Abstract
Background
Various lines of evidence including epidemiological, genetic and foetal pathogenetic models suggest a compelling role for Interleukin-6 (IL-6) in the pathogenesis of schizophrenia. IL-6 mediated inflammatory response triggered by maternal infection or stress induces disruption of prenatal hippocampal development which might contribute towards psychopathology during adulthood. There is a substantial lack of knowledge on how genetic predisposition to elevated IL-6 expression effects hippocampal structure in schizophrenia patients. In this first-time study, we evaluated the relationship between functional polymorphism rs1800795 of IL-6 and hippocampal gray matter volume in antipsychotic-naïve schizophrenia patients in comparison with healthy controls.
Methodology
We examined antipsychotic-naïve schizophrenia patients [N = 28] in comparison with healthy controls [N = 37] group matched on age, sex and handedness. Using 3 Tesla – MRI, bilateral hippocampi were manually segmented by blinded raters with good inter-rater reliability using a valid method. Additionally, Voxel-based Morphometry (VBM) analysis was performed using hippocampal mask. The IL-6 level was measured in blood plasma using ELISA technique. SNP rs1800795 was genotyped using PCR and DNA sequencing. Psychotic symptoms were assessed using Scale for Assessment of Positive Symptoms and Scale for Assessment of Negative Symptoms.
Results
Schizophrenia patients had significantly deficient left and right hippocampal volumes after controlling for the potential confounding effects of age, sex and total brain volume. Plasma IL-6 levels were significantly higher in patients than controls. There was a significant diagnosis by rs1800795 genotype interaction involving both right and left hippocampal volumes. Interestingly, this effect was significant only in men but not in women.
Conclusion
Our first time observations suggest a significant relationship between IL-6 rs1800795 and reduced hippocampal volume in antipsychotic-naïve schizophrenia. Moreover, this relationship was antithetical in healthy controls and this effect was observed in men but not in women. Together, these observations support a “differential susceptibility” effect of rs1800795 in schizophrenia pathogenesis mediated through hippocampal volume deficit that is of possible neurodevelopmental origin.
Background
Neurodevelopmental model postulates schizophrenia as a behavioural outcome of aberration in neurodevelopmental processes that begins long before the onset of clinical symptoms and is caused by a combination of genetic and environmental factors [1]. Among the various parameters that comprise gene-environment interactions, the crosstalk between genetic factors and obstetric complications has been put forth as one of the important mechanisms that increase the risk towards schizophrenia [2]. In this context, it is noteworthy that prenatal maternal infections with resultant persistent pro-inflammatory state leading to aberrant neurodevelopment are among the important obstetric complications that have been shown to confer increased risk for schizophrenia[3].
Epidemiological observations strongly support the association between elevated risk for schizophrenia in the offspring and prenatal exposure to influenza, toxoplasma, rubella, genital-reproductive infections & various other infections [4]. Research studies involving animal models examining the resultant maternal immune activation (MIA) secondary to prenatal infection has offered robust support to these epidemiologic findings through replicated observations that MIA causes phenotypes analogous to those found in patients with schizophrenia[5]. In this context, it is noteworthy that Interleukin-6 (IL-6) – a predominantly pro-inflammatory cytokine – has been shown to play a pivotal role in mediating the aberrant influence of MIA on fetal brain development leading to putative abnormalities that might possibly underlie the pathogenesis of schizophrenia [6].
In addition, various lines of evidence suggest that there is a compelling role for IL-6 to underlie the pathogenesis of schizophrenia as summarized by the following observations: i) rigorous meta-analytic studies supporting significantly higher serum levels of IL-6 in schizophrenia patients that correlate with symptom severity [7]; ii) association of schizophrenia with IL-6 gene polymorphism [8], [9]; iii) potential influence on hippocampus by peripheral IL-6 with hippocampus being the one of the most important brain regions implicated in schizophrenia[10]; iv) IL-6 playing a vital role in established models like ‘ketamine model’ of schizophrenia [11]; v) IL-6 being implicated in foetal-pathogenetic model of neurodevelopmental aberrations in schizophrenia [6].
The human IL-6 gene is located on chromosome 7p15–7p21 and regulation of IL-6 expression is influenced by subtle variations in the promoter. A functional G→C single-nucleotide polymorphism at position -174 (rs1800795) of the promoter has been described. Relationship between rs1800795 genotype and plasma levels of IL-6 is complex because of its involvement in gene × environment interaction [12]. The rs1800795 transversion is shown to affect IL-6 gene expression by modulating binding of transcription factors such as GATA1 [13], with few studies showing that the GG allele is associated with greater induction of IL-6 compared with the GC or CC alleles [14]. This modulation, however, may be subjective to presence of environmental conditions that induce the transcription factor activity [12]. Because of the necessary precondition, phenotypic manifestation of rs1800795 is not universal. For instance, impact of rs1800795 (in terms of association with increased IL-6 levels) has been found in inflammation-related conditions such as ageing [15], systemic-onset juvenile chronic arthritis [14], liver cirrhosis, hepatocellular carcinoma [16], primary Sjogren's syndrome [17] as well as in medicated schizophrenia patients [8]. However, lack of this association [18] or even reversal of such association [19] has been reported in healthy individuals, which possibly reflects absence of environmental triggers of transcriptional activity. A recent meta-analysis provides evidence that rs1800795 polymorphism is not significantly associated with circulating IL-6 levels in a normal population [20].
