hosts may increase and regions with appropriate conditions for parasite transmission to occur could expand. Thus, A. cantonensis may expand from being only a tropical concern to a more global one [34], [35].
Since the first reported cases in the Hawaiian Islands in 1960 [36], human infection by A. cantonensis has become increasingly prevalent there. From 2001 to 2012 there have been approximately 60 reported cases ([37]–[39], S. Y. Park, personal communication, November 2013). Most cases were probably caused by infection following accidental consumption of live gastropods and the consumption of produce containing infected gastropods [28].
In Hawaii, there are more than 750 recognized native land snail species, a similar number to the fauna of the continental United States and Canada combined. However a high proportion of these native species are now extinct, with most of those remaining now confined to high elevation refugia away from human disturbance [40]–[43]. The number of established non-native gastropod species in the Hawaiian Islands (43) is also the highest among the islands of the Pacific ([32], [44]–[46], Hayes et al. unpublished). Because snails are the intermediate hosts of A. cantonensis, determining which snail species carry the parasite is important for understanding the geographical spread of the disease, both on a global scale and locally from the perspective of public health management in the Hawaiian Islands. This study focused on the non-native species because these are the snails that are being transported around the world [32], [47] and are the ones likely to be spreading the disease. They may also be involved in passing the parasite on to the remaining, severely threatened native taxa, creating an additional concern from disease ecology and snail conservation perspectives as the impacts of the parasites on the snails is unknown. Therefore, the aim of this study was to screen a large cross section of the non-native gastropod species throughout the Hawaiian Islands, as well as a smaller representative number of the native species, to determine which of them act as intermediate hosts of A. cantonensis. A secondary aim was to assess the parasite loads in these species to identify those with the greatest potential for infecting people. We achieved this by screening non-native species from 12 out of the 30 families present in Hawaii, as well as native species from two of a total of ten families.
Materials and Methods
Ethics Statement
Field studies did not involve endangered or protected species. Collecting permits for all state and federal lands surveyed were provided by the Department of Land and Natural Resources and Oahu Army Natural Resources Program. Surveys in nurseries and other horticultural facilities were done with the permission of the owners.
Sampling and specimen selection
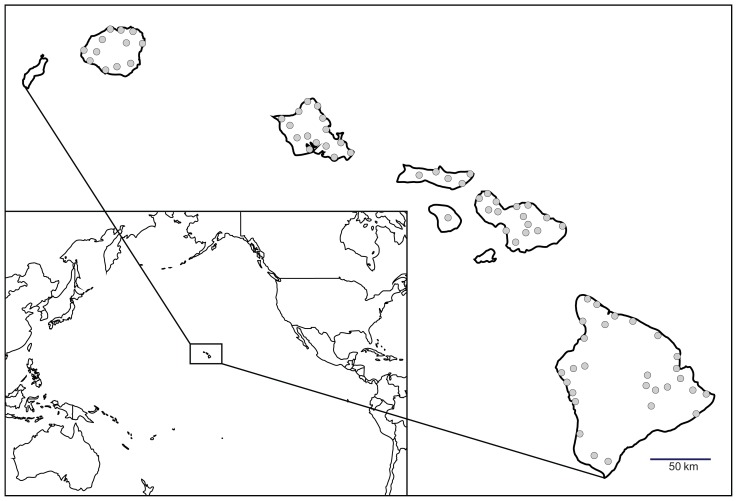
Between 2004 and 2013, surveys were conducted on the six largest Hawaiian Islands to determine the distributions of non-native and native gastropods ([32], [46], [48], unpublished data). Field studies did not involve endangered or protected species. Over 9,500 live specimens (ca 250+ species, including many undescribed native species) were collected during these surveys and preserved in 75% and 95% ethanol for morphological and molecular work, respectively. Non-native (n = 1,062) and native (n = 209) gastropod specimens were selected from these collections for screening to provide a broad coverage of species (37 species, 30 of them non-native and 7 native) and locations (182 sites) (Figure 1), including in particular species previously recorded as carriers of A. cantonensis and those known to be widespread throughout the main Hawaiian Islands according to Cowie et al. [32]. Collecting permits for all state and federal lands surveyed were provided by the Department of Land and Natural Resources and Oahu Army Natural Resources Program. Surveys in nurseries and other horticultural facilities were done with the permission of the owners.
Figure 1. The extent of gastropod sampling throughout the main Hawaiian Islands.
To show the broad geographic coverage, the map includes only sites 10 km or more away from each other.
Molecular detection of A. cantonensis
The larvae of A. cantonensis are distributed throughout the host gastropod's body, although differentially among the various organs [49], [50], and encyst in the host tissue [51]. To extract nematode DNA, total genomic DNA was isolated from ca 0.2 mg of foot tissue for the smallest snails up to ca 10 mg for the largest. Extractions were carried out using the IDPure Spin Column Plant Genomic DNA Isolation Kit following the manufacturer's instructions.
Initial tests to determine if the snails served as hosts for the nematode were done by amplifying a ca 1,134 bp fragment of 18S rDNA sequence using primers (AngioF1 and AngioR1) specific to the superfamily, Metastrongyloidea, to which A. cantonensis belongs following Qvarnstrom et al. [52]. Amplifications were performed in 25 µl reactions with a final concentration of 1X reaction buffer (ID Labs, London, ON, Canada), 0.2 mM of each dNTP, 2 mM MgCl2, 1.25 U of IDPROOF DNA polymerase (ID Labs, London, ON, Canada), 0.16 µM of each primer, 0.4 µg/µl of BSA, 0.5% DMSO and 2 µl of template DNA. A touchdown protocol was used to promote specific amplification of A. cantonensis DNA from total gastropod DNA extracts. Amplification parameters were 95°C for 5 min, 7 cycles of 95°C for 20 s, 65°C for 20 s with a 1°C decrease per cycle, and elongation at 72°C for 45 s followed by 35 cycles of 95°C for 20 s, 59°C for 20 s, and 72°C for 45 s, and a final elongation at 72°C for 10 min. Reactions were terminated with a 4°C hold for 30 min. Amplified fragments were visualized on an agarose gel to confirm size and quality. All reaction sets included a negative control and positive controls verified by previous amplifications. To evaluate the specificity of amplifications, 30% of the positive amplicons were cleaned using the IDPure Purification Kit (ID Labs, London, ON, Canada) and sequenced with the forward primer (AngioF1) at the Greenwood Molecular Biology Facility (Pacific Biosciences Research Center, University of Hawaii). The sequences were compared with known A. cantonensis sequences [53], [54].
To verify that positives from the 18S PCR assay were the result of infection by A. cantonensis, all positive samples and a random subset (ca 10%) of the negative samples were retested with a PCR assay aimed at amplifying the ribosomal internal transcribed spacer one (ITS1) region using the species-specific primers AcanITS1F1 and AcanITS1R1, following Qvarnstrom et al. [55]. These primers can detect false negatives that are missed with 18S, and reveal false positives resulting from non-specific amplification of other species of Metastrongyloidea [55]. Amplifications were performed in 25 µl reactions as for 18S with the exception of using 3 µl of template DNA. Amplification parameters were 95°C for 3 min, 45°C for 1 min, and elongation at 72°C for 1 min followed by 35 cycles of 95°C for 20 s, 48°C for 20 s, and 72°C for 35 sec with a final elongation at 72°C for 10 min. Amplicons were visualized as for 18S.
Quantification of parasite load
The parasite loads of all specimens that tested positive for A. cantonensis were quantified following the real-time, quantitative PCR (RT-PCR) TaqMan assay of Qvarnstrom et al. [55]. First, a standard curve was generated from DNA extractions containing an estimated 1.744–1,744 larvae. To do this, nematodes were isolated from tissue of a live Parmarion martensi that was known to be infected, using a modification of the protocol of Wallace and Rosen [56] with a higher pepsin concentration (S. C. Thiengo, personal communication to K. A. Hayes, November 2008). The specimen was minced, put in 50 ml of digestion solution containing 3% pepsin and 0.7% HCl and incubated at room temperature with occasional agitation. The solution was then transferred to a Baermann apparatus with a stoppered tube attached to the bottom of the funnel containing a 2×2 cm piece of medical gauze. The solution containing nematode larvae was left overnight to allow the larvae to migrate through the gauze and into the stoppered tube, after which the solution was recovered by draining the solution from the tube into a 50 ml beaker. Larvae, which remain alive and intact following digestion, were concentrated into 3 ml of solution from which five 100 µl samples were taken. The number of larvae in each 100 µl sample was counted under a stereomicroscope and the average concentration of the five samples was used to determine the total number of nematode larvae per 100 µl. Based on this estimated concentration of larvae, five samples were drawn from the remaining 2.5 ml of solution containing 1.744 (1 µl), 8.72 (5 µl), 17.44 (10 µl), 174.4 (100 µl), and 1,744 (1 ml) larvae. From each of these samples, DNA was extracted as described previously and eluted in 80 µl of elution buffer. Because of the possibility of co-isolated products inhibiting PCR all samples were diluted 1∶5 and then amplified in triplicate. Cycling conditions differed slightly from those of Qvarnstrom et al. [55], starting with 2 min at 50°C, 2 min at 90°C, and finishing with 40 cycles of 15 sec at 95°C and 1 min at 60°C. All RT-PCR assays were carried out at the State of Hawaii Department of Health using an Applied Biosystems 7500 Fast Real-time PCR System v1.4.0 and analyzed using Applied Biosystems 7500 Fast System with 21 CFR Part 11 software.
A standard curve was generated by plotting the cycle threshold (CT) values obtained from the RT-PCR of estimated quantities of parasites against the log number of estimated parasites in those samples, permitting generation of a linear equation that was used to estimate the number of larvae in a sample from its CT value. To estimate the number of larvae in each specimen, the ethanol preserved snails were patted dry in tissue paper for one minute and weighed. Shells were removed from larger snails, but for small snails the combined shell and body weight was used. The average weight of tissue used for DNA extraction of each species was also estimated by sampling a piece of tissue from five similarly sized specimens, patting them dry for one minute and weighing them. The total number of larvae was then estimated for each specimen by extrapolation.
Statistical analyses
Statistical analyses were carried out using Microsoft Excel 2013. Rates of infection of non-native and native species were compared using the chi-square test. This test was also used to assess differences in rates of infection in species with different habits as assessed in the field (primarily ground-dwelling, arboreal, freshwater, both ground-dwelling and arboreal), as ground-dwelling snails may have increased chances of encountering rat feces compared to arboreal and aquatic snails. Differences between means were considered statistically significant if the P-value was less than 0.05.
Results
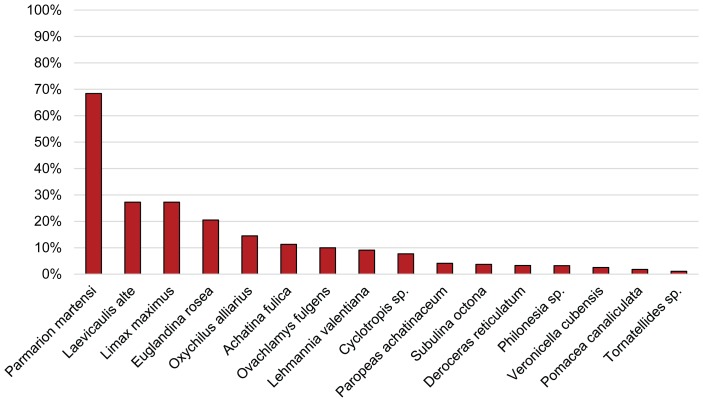
The 18S and ITS1 amplifications gave identical results. Of the 37 species, 16 tested positive for A. cantonensis, with 70 specimens testing positive out of a total of 1,271 (Table 1). Among the 30 non-native species, 14 tested positive, two being newly recorded natural hosts of A. cantonensis (Cyclotropis sp., Oxychilus alliarius). Of a total of 1,062 non-native gastropods, 6% were positive for A. cantonensis. No specimens of four non-native species (Bradybaena similaris, Deroceras laeve, Limax flavus, Melanoides tuberculata) that have been recorded in other studies as hosts of A. cantonensis (Appendix S1) tested positive for the parasite. Parmarion martensi had the highest prevalence of infection with 68% (13/19) of the specimens testing positive for A. cantonensis followed by Laevicaulis alte with an infection rate of 30% (13/44) (Table 1, Figure 2).
Table 1. Infection rates and average parasite loads (of positive specimens) in the gastropod species screened in this study.