Interestingly, the regulation of IL-6 production by this polymorphism is more pronounced in neonates than in adults [21], indicating that rs1800795 might play a prominent role in determining the inflammatory response during the phases of early environmental adversities in pathogenesis of schizophrenia. Hence, it is possible that maternal immune activation during pregnancy (which is more prevalent in schizophrenia patients compared to healthy controls [22], [23]) might result in hyper-inflammatory IL-6 response in individuals with GG allele. Further, rs1800795 can moderate the impact of alterations in functioning of HPA axis and sympathetic nervous system which might result from neonatal immune challenge, such that C allele carriers would be protected from the heightened inflammation related to adverse environmental conditions [12]. Though these background works suggest a critical role for IL-6 in the pathogenesis of schizophrenia, one has to acknowledge this is clearly a candidate gene approach since currently there is only a limited support for the proposed SNP in terms of evidence from genome wide association studies on schizophrenia pathophysiology.
In relation to IL-6 gene polymorphisms, various lines of evidence support a significant relationship between rs1800795 and pre-term birth - another important obstetric complication that has been associated with elevated risk for schizophrenia [24], [25]. Moreover, prematurity at birth has been consistently shown to be linked with hippocampal volume deficit in a recent meta-analysis [26]; in addition, it has been demonstrated such hippocampal volume aberrations in preterm infant might have long lasting adverse impact on cognitive functions [27]. These observations become significant with relevance to schizophrenia, since hippocampus is a critical brain region implicated in the pathogenesis of this disorder [28], [29], [30], [31]; more specifically, hippocampal volume deficit in schizophrenia has been shown to be significantly influenced by obstetric complications [32].
Relationship between peripheral IL-6 and hippocampal volume has been reported by previous studies. One study that has examined middle-aged, healthy individuals demonstrated a significant inverse relationship between the plasma IL-6 level and left hippocampal gray matter volume [10]. Another study has shown that in patients with first-episode psychosis, IL-6 expression in the peripheral leucocyte significantly predicted a smaller left hippocampal volume [33].
Thus, several lines of evidence as reviewed above implicate a compelling role for elevated IL-6 in the pathogenesis of schizophrenia possibly through disruption of prenatal hippocampal development. Increased level of IL-6 has been associated with smaller hippocampal volume. In the context of IL-6 gene promoter polymorphism, since GG allele is associated with increased expression of IL-6 gene in the presence of environmental conditions, one would predict this allele to be associated with smaller volume of hippocampus in schizophrenia patients. However, to the best of our knowledge, the effect of IL-6 promoter polymorphism on hippocampal volume is yet to be evaluated in schizophrenia. In this study, we evaluated the relationship between IL-6 promoter polymorphism and hippocampal gray matter volume in antipsychotic-naïve schizophrenia patients (N = 28) in comparison with healthy controls (N = 37). We hypothesized that schizophrenia patients with GG allele will demonstrate significantly deficient hippocampus volume in comparison with healthy controls.
Methods
Patients attending the clinical services of the National Institute of Mental Health & Neurosciences (India), who fulfilled DSM-IV criteria for schizophrenia and were never treated with any psychotropic medications including antipsychotics & not having substance abuse [n = 28; age = 29.9±5.7; 14-males], were examined in this study. The diagnosis of schizophrenia was established using Mini International Neuropsychiatric Interview Plus [34], which was confirmed by another psychiatrist through an independent clinical interview. The details related to illness onset and antipsychotic-naïve status were carefully ascertained by reliable information obtained from at least one reliable adult relative. Psychotic symptoms were assessed using Scale for Assessment of Positive Symptoms (SAPS) and Scale for Assessment of Negative Symptoms (SANS). Healthy controls (N = 37) (age = 27.4±5.6 years; 20-males), who volunteered for study, were screened to rule out any psychiatric diagnosis using the MINI as well as a comprehensive mental status examination. None of the controls had family history of psychiatric disorder in first-degree relatives. To avoid the potential confounding effect of differential handedness, only right handed subjects were included in this study.
Patients and controls did not have features suggestive of alcohol abuse/dependence. None used stimulant or opiate drug. None had history or clinical feature suggestive of neurological/medical disorder. None had abnormal movements as assessed by Abnormal Involuntary Movements Scale. Clinical assessments and blood sample collection were performed on the same day before starting antipsychotics. After complete description of study to the subjects, written informed consent was obtained. The research protocol was reviewed and approved by the National Institute of Mental Health and Neurosciences (NIMHANS) ethics committee.
MRI Acquisition
MRI was done with 3.0 Tesla scanner (Achieva, Philips, Best, The Netherlands). T1 weighted images were acquired using the following parameters: TR = 8.1 msec, TE = 3.7 msec, nutation angle = 8 degree, FOV = 256 mm, slice thickness = 1 mm without inter-slice gap, NEX = 1, matrix = 256×256. The images were transferred on to a personal computer (PC) platform. They were stored with coded identification.
Hippocampus Volumetry
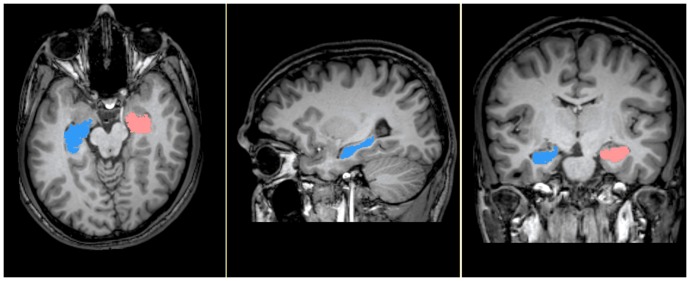
Bilateral hippocampi were measured using MRI scans with the software ‘3D Slicer 3.4’ (http://www.slicer.org/). The structure was outlined by the rater using the computer mouse controlled pointer and measured using semi-automated three-dimensional interactive method (Figure-1). The gray matter area was marked in each consecutive coronal slice along the anterior to posterior using anatomical landmarks validated using review of literature [35]. Anteriorly, first appearance of alveus was identified to reliably differentiate hippocampus from amygdala [36].Superior border of hippocampus is covered by the alveus and adjoins to cerebrospinal fluid while the inferior border was identified by the white matter of the parahippocampal gyrus below the subiculum. The lateral ventricle CSF was used as an external landmark to define the lateral extent. Medial border was defined superiorly by the CSF of the cisterna ambiens and inferiorly by vertical arbitrary line placed at the dorsomedial tip of the white matter of the parahippocampal gyrus[37]. Coronal slice where an ovoid gray matter starts to appear inferiomedially to the trigone of the lateral ventricle marked the posterior end [38]. Sagittal and axial sections were used to confirm the anatomical landmarks. Further, after tracing on coronal section, imperfections were rectified on sagittal plane, such as excluding any areas above hippocampal body (fimbria) and confirming the posterior extent of the hippocampal tail. The total brain volume, which was used as a covariate in statistical analyses to control for the potential confounding effect of the global brain size, was automatically computed using established methods (FMRIB Software Library (FSL); www.fmrib.ox.ac.uk/fsl/). In order to assess reliability of the measures, another trained rater blind to diagnosis of subject, independently carried out hippocampal volumetry in a random subset of data (n = 10). Excellent reliability was ascertained with intra-class correlation coefficient of more than 0.95.
Figure 1. Cerebral MRI depicting manual segmentation of hippocampus.
Figure shows MRI with segmented hippocampus in axial, sagittal & coronal sections within 3D-Slicer software interface.
Voxel Based Morphometry [VBM]
T1-weighted images were processed using SPM8 (Wellcome Trust Centre for Neuroimaging, UCL, London; UK; http://www.fil.ion.ucl.ac.uk/spm) implemented in the VBM Toolbox 8 (http://dbm.neuro.uni-jena.de/vbm.html) under MatLab 7.8.0 (The MathWorks Inc., Sherborn, MA, USA). Standard routines and default parameters of the VBM 8 toolbox were applied. After setting the image origin to the anterior commissure, images were bias corrected, pre-registered to standardized International consortium for brain mapping (ICBM- East Asian brains) space using affine transformation with regularization and segmented using the “unified segmentation” approach [39]. SPM8 segmentation was based on a modified gaussian mixture model to avoid misclassification. Spatial adaptive non local means (SANLM) filter was applied to remove noise while preserving edges. To remove isolated voxels of one tissue class within a cluster of voxels belonging to a different tissue class, a hidden Markov random field model weighting was used. Spatial normalization was performed with high-dimensional Diffeomorphic Anatomical Registration using the Exponentiated Lie algebra (DARTEL) template [40]. Grey matter segments were modulated by the Jacobian determinants of the deformations to account for local expansion and compression introduced by non-linear transformation. Finally, the gray matter images were smoothed with an 8-mm full-width at half-maximum (FWHM) isotropic gaussian kernel. This was done to reduce errors related to inter-subject variability in local anatomy and to render the imaging data more normally distributed. Bilateral hippocampal mask was created using the Wake Forest University school of medicine (WFU) Pickatlas [41].
Total brain tissue volume was estimated using Sienax tool of FSL package [42]. Statistical parametric maps were examined for effect of diagnosis as well as diagnosis by genotype interaction of voxel-wise hippocampal gray matter volume using ANCOVA model controlling for confounding effects of sex, age and total intracranial volume. In order to avoid possible edge effects between different tissue types, all voxels with gray matter values of less than 0.1 (absolute threshold masking) were excluded. Further, threshold of uncorrected p<0.05 was applied, since GLM analyses were limited to a priori region contained within the hippocampal mask.
IL-6 Assay
Blood samples were collected between 0800 and 0900 hrs. (A.M) after 12-hour overnight fast from ante-cubital vein into K2 EDTA vacutainer tubes (Becton & Dickinson, U.S.A). Plasma were collected within 30 minutes of collection by centrifugation for15 minutes at 1000 x g, which were then aliquoted and stored at −80°C. Quantitative sandwich enzyme immunoassay for IL-6 was done using commercial ELISA method with sensitivity <0.70 pg/mL (R&D Systems, MN, USA) following manufacturer's instructions. All samples were coded; thawed only once and analyzed by the same investigator, who was blind to the clinical situation.
SNP Genotyping
DNA extraction was carried out using commercial spin column method (Qiagen, Inc.). First, the samples were treated with protease and then with WBC lysis buffer by incubating the mixture at 56 C for 10 min. Later, digested samples were treated with ethanol and then, subjected to spin column for isolation of genomic DNA. The columns were washed twice and then DNA is eluted in elution buffer. Extracted DNA was checked on agarose gel and stored at -80 C for later use.
The PCR amplification of the IL-6 promoter was performed with published primers [43] using True Allele PCR Mix protocol in Veriti Thermal Cycler (Applied Biosystems). The PCR products were checked on agarose gel, purified using silica spin column (Invitrogen) and sequenced using Big Dye Terminator v3.1. cycle sequencing kit (Applied Biosystems). Sequencing was carried out on 3730XL Genetic Analyzer (Applied Biosystems) with PCR primers as well as two additional internal primers published previously [44]. SeqScape v2.7 software (Applied Biosystems) was used for the assembly of the sequence data. The reference sequence of the IL-6 promoter region used for analysis was downloaded from the Ensembl Genome Browser release 67 - GRCh37, May 2012 [45].
All subjects were of Indian origin. Expected minor (C) allele frequency of rs1800795 in Indian population is 0.12 [GIH population - HapMap database Release 27]. (http://hapmap.ncbi.nlm.nih.gov/cgi-perl/gbrowse/hapmap27_B36/?name=SNP%3Ars1800795) [46]. Due to the low prevalence of the rs1800795 C allele in our population, only 2 CC homozygotes out of total 65 subjects were detected. Hence, the genotypes are grouped into two as “GG” and “GC/CC” using the dominant model, similar to some of the earlier rs1800795 studies[47]. Data were tested for normality using the Shapiro–Wilks test. Data analysis was performed using the SPSS-11.0 using the following statistics: student's t-test (two-tailed), chi-square test, Pearson's correlation, and general linear model based multivariate & univariate analysis of covariance (ANCOVA).