| Family | Species | Habit | No. tested | No. (%) positive | No. sites positive (total) | Average CT 1∶5 dilution value (range) | Average no. of parasites per 5 mg of snail tissue | Average no. of parasites in entire specimen (range) |
| Achatinellidae | Auriculella spp. | A | 31 | 0 | 0 (8) | - | - | - |
| Elasmias spp. | B | 18 | 0 | 0 (9) | - | - | - | |
| Lamellidea spp. | B | 25 | 0 | 0 (13) | - | - | - | |
| Tornatellides spp. | B | 90 | 1 (1) | 1 (30) | − (24.66) | - | − (2,379) | |
| Achatinidae | Achatina fulica* | G | 62 | 7 (11) | 4 (21) | 25.66 (20.16–31.26) | 237 | 213,515 (14,379–870,868) |
| Agriolimacidae | Deroceras laeve* | G | 79 | 0 | 0 (27) | - | - | - |
| Deroceras reticulatum* | G | 61 | 2 (3) | 1 (23) | 25.24 (23.54–26.94) | 1,564 | 9,789 (3,500–16,078) | |
| Ampullariidae | Pomacea canaliculata* | F | 56 | 1 (2) | 1 (15) | − (23.78) | - | − (68,133) |
| Arionidae | Arion intermedius | G | 20 | 0 | 0 (8) | - | - | - |
| Arion subfuscus | G | 8 | 0 | 0 (4) | - | - | - | |
| Ariophantidae | Parmarion martensi* | G | 19 | 13 (68) | 5 (8) | 24.23 (17.28–29.43) | 912 | 55,852 (850–341,828) |
| Assimineidae | Cyclotropis sp. | G | 13 | 1 (8) | 1 (3) | − (28.60) | - | − (154) |
| Bradybaenidae | Bradybaena similaris* | G | 65 | 0 | 0 (16) | - | - | - |
| Euconulidae | Liardetia doliolum | G | 8 | 0 | 0 (5) | - | - | - |
| Gastrodontidae | Zonitoides arboreus | G | 18 | 0 | 0 (5) | - | - | - |
| Helicarionidae | Kaala subrutila | G | 2 | 0 | 0 (1) | - | - | - |
| Ovachlamys fulgens* | G | 10 | 1 (10) | 1 (4) | − (22.42) | - | − (11,118) | |
| Philonesia sp. | A | 31 | 1 (3) | 1 (11) | − (24.57) | - | − (4,823) | |
| Helicidae | Cornu aspersum | G | 25 | 0 | 0 (8) | - | - | - |
| Limacidae | Lehmannia valentiana* | G | 11 | 1 (9) | 1 (6) | − (22.19) | - | − (24,819) |
| Limax flavus* | G | 8 | 0 | 0 (4) | - | - | - | |
| Limax maximus* | G | 11 | 3 (27) | 2 (4) | 22.22 (20.36–24.35) | 1,960 | 398,160 (170,067–566,582) | |
| Lymnaeidae | Fossaria viridis | F | 18 | 0 | 0 (4) | - | - | - |
| Milacidae | Milax gagates | G | 22 | 0 | 0 (7) | - | - | - |
| Orthalicidae | Bulimulus guadalupensis | G | 10 | 0 | 0 (2) | - | - | - |
| Oxychilidae | Oxychilus alliarius | G | 69 | 10 (14) | 6 (17) | 25.69 (20.22–34.41) | 1,922 | 13,382 (63–55,807) |
| Planorbidae | Planorbella duryi | F | 20 | 0 | 0 (6) | - | - | - |
| Physidae | Physa spp. | F | 27 | 0 | 0 (6) | - | - | - |
| Spiraxidae | Euglandina rosea* | G | 39 | 8 (21) | 5 (16) | 27.66 (24.37–33.13) | 166 | 43,687 (1,244–113,645) |
| Streptaxidae | Gonaxis kibweziensis | G | 11 | 0 | 0 (5) | - | - | - |
| Subulinidae | Paropeas achatinaceum* | G | 73 | 3 (4) | 2 (18) | 27.16 (25.77–29.36) | 1,518 | 11,421 (1,724–21,087) |
| Subulina octona* | G | 54 | 2 (4) | 1 (13) | 24.60 (23.21–25.99) | 3,548 | 39,114 (15,835–62,392) | |
| Succineidae | Succinea caduca | G | 12 | 0 | 0 (4) | - | - | - |
| Succinea tenella | G | 25 | 0 | 0 (6) | - | - | - | |
| Thiaridae | Melanoides tuberculata* | F | 17 | 0 | 0 (5) | - | - | - |
| Veronicellidae | Laevicaulis alte* | G | 44 | 13 (30) | 11 (21) | 24.99 (17.32–31.42) | 1,592 | 342,971 (4,127–2,801,566) |
| Veronicella cubensis* | G | 159 | 4 (3) | 3 (45) | 23.40 (21.35–24.93) | 531 | 116,891 (28,931–253,909) | |
| Total | 1271 | 71 (6) | 40 (182) |
Previously recorded as a host in the Hawaiian Islands and/or elsewhere.
Native Hawaiian gastropod species. Habits are ground-dwelling (G), arboreal (A), both ground-dwelling and arboreal (B) and freshwater (F).
Figure 2. Infection rates for gastropod species that tested positive in this study.
Levels of infection vary considerably from 68% infection in Parmarion martensi to 1% in Tornatellides sp.
Of the seven native Hawaiian species screened, two tested positive (one individual of each of Philonesia sp. and Tornatellides sp.). The proportion of native snails found to carry A. cantonensis was significantly smaller than the proportion of non-native snails that tested positive (χ2 = 9.95, df = 1, P = 0.002). The proportions of all snails infected were significantly related to their habit (χ2 = 18.5, df = 3, P = 0.0004): 7% (67/938) of the individuals of ground-dwelling species tested positive; arboreal and freshwater snails were less susceptible at 2% (1/62) and 0.7% (1/138), respectively; and 0.8% (1/133) of the individuals of species that are both ground-dwelling and arboreal tested positive. Of 182 sites from which snails were screened, 40 had snails that tested positive for A. cantonensis (Table 1). Infected L. alte were found at more sites (11) than any other species.
The linear equation derived from RT-PCR standard curve data was
where y is the CT value and x is the number of larvae in the sample. The numbers of parasites in each DNA extraction were estimated using this equation and ranged from 2 to 6,427. Extrapolating from these data to the intensity of infection (number in 5 mg of tissue) and the total number in each individual, showed that parasite load varied widely both within and among species (Table 1). Subulina octona had the highest average concentration of parasites (3,548 per 5 mg tissue). However, a L. alte specimen had the highest individual parasite concentration (8,147 parasites per 5 mg of tissue), followed by an O. alliarius (7,068) and a P. martensi (6,639). The two caenogastropod species, Pomacea canaliculata and Cyclotropis sp. had the lowest average parasite concentrations, more than 20 times less than that in S. octona, while an A. fulica had the lowest individual parasite concentration at 6 parasites per 5 mg of tissue. Average total parasite loads were highest in L. maximus, L. alte and A. fulica (398,160, 342,971 and 213,515, respectively) and individuals of these species also had the highest individual loads (566,582, 2,801,566 and 870,867, respectively). These high numbers are based on significant extrapolation beyond the standard curve. Theoretically the curve should be linear but at such high levels of infection it may not be and these numbers may be over-estimates. Also, the concentration of larvae may differ among the various parts of the snails' bodies [49], [50]. (Such caveats are also applicable to studies that extrapolated from samples in which larvae were counted visually.) The species with the lowest average parasite load was Cyclotropis sp. (154 parasites), but an O. alliarius specimen had the lowest individual load (63).
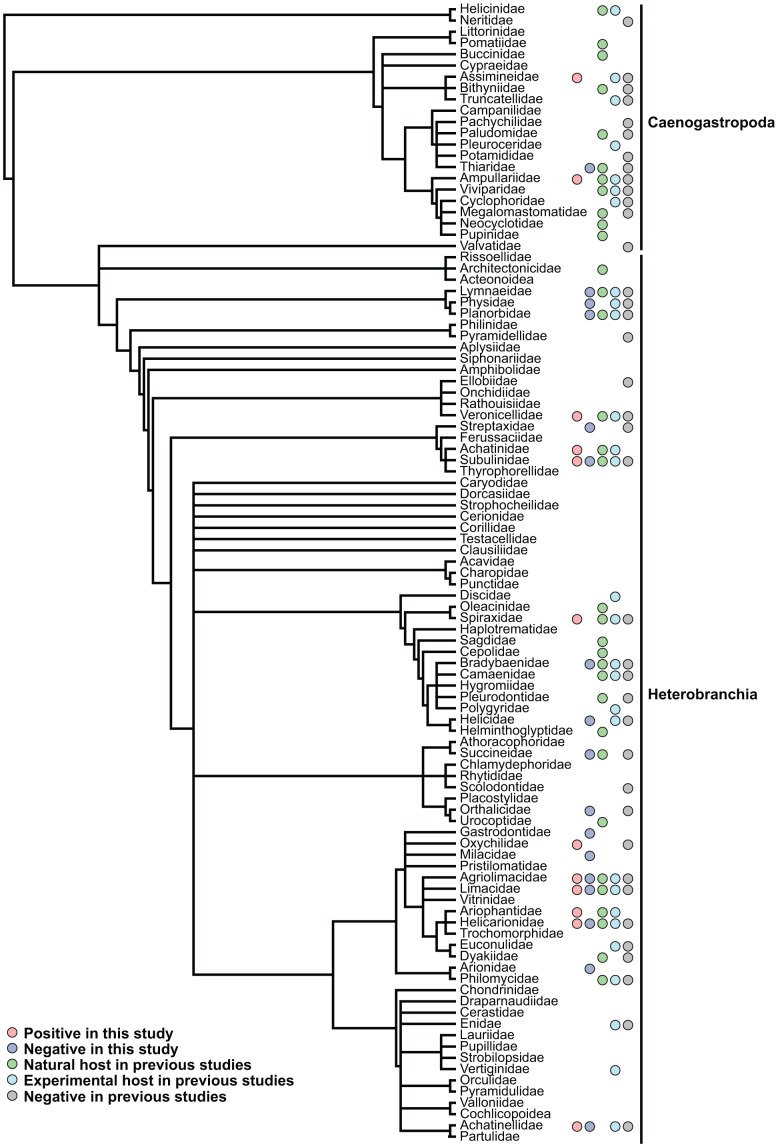
We have now shown that nearly a third of the non-native snail species established in the Hawaiian Islands are carriers of A. cantonensis along with two native species (Philonesia sp., Tornatellides sp.). Others may also act as carriers but were just not recorded as such in this study. In addition, many other species act as hosts in other parts of the world (Appendix S1). Including the current study, species from 58 families (out of 409 extant gastropod families) [59] have now been evaluated for their potential to carry A. cantonensis, and all but 12 of these families contained taxa that were capable of carrying the nematode (Figure 3).
Figure 3. Phylogeny of mollusc families showing which have been recorded as intermediate hosts of Angiostrongylus cantonensis.
Phylogeny constructed using the classifications and phylogenies of Bouchet and Rocroi, Aktipis et al. and Strong et al. [59]–[61], indicating the diversity of families in which mollusc species have been shown to act as hosts of Angiostrongylus cantonensis. Bars at the right of the tree indicate the taxonomic group that the families belong to.
Discussion
The diversity of gastropods now known to carry Angiostrongylus cantonensis encompasses a broad phylogenetic range of both terrestrial and freshwater species (Figure 3; Appendix S1). A single fully marine species (Discotectonica acutissima: Architectonicidae) has also been reported as testing positive for A. cantonensis [57] but this is a sublittoral (50–200 m depth) species [58] and the finding may be incorrect. Previous studies had identified species in 33 families as natural hosts of A. cantonensis, with the present study adding three more: Achatinellidae, Assimineidae and Oxychilidae. Species in another 10 families have been shown experimentally to be capable of acting as hosts. Individuals from 12 families were positive for A. cantonensis in the current study. These included not only highly divergent heterobranchs (which includes approximately 80% of all land snails) such as Tornatellides sp. (Achatinellidae), V. cubensis (Veronicellidae) and P. martensi (Ariophantidae) but also caenogastropods (the largest gastropod group, mostly marine), i.e. Cyclotropis sp. and P. canaliculata. Based on dietary habits, detritivores, which more often come in contact with rat feces, might be anticipated to have a higher incidence of infection. However, herbivorous species such as Veronicella cubensis [62], [63], predatory species like Euglandina rosea [64], and detritivores like S. octona [65], all tested positive, despite their dietary differences. Although the molecular approach used in this study was not able to distinguish larval stages of the parasite and therefore whether the parasite can develop to the infective stage in all species that tested positive, the majority of studies listed in Appendix S1 were morphological and detected third stage larvae. Therefore, the extremely broad diversity of gastropods in which A. cantonensis has been found, indicates that almost any terrestrial or freshwater gastropod may have the potential to carry and transmit the parasite, which has broad implications for its continued spread.
Two families (Achatinidae, Ariophantidae) exhibited a high infection rate, inasmuch as they included species that were reported as natural hosts and/or had been successfully infected experimentally in this and all previous studies that screened them for A. cantonensis. Species in two families (Orthalicidae and Streptaxidae) tested negative in this and all previous studies, perhaps because they have low susceptibility to infection or the individuals tested came from localities where the frequency of infection of intermediate hosts was low. Ten families were represented by species that were only reported as having been infected experimentally, including the Truncatellidae, which live close to the sea shore where the probability of transmission may be low because wave action washes rat feces away.
As predicted, ground-dwelling species tested positive for A. cantonensis more frequently than arboreal and freshwater species, probably because of their more ready access to rat feces. Four freshwater species (Fossaria viridis, Melanoides tuberculata, Planorbella duryi, Physa sp.) tested negative for A. cantonensis in this study and only 1 of 56 (2%) P. canaliculata tested positive. It may be more difficult for snails in freshwater habitats to acquire the parasite as access to rat feces in streams and rivers may be limited [66]. However, other studies have identified a number of freshwater species able to carry A. cantonensis, including P. canaliculata and M. tuberculata (Appendix S1). The rate of infection of M. tuberculata was often less than 1%, whereas P. canaliculata showed higher rates of up to 40% [23], [67]–[71]. The low infection rate in P. canaliculata in the present study could be due to the majority of these specimens being from irrigated areas such as taro patches, some with flowing water, where incidence of infection may be low because of lower concentrations of parasites in the water than in intact rat feces in terrestrial situations [72]. In Asia, P. canaliculata is commonly identified as the source of infection in human cases of angiostrongyliasis, not necessarily because of the high percentage of infected snails, but because of its popularity as a food source [73], [74].
A number of terrestrial snails also did not test positive for A. cantonensis. For instance, none of the 65 Bradybaena similaris specimens tested positive in this study. It is possible that B. similaris is naturally less susceptible to A. cantonensis infection than other species, as in a previous study only 8 of 281 (4%) B. similaris specimens from Oahu were infected [15]. All Arion intermedius, Arion subfuscus and Cornu aspersum specimens tested were from sites higher than 600 m above sea level, where the parasite may not yet be at high densities or may be limited by temperature. Alternatively, at least for C. aspersum, nematode inhibitors that prevent maturation or reproduction and that have been isolated from this species [76], [77], may explain its low infection rate. In another study, no parasites were recovered from several hundred C. aspersum in New Caledonia [78], which lends support to this possibility.
Achatina fulica is well known as an intermediate host of A. cantonensis (e.g. [11], [74], [79], [80]). However, the level of infection of A. fulica varies widely among localities. In the present study only 7 of 62 (11%) A. fulica tested positive. In one study in Brazil, Neuhauss et al. [81] found only one infected A. fulica out of 244 (0.4%) screened, while in another Thiengo et al. [26] found 14 among 33 (42%) screened. The level of infection at different locations in Guangdong, China, varied widely from 0 to 45.4% [82]. The most likely explanation for this variability may be the variation in presence and abundance of A. cantonensis in different environments, possibly related to abiotic factors such as temperature and humidity, but perhaps also to the distribution of infected rats, the species of rats present or differences in the interactions between rats and gastropods.
Among the newly recorded hosts, Oxychilus alliarius is a widespread European species. While A. cantonensis is primarily a tropical and subtropical parasite, presumably because it is constrained by ambient temperatures (which determine the temperature of its poikilothermic gastropod intermediate hosts), the fact that it can infect temperate gastropod species indicates that global warming trends may allow it to establish more widely in locations where such hosts are already present.