Results
The age and sex ratio in both patient and control groups was similar. The genotypes were consistent with Hardy-Weinberg equilibrium proportions in both the groups [Patient: χ2 = 0.6; p = 0.4; Control: χ2 = 1.5; p = 0.2]. Distribution of the minor allele and genotypes for rs1800795 polymorphism are presented in Table-1. There was no significant difference in the frequency distribution of rs1800795 alleles and genotypes.
Table 1. Comparative Profile of rs1800795 genotype distribution between patients & controls.
Schizophrenia patients had significantly deficient left and right hippocampal volumes after controlling for the potential confounding effects of age, sex and total brain volume (Table- 2) Also, plasma IL-6 levels were significantly higher in patients than controls. However, the levels did not differ between GG and GC/CC genotypes [Patient: t = 0.3; p = 0.9; Control: t = 0.2; p = 0.8].
Table 2. Comparative profile of schizophrenia patients and healthy controls.
| Characteristic | Patients | Controls | Statistic | p |
| N | 28 | 37 | ||
| Age [Years] * | 29.9±5.7 | 27.4±5.6 | t = 1.8 | 0.1 |
| Sex Ratio [M:F] $ | 14∶14 | 20∶17 | ?2 = 0.1 | 0.7 |
| Left Hippocampal Volume [mL]# | 2.4±0.4 | 2.7±0.3 | F = 9.8 | 0.003 |
| Right Hippocampal Volume [mL]# | 2.6±0.5 | 2.9±0.3 | F = 10.5 | 0.002 |
| Plasma IL-6 [pg/mL] * ,£ | 2.2±1.8 | 1.4±0.8 | t = 2.3 | 0.03 |
| IL-6 Genotype [GG: GC/CC]$ | 21∶7 | 27∶10 | ?2 = 0.03 | 0.9 |
* - Independent samples t-test;
- Chi-Square test; # - ANCOVA.
£ - data was available for 25 patients and 33 controls.
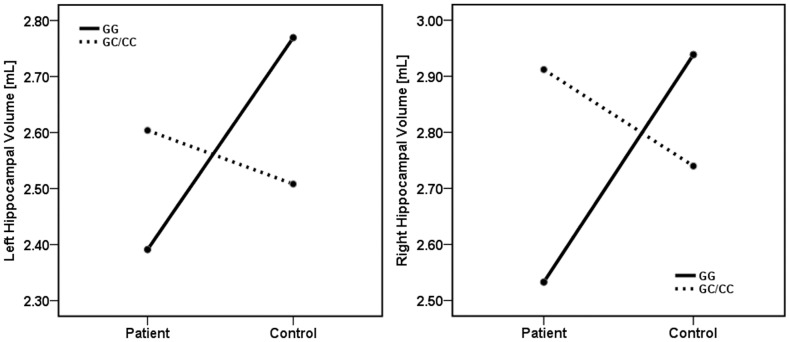
Multivariate ANCOVA on bilateral hippocampal volume, controlling for the potential confounding effects of age, sex and total brain volume revealed a significant ‘diagnosis by genotype’ interaction [F = 5.0; p = 0.01] involving both right [F = 10.2; p = 0.002] and left [F = 6.6; p = 0.013] hippocampal volumes (Figure-2 & Figure-3). Follow-up analyses to uncover the effect of genotype identified that in the subgroup of subjects who carried “GG” genotype, the hippocampal volumes were significantly deficient in patients in comparison with healthy controls [Right: F = 23.2; p<0.001; Left: F = 20.5; p<0.001], whereas in the subgroup with “GC/CC” genotype, the hippocampal volumes did not differ significantly [Right: F = 0.7; p = 0.4; Left: F = 0.7; p = 0.4]. Within healthy controls, GG homozygotes had significantly larger bilateral hippocampal volume compared to GC/CC carriers [Right: F = 5.2; p = 0.03; Left: F = 4.6; p = 0.04]; on the contrary, the reverse was true in patients [but significant only for right side; Right: F = 5.5; p = 0.03; Left: F = 1.7; p = 0.2]. [ As the control group consisted of a larger number of subjects than the patients' group, it is possible that this might have differentially influenced the variance of the measurements between groups. To explore this further, we performed additional analyses involving patients (N = 28) and equal number of controls (N = 28) with one-to-one matching. The results of these analyses did not differ from the observations from the whole group (supplementary material - S1)].
Figure 2. Significant diagnosis-by-genotype interaction on hippocampal volumes.
Figure shows significant diagnosis by genotype interaction involving both right and left hippocampal volumes – i.e. the effect of rs1800795 genotypes [GG & GG/GC] on hippocampal volume was found to be antithetical between patients and controls.
Figure 3. Box plot depicting significant diagnosis-by-genotype interaction on hippocampus volume between patients and controls.