This is the first report of native Hawaiian snails carrying A. cantonensis. Most extant native snails are confined to high elevation habitat and rarely encountered by people. They are therefore unlikely to be important in transmission of A. cantonensis to humans. However, this finding may have negative implications for the health of the native Hawaiian snail fauna, which is especially vulnerable to additional threats. Once consisting of over 750 species [42], the fauna has declined drastically and is being replaced by a much smaller number of alien species [32], [43], [46], [48]. The deliberate introduction of predatory snails, notably Euglandina rosea, for use as biocontrol agents in ill-conceived efforts to control Achatina fulica has had a devastating effect on the native snails [43], [44], [83]. Habitat destruction has also been of major significance in the decline of the native fauna [84]. The snail fauna may be facing an additional threat if infection with A. cantonensis reduces the snails' fitness. Native snails are important in the functioning of healthy ecosystems and the possible impacts on the native fauna could have serious implications for ecosystem health. Native snails in Jamaica also carry A. cantonensis, but little is known about the effect of the parasite on their fitness [13], [85]. Richards and Merritt [51] recorded a clear tissue reaction to the parasite in Biomphalaria glabrata. This type of response to parasitic infection may be costly, using energy usually allocated towards survival and reproduction [86], [87]. Wallace and Rosen [75] showed that mortality was higher in Physa elliptica experimentally infected with A. cantonensis than in uninfected controls.
Although only 2 out of 210 specimens of native species tested positive for the parasite, this does indicate that the parasite may now have become sufficiently widespread and abundant to begin to infect native snail populations. Most native Hawaiian snails are very sparsely distributed, mostly at high elevations where A. cantonensis may not be able to survive in rat feces or develop in the snails. Nonetheless, these high elevation refugia generally support more than one species of native snail, so native species other than those testing positive in this study may yet be found to be susceptible to infection.
Susceptibility to infection may be related to the snails' behavior, location or physiology [78]. For example, P. martensi, a species that is mainly ground-dwelling is highly susceptible, with 68% of the specimens in this study testing positive, and often heavily infected [50], [52], [55], [88], [89]. However it will readily climb and is often found in trash cans and compost piles, where contact with rats and rat feces is probably a common occurrence [89]. These factors may also affect parasite load, which differed greatly within and among species. One P. martensi specimen had one of the highest parasite concentrations in its tissue and had on average as many larvae in its entire body as other species four to six times its mass (Table 1). Jarvi et al. [50] also showed that this species can support high levels of A. cantonensis. Parmarion martensi is an invasive species found in the Hawaiian Islands only on the islands of Oahu and Hawaii and is the species most frequently implicated in transmission of the parasite to humans in the Islands [89]. Predatory behavior of some snails, such as A. fulica which feed on slugs [90] and P. canaliculata observed to prey on other snails [91] may lead to infection in these snails via an alternative pathway other than feces.
Although the two caenogastropod species, P. canaliculata and Cyclotropis sp., had the lowest average parasite concentrations, this was based on only one positive individual for each species. These low parasite loads in P. canaliculata may again be due to the lower chance of this species ingesting larvae in a stream or pond than on land. Even though A. fulica is a relatively large (heavy) species, it harbored on average as many or fewer parasites than species only 15–25% of its mass, and one A. fulica had the lowest individual parasite concentration. This could be due to the muscular foot tissue being relatively poorly oxygenated and thus less preferred by A. cantonensis [49]. Despite Tornatellides sp. having a high parasite load per mg of tissue, even if a whole specimen (ca 2 mm in length) were consumed only ca 2,000 larvae (an overestimate due to the added weight of its shell, which was not removed for this extrapolation) would be ingested, while consuming a whole L. alte could result in ingestion of up to a thousand times more larvae.
A diverse assemblage of gastropods can thus serve as hosts of A. cantonensis to varying degrees. In the future, monitoring and quarantine efforts should take this into account. In Hawaii there has been a distinct focus on P. martensi [28], [52], [50], [55], [89], and although this species is definitely one of the most important hosts in Hawaii, it is by no means the only one. A number of species commonly found in highly populated areas at lower elevations were positive for A. cantonensis. Species such as A. fulica, E. rosea, L. alte, P. achatinaceum, S. octona and V. cubensis are commonly found in Hawaii in nurseries, farms and home gardens, where contact with produce can be a regular occurrence, and inadvertent consumption of infected hosts on produce is thought to be a major pathway of infection [29]. Also it is difficult to remove snails from produce [92].
Both tropical and temperate snails can carry A. cantonensis, indicating the potential for future expansion of the parasite's range under climate change and the need for continued concern about angiostrongyliasis as an emerging infectious disease. Since many species are potential hosts, it is likely that abiotic factors, particularly temperature and perhaps humidity, have a greater influence on infection rates and continued range expansion of the parasite than does the spread of particular host species. Knowledge of the possible vectors of A. cantonensis and their parasite loads is important for public health management.
Supporting Information
Known gastropod hosts of Angiostrongylus cantonensis , associated localities and corresponding key references.* References reporting only experimental laboratory infection - no locality is given for such studies. ** References reporting both natural and experimental infection.
(DOCX)
Acknowledgments
We acknowledge Christopher Lepczyk and Kenton Kramer for their constructive comments in editing previous drafts of this manuscript. We thank all the people who participated in the collection, identification and curation of the specimens used in this study, especially previous and present members of the Cowie Lab and Kay Howe. We also thank the Hawaii Department of Health, notably Christian Whelen, Caitlin Saucier and Precilia Calimlim, for advice and the technical support to complete the parasite load component of this study. Maps used in Figure 1 were obtained from http://d-maps.com/carte.php?num_car=3235&lang=en and http://d-maps.com/carte.php?num_car=6903&lang=en.