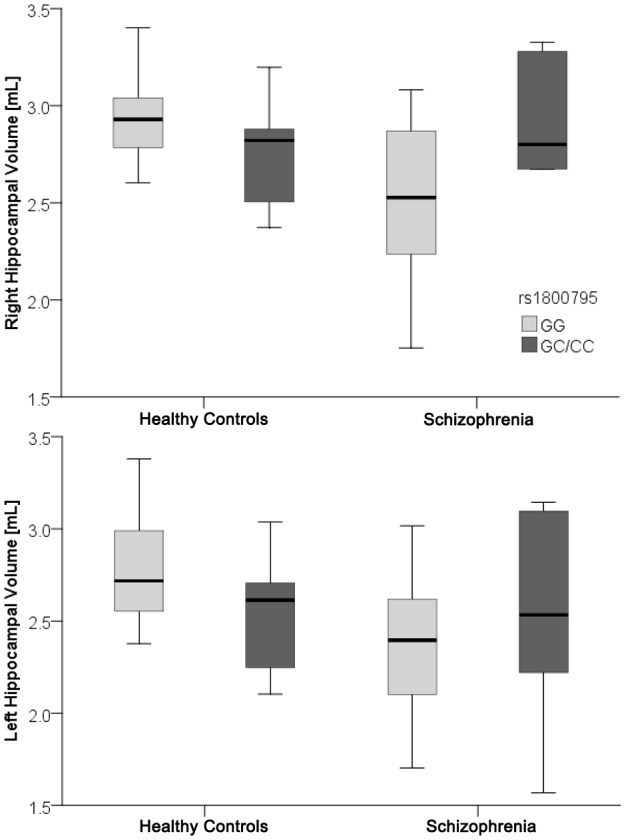
Figure shows box plot depicting significant diagnosis by genotype interaction involving both right and left hippocampal volumes – i.e. the effect of rs1800795 genotypes [GG & GG/GC] on hippocampal volume was found to be antithetical between patients and controls; the interaction boxplot shows the five statistics of hippocampal volume (minimum, first quartile, median, third quartile, and maximum) for each genotype per group.
Further, exploratory analyses of ‘diagnosis by genotype by sex’ interaction was found to be significant for left [F = 5.6; p = 0.02] but only showed a trend towards significance for right [F = 3.6; p = 0.06] hippocampal volumes. The significance of this three-way interaction was contributed by presence of ‘diagnosis by genotype’ interaction only in males but not in females. The observed post- hoc power of the study at α = 0.05 for bilateral volumetric differences between diagnostic groups was found to be 87%. However, the observation pertaining how sex modulates the effect of genotype and diagnosis on hippocampal volume should be treated with caution, given the small sample sizes in individual cells [Male GG: GC/CC = 9∶5 (Patients), 14∶6 (Controls); Female GG: GC/CC = 12∶2 (Patients), 13∶4 (Controls)].
In parallel to manual morphometry, automated VBM analysis also showed main effect of diagnosis as well as diagnosis-by-genotype interaction effect on hippocampal gray matter in the same direction (Figure-4). Coordinates, cluster size and peak t value for the VBM analysis of main and interaction effect are presented in Table-3. Further, hippocampal volumes obtained from manual and voxel wise measurements showed a significant and strong positive correlation [Right: r = 0.8; p<0.001; Left: r = 0.8; p<0.001].
Table 3. VBM analysis of hippocampal gray matter.
| Comparison | Side | MNI Coordinates [X,Y,Z] | Cluster Size | T | df | p |
| Main effect of diagnosis | ||||||
| Controls > Patients | Left | −35 −18 −18 | 2049 | 5.13 | 60 | <0.001 |
| Right | 18 −30 −5 | 2148 | 5.24 | 60 | <0.001 | |
| Patients > Controls | Left | Nil | - | - | - | - |
| Right | Nil | - | - | - | - | |
| Diagnosis-by- genotype interaction effect | ||||||
| (GG - controls & GC/CC - patients) > (GC/CC- controls & GG - patients) | ||||||
| Left | −30 −19 −18 | 61 | 2.03 | 58 | 0.023 | |
| Right | 24 −1 −24 | 37 | 1.99 | 58 | 0.025 | |
| Right | 41 −19 −15 | 29 | 1.92 | 58 | 0.029 | |
| (GC/CC - controls & GG - patients) > (GG - controls & GC/CC - patients) | ||||||
| Left | Nil | - | - | - | - | |
| Right | Nil | - | - | - | - |
Figure 4. Significant effect of diagnosis-by-genotype interaction on hippocampus volume as demonstrated by voxel based morphometry analysis.
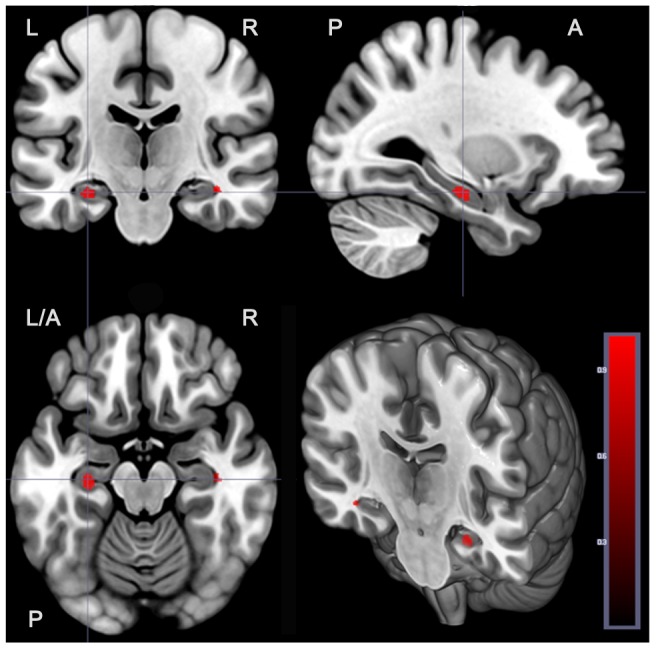
Red blobs in the figures show significant diagnosis by genotype interaction involving both right and left hippocampal volumes by VBM in parallel to those that were observed in manual analyses.