Funding Statement
Funding for this work was provided by the United States Department of Agriculture Cooperative Agricultural Pest Survey program (http://www.usda.gov/wps/portal/usda/usdahome), National Science Foundation (DEB-1120906; http://www.nsf.gov/), Watson T. Yoshimoto Foundation through the Ecology, Evolutionary and Conservation Biology program at the University of Hawaii (http://www.hawaii.edu/eecb/), American Malacological Society (http://www.malacological.org/) and Hawaiian Malacological Society. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Wallace GD, Rosen L (1969a) Studies on eosinophilic meningitis: Experimental infection of rats and other homeothermic vertebrates with Angiostrongylus cantonensis . Am J Epidemiol 89: 331. [DOI] [PubMed] [Google Scholar]
- 2. Duffy MS, Miller CL, Kinsella JM, de Lahunta A (2004) Parastrongylus cantonensis in a nonhuman primate, Florida. Emerg Infect Dis 10: 2207–2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lindo JF, Escoffery CT, Reid B, Codrington G, Cunningham-Myrie C, et al. (2004) Fatal autochthonous eosinophilic meningitis in a Jamaican child caused by Angiostrongylus cantonensis . Am J Trop Med Hyg 70: 425–428. [PubMed] [Google Scholar]
- 4. Monks DJ, Carlisle MS, Carrigan M, Rose K, Spratt D, et al. (2005) Angiostrongylus cantonensis as a cause of cerebrospinal disease in a yellow-tailed black cockatoo (Calyptorhynchus funereus) and two tawny frogmouths (Podargus strigoides). J Avian Med Surg 19: 289–293. [Google Scholar]
- 5. Lunn JA, Lee R, Smaller J, MacKay BM, King T, et al. (2012) Twenty two cases of canine neural angiostrongyliosis in eastern Australia (2002–2005) and a review of the literature. Parasit Vectors 5: 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Murphy GS, Johnson S (2013) Clinical aspects of eosinophilic meningitis and meningoencephalitis caused by Angiostrongylus cantonensis, the rat lungworm. Hawaii J Med Public Health 72 (Supplement 2)35–40. [PMC free article] [PubMed] [Google Scholar]
- 7. Diaz JH (2010) The helminthic eosinophilic meningitides: emerging zoonotic parasitic diseases worldwide. Trop Med Health 38: 115–126. [Google Scholar]
- 8. Wang Q-P, Lai D-H, Zhu X-Q, Chen X-G, Lun Z-R (2008) Human angiostrongyliasis. Lancet 8: 621–630. [DOI] [PubMed] [Google Scholar]
- 9. Cowie RH (2013a) Biology, systematics, life cycle, and distribution of Angiostrongylus cantonensis, the cause of rat lungworm disease. Hawaii J Med Public Health 72 (Supplement 2)6–9. [PMC free article] [PubMed] [Google Scholar]
- 10. Aguiar PH, Morera P, Pascual J (1981) First record of Angiostrongylus cantonensis in Cuba. Am J Trop Med Hyg 30: 963–965. [DOI] [PubMed] [Google Scholar]
- 11. Kliks MM, Palumbo NE (1992) Eosinophilic meningitis beyond the Pacific Basin: the global dispersal of a peridomestic zoonosis caused by Angiostrongylus cantonensis, the nematode lungworm of rats. Soc Sci Med 34: 199–212. [DOI] [PubMed] [Google Scholar]
- 12. New D, Little MD, Cross J (1995) Angiostrongylus cantonensis infection form eating raw snails. N Engl J Med 332: 1105–1106. [DOI] [PubMed] [Google Scholar]
- 13. Lindo JF, Waugh C, Hall J, Cunningham-Myrie C, Ashley D, et al. (2002) Enzootic Angiostrongylus cantonensis in rats and snails after an outbreak of human eosinophilic meningitis, Jamaica. Emerg Infect Dis 8: 324–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wallace GD, Rosen L (1965) Studies on eosinophilic meningitis: I. Observations on the geographic distribution of Angiostrongylus cantonensis in the Pacific area and its prevalence in wild rats. Am J Epidemiol 81: 52–62. [DOI] [PubMed] [Google Scholar]
- 15. Wallace GD, Rosen L (1969b) Studies on eosinophilic meningitis. V. Molluscan hosts of Angiostrongylus cantonensis on Pacific islands. Am J Trop Med Hyg 18: 206–216. [PubMed] [Google Scholar]
- 16.Cross JH, Chen ER (2007) Angiostrongyliasis. In: Murrell KD, Fried B, editors. Food-borne parasitic zoonoses: fish and plant-borne parasites. New York: Springer Science + Business Media, LLC. pp. 263–290.
- 17. Chen D, Zhang Y, Shen H, Wei Y, Huang D, et al. (2011a) Epidemiological survey of Angiostrongylus cantonensis in the west-central region of Guangdong Province, China. Parasitol Res 109: 305–314. [DOI] [PubMed] [Google Scholar]
- 18. Yong HS, Eamsobhana P (2013) Definitive rodent hosts of the rat lungworm Angiostrongylus cantonensis . Raffles Bull Zool 29: 111–115. [Google Scholar]
- 19. Prociv P, Spratt DM, Carlisle MS (2000) Neuro-angiostrongyliasis: unresolved issues. Int J Parasitol 30: 1295–1303. [DOI] [PubMed] [Google Scholar]
- 20. Graeff-Teixeira C, da Silva ACA, Yoshimura K (2009) Update on eosinophilic meningoencephalitis and its clinical relevance. Clin Microbiol Rev 22: 322–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang J, Qi H, Diao Z, Zheng X, Li X, et al. (2010) An outbreak of Angiostrongyliasis cantonensis in Beijing. J Parasitol 96: 377–381. [DOI] [PubMed] [Google Scholar]
- 22. Punyagupta S, Juttijudata P, Bunnag T (1975) Eosinophilic meningitis in Thailand. Am J Trop Med Hyg 24: 921–931. [PubMed] [Google Scholar]
- 23. Ibrahim MM (2007) Prevalence and intensity of Angiostrongylus cantonensis in freshwater snails in relation to some ecological and biological factors. Parasite 14: 61–70. [DOI] [PubMed] [Google Scholar]
- 24. Tsai H-C, Liu Y-C, Kunin CM, Lee SS-J, Chen Y-S, et al. (2001) Eosinophilic meningitis caused by Angiostrongylus cantonensis: report of 17 cases. Am J Med 111: 109–114. [DOI] [PubMed] [Google Scholar]
- 25. Tesana S, Srisawangwong T, Sithithaworn P, Laha T, Andrews R (2009) Prevalence and intensity of infection with third stage larvae of Angiostrongylus cantonensis in mollusks from northeast Thailand. J Trop Med Hyg 80: 983–987. [PubMed] [Google Scholar]
- 26. Thiengo SC, Maldonado A, Mota EM, Torres EJL, Caldeira R, et al. (2010) The giant African snail Achatina fulica as natural intermediate host of Angiostrongylus cantonensis in Pernambuco, northeast Brazil. Acta Trop 115: 194–199. [DOI] [PubMed] [Google Scholar]
- 27. Slom TJ, Cortese MM, Gerber SI, Jones RC, Holtz TH, et al. (2002) An outbreak of eosinophilic meningitis caused by Angiostrongylus cantonensis in travelers returning from the Caribbean. N Engl J Med 346: 668–675. [DOI] [PubMed] [Google Scholar]
- 28.Hollyer JR, Troegner VA, Cowie RH, Hollingsworth RG, Nakamura-Tengan L, et al. (2010) Best on-farm food safety practices: reducing risks associated with rat lungworm infection and human eosinophilic meningitis. Food Saf Technol, College of Agriculture and Human Resources, University of Hawaii at Manoa 39, 8 p.
- 29. Cowie RH (2013b) Pathways for transmission of angiostrongyliasis and the risk of disease associated with them. Hawaii J Med Public Health 72 (Supplement 2)70–74. [PMC free article] [PubMed] [Google Scholar]
- 30. Jin E, Ma D, Liang Y, Ji A, Gan S (2005) MRI findings of eosinophilic myelomeningoencephalitis due to Angiostrongylus cantonensis . Clin Radiol 60: 242–250. [DOI] [PubMed] [Google Scholar]
- 31.Cowie RH, Robinson DG (2003) Pathways of introduction of nonindigenous land and freshwater snails and slugs. In: Ruiz GM, Carlton JT, editors. Invasive Species: Vectors and Management Strategies. Washington (DC): Island Press. pp. 93–122.
- 32. Cowie RH, Hayes KA, Tran CT, Meyer WM III (2008) The horticultural industry as a vector of alien snails and slugs: widespread invasions in Hawaii. Int J Pest Manage 54: 267–276. [Google Scholar]
- 33. Chen M-X, Zhang R-L, Ai L, Chen J-X, Chen S-H, et al. (2011b) Seroprevalence of Angiostrongylus cantonensis infection in humans in China. J Parasitol 97: 144–145. [DOI] [PubMed] [Google Scholar]
- 34. Lafferty KD (2009) The ecology of climate change and infectious diseases. Ecology 90: 888–900. [DOI] [PubMed] [Google Scholar]
- 35. Lv S, Zhang Y, Steinmann P, Yang G-J, Yang K, et al. (2011) The emergence of angiostrongyliasis in the People's Republic of China: the interplay between invasive snails climate change and transmission dynamics. Freshw Biol 56: 717–734. [Google Scholar]
- 36. Horio SR, Alicata JE (1961) Parasitic meningo-encephalitis in Hawaii. A new parasitic disease of man. Hawaii Med J 21: 139–140. [PubMed] [Google Scholar]
- 37. Hochberg NS, Park SY, Blackburn BG, Sejvar JJ, Gaynor K, et al. (2007) Distribution of eosinophilic meningitis cases attributable to Angiostrongylus cantonensis, Hawaii. Emerg Infect Dis 13: 1675–1680. [DOI] [PubMed] [Google Scholar]
- 38. Hochberg NS, Blackburn BG, Park SY, Sejvar JJ, Effler PV, et al. (2011) Eosinophilic meningitis attributable to Angiostrongylus cantonensis infection in Hawaii: clinical characteristics and potential exposures. Am J Trop Med Hyg 85: 685–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.State of Hawaii Department of Health (2013) 10-year summary of reported cases of notifiable diseases. Hawai'i, 2003–2012. Available: http://health.hawaii.gov/docd/files/2013/07/dib_disease_counts_rates.pdf. Accessed 19 November 2013.
- 40. Pilsbry HA (1948) Land Mollusca of North America (north of Mexico). Acad Nat Sci 2: 521–1113. [Google Scholar]
- 41.Solem A (1984) A world model of land snail diversity and abundance. In: Solem A, van Bruggen AC, editors. World-wide snails. Leiden: Brill/Backhuys. pp. 6–22.
- 42. Cowie RH (1995) Variation in species diversity and shell shape in Hawaiian land snails: in situ speciation and ecological relationships. Evolution 49: 1191–1202. [DOI] [PubMed] [Google Scholar]
- 43. Cowie RH (1998) Patterns of introduction of non-indigenous non-marine snails and slugs in the Hawaiian Islands. Biodivers Conserv 7: 349–368. [Google Scholar]
- 44. Cowie RH (2001) Invertebrate invasions on Pacific Islands and the replacement of unique native faunas: a synthesis of the land and freshwater snails. Biol Invasions 3: 119–136. [Google Scholar]
- 45. Christensen CC, Yeung NW, Hayes KA (2012) First records of Paralaoma servilis (Shuttleworth, 1852) (Gastropoda: Pulmonata: Punctidae) in the Hawaiian Islands. Bishop Mus Occas Pap 112: 3–7. [Google Scholar]
- 46. Hayes KA, Yeung NW, Kim JR, Cowie RH (2012) New records of alien Gastropoda in the Hawaiian Islands: 1996–2010. Bishop Mus Occas Pap 112: 21–28. [Google Scholar]
- 47.Bergey EA, Figueroa LL, Mather CM, Martin RJ, Ray EJ, et al. (2013) Trading in snails: plant nurseries as transport hubs for non-native species. Biol Invasions 1–11. Available: http://link.springer.com.eres.library.manoa.hawaii.edu/article/10.1007/s10530-013-0581-1/fulltext.html. Accessed 20 December 2013.
- 48. Hayes KA, Tran CT, Cowie RH (2007) New records of alien Mollusca in the Hawaiian Islands: nonmarine snails and slugs (Gastropoda) associated with the horticultural trade. Bishop Mus Occas Pap 96: 54–63. [Google Scholar]
- 49. Brockelman CR, Chusatayanond W, Baidikul V (1976) Growth and localization of Angiostrongylus cantonensis in the molluscan host, Achatina fulica . Southeast Asian J Trop Med Public Health 7: 30–37. [PubMed] [Google Scholar]
- 50. Jarvi SI, Farias MEM, Howe K, Jacquier S, Hollingsworth R, et al. (2012) Quantitative PCR estimates Angiostrongylus cantonensis (rat lungworm) infection levels in semi-slugs (Parmarion martensi). Mol Biochem Parasitol 185: 174–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Richards CS, Merritt JW (1967) Studies on Angiostrongylus cantonensis in molluscan intermediate hosts. J Parasitol 53: 382–388. [PubMed] [Google Scholar]
- 52. Qvarnstrom Y, Sullivan JJ, Bishop HS, Hollingsworth R, da Silva AJ (2007) PCR-based Detection of Angiostrongylus cantonensis in Tissue and Mucus Secretions from Molluscan Hosts. Appl Environ Microbiol 73: 1415–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Carreno RA, Nadler SA (2003) Phylogenetic analysis of the Metastrongyloidea (Nematoda: Strongylida) inferred from ribosomal RNA gene sequences. J Parasitol 89: 965–973. [DOI] [PubMed] [Google Scholar]
- 54. Tokiwa T, Harunari T, Tanikawa T, Komatsu N, Koizumi N, et al. (2012) Phylogenetic relationships of rat lungworm, Angiostrongylus cantonensis, isolated from different geographical regions revealed widespread multiple lineages. Parasitol Int 61: 431–436. [DOI] [PubMed] [Google Scholar]
- 55. Qvarnstrom Y, da Silva ACA, Teem JL, Hollingsworth R, Bishop H, et al. (2010) Improved molecular detection of Angiostrongylus cantonensis in mollusks and other environmental samples with a species-specific internal transcribed spacer 1-based TaqMan assay. Appl Environ Microbiol 76: 5287–5289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wallace GD, Rosen L (1969c) Techniques for recovering and identifying larvae of Angiostrongylus cantonensis from molluscs. Malacologia 7: 427–438. [Google Scholar]
- 57. Yang X, Qu Z, He H, Zheng X, He A, et al. (2012) Enzootic angiostrongyliasis in Guangzhou, China, 2008-2010. Am J Trop Med Hyg 86: 846–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Bieler R (1993) Architectonicidae of the Indo-Pacific (Mollusca, Gastropoda). Abh Natwiss Ver Hambg (NF) 30: 1–377. [Google Scholar]
- 59. Bouchet P, Rocroi J-P (2005) Classification and nomenclator of gastropod families. Malacologia 47: 85–397. [Google Scholar]
- 60.Aktipis SW, Giribet G, Lindberg DR, Ponder WF (2008) Gastropoda: an overview and analysis. In: Ponder WF, Lindberg DR, editors. Phylogeny and evolution of the Mollusca. Berkeley and Los Angeles : University of California Press.pp. 201–238.
- 61. Strong EE, Gargominy O, Ponder WF, Bouchet P (2008) Global diversity of gastropods (Gastropoda; Mollusca) in freshwater. Hydrobiologia 595: 149–166. [Google Scholar]
- 62. Hata TY, Hara AH, Hu BK-S (1997) Molluscicides and mechanical barriers against slugs, Vaginula plebeia Fischer and Veronicella cubensis (Pfeiffer) (Stylommatophora: Veronicellidae). Crop Prot 16: 501–506. [Google Scholar]
- 63.Rueda A, Caballero R, Kamnsky R, Andrews KL (2002) Vaginulidae in Central America, with emphasis on the bean slug Sarasinula plebeia (Fischer). In: Barker GM, editor. Molluscs as Crop Pests. Wallingford, U. K.: CABI Publishing. pp. 115–144.