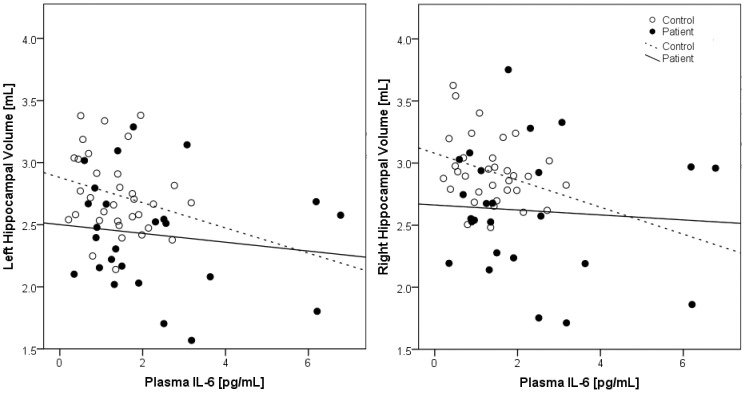
Plasma IL-6 level had a significant negative correlation with left hippocampal volume (r = −0.3; p = 0.04) and trend-level negative correlation with right hippocampal volume (r = −0.2; p = 0.08) in analyses involving all subjects (patients & controls). Sub-group analyses revealed non-significant negative correlation between IL-6 and bilateral hippocampal volume in controls [Right: r = −0.3; p = 0.09; Left: r = −0.2; p = 0.2] as well as patients [Right: r = −0.1; p = 0.7; Left: r = −0.1; p = 0.5] (Figure-5).
Figure 5. Scatter plot depicting the relationship between plasma IL-6 level and hippocampal volumes in schizophrenia patients and healthy controls.
The psychopathology scores of the entire patients sample were: Total SAPS score = 34.9±14.6; Total SANS score = 55.5±26.5. The genotype sub-groups of patients (i.e. GG vs. [GC/CC]) did not significantly differ in total symptoms scores [SAPS: t = 0.9; p = 0.3; SANS: t = 0.5; p = 0.6]. No significant correlations were found between total symptoms scores and IL-6 levels [SAPS: r = 0.2; p = 0.3; SANS: r = 0.2; p = 0.4]. There was a trend towards significant negative correlation between SAPS and hippocampal volume [Right: r = −0.3; p = 0.1; Left: r = −0.4; p = 0.05], but no correlation was found between SANS and hippocampal volume [Right: r = 0.09; p = 0.7; Left: r = 0.2; p = 0.4]
Discussion
The study observations offer novel insights into the effect of rs1800795 polymorphism on the hippocampal volume in schizophrenia in comparison with healthy controls. We observed that schizophrenia patients had significantly deficient left and right hippocampal volumes as well as higher plasma IL-6 in comparison with healthy controls. There was a significant diagnosis by genotype interaction involving both right and left hippocampal volumes– i.e. the effect of rs1800795 genotypes [GG & GG/GC] on hippocampal volume was found to be antithetical between patients and controls. Also, hippocampal volume was significantly deficient only in the subset of patients with “GG” genotype and not with “GG/GC”, in comparison to genotype-matched controls. However, plasma IL-6 showed only weak correlation with hippocampal volumes; there was no significant difference in the frequency distribution of rs1800795 genotypes between patients and controls; also, IL-6 levels did not show any significant relationship with genotypes.
Various lines of evidence argue for a critical role of hippocampus & its interactions with other brain regions to underlie the pathogenesis of schizophrenia [28], [31], [48], [49], [50]. Interestingly, the influence of hippocampus becomes especially significant given the ‘stress-diathesis’ paradigm as an important explanatory model for schizophrenia [51], [52]. This ‘stress-diathesis’ paradigm invokes the impact of various immunobiological parameters (for example – IL-6 and various other cytokines) [53]. Contextually, our study finding of hippocampal volume deficit is in tune with this proposition.
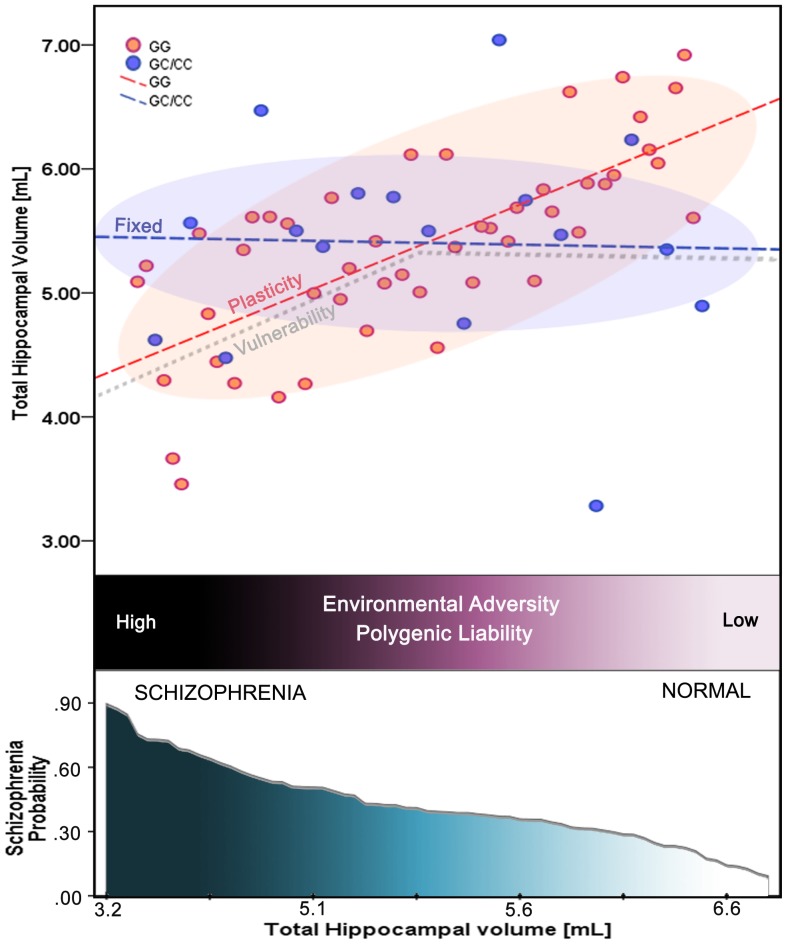
In a recent study examining healthy subjects, the polymorphism rs1800795 showed a strong main effect of genotype with the volume of the right hippocampus head. Homozygous carriers of the G-allele had significantly larger hippocampal gray matter volumes compared to_heterozygous subjects [54]. Our study observation in healthy control group replicates this finding. On the contrary, in patients, the direction of association was reverse – i.e. – those with “GG” genotype had overall smaller hippocampal volume. Thus, these observations pertinent to influence of rs1800795 promoter polymorphism on the volume of hippocampus are antithetical in controls and patients. Such an antithetical influence which is diametrically differential between patients and controls might support the “differential susceptibility” model (Figure-6). The “differential susceptibility” postulate argues that the same genes that convey increased vulnerability to psychopathology under adverse environmental conditions have the potential to act in advantageous ways on psychological functioning when the environment is supportive or enriched [55], [56]. Interestingly, there is some support that rs1800795 promoter polymorphism show differential susceptibility in that a study that has applied computational identification of gene–social environment interaction at the human IL-6 locus showed that this polymorphism might have functional impact on gene expression only in the presence of adverse environmental/social conditions [12]. The underlying molecular mechanism has been demonstrated to be mediated through modulation of β-adrenergic activation of GATA1 and particularly GG genotype of rs1800795 is shown to be GATA1 sensitive. Interestingly, the transcription factor GATA1, is implicated in reduction in brain volume and a decrease in the size and density of neurons [57]. Thus, it is possible that under the influence of environmental factors, the GG polymorphism might have resulted in deleterious impact on neurodevelopment leading to decreased hippocampal volume.