- 64. Meyer WM III, Cowie RH (2010) Feeding preferences of two predatory snails introduced to Hawaii and their conservation implications. Malacologia 53: 135–144. [Google Scholar]
- 65. Juřičková L (2006) Subulina octona (Bruguière, 1798) – a new greenhouse species for the Czech Republic (Mollusca: Gastropoda: Subulinidae). Malacol Bohemoslov 5: 1–2. [Google Scholar]
- 66. Morley NJ (2010) Aquatic molluscs as auxiliary hosts for terrestrial nematode parasites: implications for pathogen transmission in a changing climate. Parasitol 137: 1041–1056. [DOI] [PubMed] [Google Scholar]
- 67. Crook JR, Fulton SE, Supanwong K (1968) Ecological studies on the intermediate and definitive hosts of Angiostrongylus cantonensis (Chen, 1935) in Thailand. Ann Trop Med Parasitol 62: 27–44. [DOI] [PubMed] [Google Scholar]
- 68. Hu X-M, Tong C-J, Liu J (2007) Survey of epidemic focus of Angiostrongylus cantonensis in Hainan Province. China Trop Med 7: 1995–1996. [Google Scholar]
- 69. Liu J, Hu X-M, Wang S-Q (2007) Survey of natural infectious foci of Angiostrongylus cantonensis in Dingan County, Hainan. China Trop Med 7: 408–409. [Google Scholar]
- 70. Zhang R-L, Chen M-X, Gao S-T, Geng Y-J, Huang D-N, et al. (2008) Enzootic angiostrongyliasis in Shenzhen, China. Emerg Infect Dis 14: 1955–1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Zhang Y, Lv S, Yang K, Liu H-X, Hu L, et al. (2009) The first national survey on natural nidi of Angiostrongylus cantonensis in China. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi 27: 508–512. [PubMed] [Google Scholar]
- 72. Yen CM, Chen ER, Cheng CW (1990) A survey of Ampullarium canaliculatus for natural infection of Angiostrongylus cantonensis in south Taiwan. J Trop Med Hyg 93: 347–350. [PubMed] [Google Scholar]
- 73. Nishimura K, Mogi M, Okazawa T, Sato Y, Toma H, et al. (1986) Angiostrongylus cantonensis infection in Ampullarius canaliculatus (Lamarck) in Kyushu, Japan. The Southeast Asian J Trop Med Public Health 17: 595–600. [PubMed] [Google Scholar]
- 74. Lv S, Zhang Y, Steinmann P, Zhou X-N (2008) Emerging angiostrongyliasis in mainland China. Emerg Infect Dis 14: 161–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Wallace GD, Rosen L (1969d) Experimental infection of Pacific island mollusks with Angiostrongylus cantonensis . Am J Trop Med Hyg 18: 13–19. [PubMed] [Google Scholar]
- 76. Ratanarat-Brockelman C (1975) Inhibition of Rhabditis maupasi (Rhabditidae; Nematoda) maturation and reproduction by factors from the snail host Helix aspersa . J Invertebr Pathol 25: 229–237. [DOI] [PubMed] [Google Scholar]
- 77. Ratanarat-Brockelman C (1977) Isolation of nematode inhibitor from hemolymph of the snail, Helix aspersa . Biol Bull 152: 406–414. [DOI] [PubMed] [Google Scholar]
- 78. Ash LR (1976) Observations on the role of mollusks and planarians in the transmission of Angiostrongylus cantonensis infection to man in New Caledonia. Rev Biol Trop 24: 163–174. [Google Scholar]
- 79. Alicata JE (1966) The presence of Angiostrongylus cantonensis in islands of the Indian Ocean and probable role of the giant African snail, Achatina fulica, in dispersal of the parasite to the Pacific Islands. Can J Zool 44: 1041–1049. [DOI] [PubMed] [Google Scholar]
- 80.Maldonado A, Simões R, Thiengo SC (2012) Angiostrongyliasis in the Americas. In: Lorenzo-Morales J, editor Zoonosis. InTech. pp. 303–320.
- 81. Neuhauss E, Fitarelli M, Romanzini J, Graeff-Teixeira C (2007) Low susceptibility of Achatina fulica from Brazil to infection with Angiostrongylus costaricensis and A. cantonensis . Mem Inst Oswaldo Cruz 102: 49–52. [DOI] [PubMed] [Google Scholar]
- 82. Deng Z-H, Zhang Q-M, Huang S-Y, Jones JL (2012) First provincial survey of Angiostrongylus cantonensis in Guangdong Province, China. Trop Med Int Health 17: 119–122. [DOI] [PubMed] [Google Scholar]
- 83. Hadfield MG, Miller SE, Carwile AH (1993) The decimation of endemic Hawaiian tree snails by alien predators. Am Zool 33: 610–622. [Google Scholar]
- 84. Lydeard C, Cowie RH, Ponder WF, Bogan AE, Bouchet P, et al. (2004) The global decline of nonmarine mollusks. Bioscience 54: 321–330. [Google Scholar]
- 85. Rosenburg G, Muratov IV (2006) Status report on the terrestrial Mollusca of Jamaica. Proc Acad Nat Sci Philadelphia 155: 117–161. [Google Scholar]
- 86. Agnew P, Koella JC, Michalakis Y (2000) Host life history responses to parasitism. Microbes Infect 2: 891–896. [DOI] [PubMed] [Google Scholar]
- 87. Rolff J, Siva-Jothy MT (2003) Invertebrate ecological immunology. Science 301: 472–475. [DOI] [PubMed] [Google Scholar]
- 88. Asato R, Taira K, Nakamura M, Kudaka J, Itokazu K, et al. (2004) Changing epidemiology of angiostrongyliasis cantonensis in Okinawa Prefecture, Japan. Jpn J Infect Dis 57: 184–186. [PubMed] [Google Scholar]
- 89. Hollingsworth RG, Kaneta R, Sullivan JJ, Bishop HS, Qvarnstrom Y, et al. (2007) Distribution of Parmarion cf. martensi (Pulmonata: Helicarionidae), a new semi-slug pest on Hawaii Island, and its potential as a vector for human angiostrongyliasis. Pac Sci 61: 457–467. [Google Scholar]
- 90. Meyer WM III, Hayes KA, Meyer AL (2008) Giant African snail. Achatina fulica, as a snail predator. Am Malacol Bull 24: 117–119. [Google Scholar]
- 91. Kwong K-L, Chan RKY, Qiu J-W (2009) The potential of the invasive snail Pomacea canaliculata as a predator of various life-stages of five species of freshwater snails. Malacologia 51: 343–356. [Google Scholar]
- 92. Yeung NW, Hayes KA, Cowie RH (2013) Effects of washing produce contaminated with the snail and slug hosts of Angiostrongylus cantonensis with three common household solutions. Hawaii J Med Public Health 72 (Supplement 2)83–85. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Known gastropod hosts of Angiostrongylus cantonensis , associated localities and corresponding key references.* References reporting only experimental laboratory infection - no locality is given for such studies. ** References reporting both natural and experimental infection.
(DOCX)