Figure 6. Illustration of the differential susceptibility in contrast to diathesis-stress model.
Total hippocampal volume is represented against environmental adversity and polygenic liability that are known to be associated with schizophrenia (not quantified in this study). Pink and blue lines depict homozygous G and carrier of C rs1800795 genotypes respectively, that differ in their responsiveness to environmental and epistatic factors: the “plasticity” conferred by homozygous G is disproportionately more affected by influences compared to the “fixed” C carrier group. The gray line depicts “vulnerability”, that is affected only when exposed to an adversity, and this diathesis-stress view is not supported by data in the current study. In relation to these models, the bottom panel shows predicted probability of 'schizophrenia' outcome as the function of total hippocampal volume.
Hippocampus is among the brain regions with the most pronounced expression of IL-6 receptors [58] and is sensitive to IL-6 in the peripheral blood, which can enhance dopamine turnover in the hippocampus [59] . Animal studies of prenatal immune activation have shown that maternal IL-6 can cross the placenta [60] and disrupt fetal brain development [61] by potentially inhibiting neuronal survival [62], dendritic outgrowth and branching of hippocampal and cortical neurons [63]. Further, fetal exposure to IL-6 in rats can lead to increased IL-6 levels in the peripheral circulation and hippocampus as well as abnormalities of hippocampal structure, morphology and function during adulthood [64].
On the other hand, in absence of inflammatory response, IL-6 can act as neuropoietic cytokine and neurotrophic agent [65], plays critical role in neurite outgrowth, survival, proliferation and gene expression [66]. Further, pathological increase or complete deficiency of IL-6 both can result in impairment of hippocampal dependent function like learning in mice [67] implicating that perhaps an appropriate IL-6 balance might be crucial for normal neurodevelopment and function.
We observed that, in the subgroup with “GC/CC” genotype, the hippocampal volumes were not significantly deficient in patients in comparison to genotype matched controls. Perhaps, this could be seen as neuroprotective effect of “C” allele in schizophrenia patients. However, this effect was seen only in male patients. In this context, it is noteworthy that exposure to sex hormones like androgen and estrogen potentially modulates IL-6 production [68], [69]. Moreover, genetic predisposition to produce high levels of IL-6 by rs1800795 could itself be sex-dependent with the earlier study reporting the effect of this SNP on serum IL-6 in men, but not in women [70]. Further, another study has shown sex differences in terms of hippocampal sub regions which were affected in rats prenatally exposed to IL-6 [64]. However, it is important to note that the sample size of this study is very small given the difficulty in recruiting antipsychotic-naïve schizophrenia patients for these studies. Hence, the potential modulatory influences of the sex on the effect of genotype and diagnosis on hippocampal volume should be treated with caution, given the small sample sizes in individual cells.
The image analysis methodologies used in this study merit further discussion. While manual morphometry reliably informs about overall volume differences over a specified region, VBM provides more detail regarding the specific points of maximal change within a structure. Both of these methods have been demonstrated to be capable of detecting differences in small areas such as the hippocampus [71], [72], [73], [74], [75]. Particularly, VBM is free of rater and anatomic variability related reliability issues and biases [76]. However, VBM is known to be sensitive to optimization methods (e.g. creation of study-specific template), smoothing parameters (FWHM of Gaussian kernel) [77] and may be prone to errors such as mis-registration of small regions of interest and tissue misclassification [78].
On the contrary, manual volumetric techniques are prone for rater-dependent errors. To minimize such errors, in this study, we have ascertained optimal inter-rater reliability. Moreover, the rater who performed the volumetry was blind to the status of the subject since the analysis was performed on coded images. In addition, it is important to note that hippocampal volumes reported in our study range from 2.4 to 3.6 ml (right) and 2.1 to 3.4 ml (left) for healthy controls, 1.7 to 3.8 ml (right) and 1.6 to 3.3 ml (left) for patients [Mean ± SD given in Table-2]; this is comparable with various earlier studies that have examined hippocampal volume of schizophrenia patients and normal individuals [79], [80], [81], [82]. Nonetheless, it is noteworthy that a large discrepancy in mean hippocampal volume can be seen in available literature, due to factors such as heterogeneous procedures for anatomic definition of the hippocampus, different approaches to measure volume, i.e. manual vs. automated methods, inclusion of white and gray matter in the definition of the region etc. Data from online database - ‘The Internet Brain Volume Database’ (http://www.cma.mgh.harvard.edu/ibvd) shows that average unadjusted hippocampal volumes among normal individuals in the age group similar to that of our study (17–42 yr.), from 67 studies published from 2000–2010 are 3.3 ml for left (ranging from 2.1 to 4.5 ml) and 3.4 ml for right (ranging from 2.1 to 4.7 ml). We find that hippocampal volumes of healthy controls in_ our study approximately correspond to the first quartile of these reported values (left − 2.8 ml, right – 3.0 ml). To summarize, the hippocampal volumes obtained in this study is comparable with many previous studies in this area. It has been suggested earlier [83] that application of a combination of both of these methods (manual as well as VBM) might provide highest reliability and accuracy. Hence, combined application of these image analysis methods has added to the methodological strength of this study.
In this study, we have observed a significant impact of IL-6 genotype on bilateral hippocampus in healthy controls with the directionality of effect in tune with a previous observation [54]; on the other hand, in schizophrenia patients the effect of G-allele on the hippocampal volume was restricted to the right side. With regards to the lateralization of hippocampus volume deficits in schizophrenia, while few studies have shown significant volume deficits in bilateral hippocampi in antipsychotic-naive schizophrenia [84], [85], others have shown deficits to be significantly pronounced in one of the hemisphere, either right [86], [87] or left [88]. Keeping this in mind, we have examined right and left hemisphere separately. An important future direction would be to explore for any consistent observation to support status-differential genotypic effects with regards to the laterality of hippocampal involvement in larger samples.
While our study observations have implicated global hippocampal deficits to be linked IL-6 promoter polymorphism, one potential area of further enquiry would be the specific relationship of sub-regions of hippocampus in terms of any differential associations. We used 3Tesla MR images with 1 mm resolution, which allows efficient segmentation of hippocampus in manual morphometry as well as VBM. However, the methods used in this study do not permit the accurate localization of maximal volume change within a relatively small brain structure such as hippocampus. We believe that this is an important aspect that needs to be evaluated in future studies. Also, it is noteworthy that the correlation between hippocampus and IL-6 in the whole sample seems weak despite the observations that the hippocampi are smaller in patients with concurrent greater levels of plasma IL-6. Perhaps, examination of a larger sample might facilitate further elucidation of this relationship. This is another important future direction.
In summary, our first time observations suggest significant relationship between GG allele of IL6 promoter polymorphism rs1800795 and reduced hippocampal volume in antipsychotic-naïve schizophrenia patients. Moreover, this relationship was antithetical in healthy controls, and this effect was observed in men but not in women. Together, these observations support a “differential susceptibility” effect of rs1800795 in schizophrenia pathogenesis mediated through hippocampal volume deficit that is of possible neurodevelopmental origin.
Supporting Information
Comparative analysis of one-to-on matched samples of patients and controls.
(DOCX)
Funding Statement
This study is supported by the Innovative Young Biotechnologist Award by the Department of Biotechnology (Government of India) to GV (BT/04/IYBA/2011) as well as by the Wellcome Trust/DBT India Alliance Senior Fellowship research grant to GV (500236/Z/11/Z). SVK is supported by the Wellcome Trust/DBT India Alliance. VS is supported by the Indian Council of Medical Research. AS & DAJ are supported by the Department of Science & Technology (Government of India). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Rapoport JL, Addington AM, Frangou S, Psych MR (2005) The neurodevelopmental model of schizophrenia: update 2005. Mol Psychiatry 10: 434–449. [DOI] [PubMed] [Google Scholar]
- 2. Nicodemus KK, Marenco S, Batten AJ, Vakkalanka R, Egan MF, et al. (2008) Serious obstetric complications interact with hypoxia-regulated/vascular-expression genes to influence schizophrenia risk. Molecular psychiatry 13: 873–877. [DOI] [PubMed] [Google Scholar]
- 3. Brown AS, Derkits EJ (2010) Prenatal infection and schizophrenia: a review of epidemiologic and translational studies. The American journal of psychiatry 167: 261–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brown AS (2011) Exposure to prenatal infection and risk of schizophrenia. Frontiers in psychiatry/ Frontiers Research Foundation 2: 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Boksa P (2010) Effects of prenatal infection on brain development and behavior: a review of findings from animal models. Brain, behavior, and immunity 24: 881–897. [DOI] [PubMed] [Google Scholar]
- 6. Smith SE, Li J, Garbett K, Mirnics K, Patterson PH (2007) Maternal immune activation alters fetal brain development through interleukin-6. J Neurosci 27: 10695–10702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Potvin S, Stip E, Sepehry AA, Gendron A, Bah R, et al. (2008) Inflammatory cytokine alterations in schizophrenia: a systematic quantitative review. Biol Psychiatry 63: 801–808. [DOI] [PubMed] [Google Scholar]
- 8. Zakharyan R, Petrek M, Arakelyan A, Mrazek F, Atshemyan S, et al. (2012) Interleukin-6 promoter polymorphism and plasma levels in patients with schizophrenia. Tissue Antigens 80: 136–142. [DOI] [PubMed] [Google Scholar]
- 9. Paul-Samojedny M, Owczarek A, Kowalczyk M, Suchanek R, Palacz M, et al. (2013) Association of interleukin 2 (IL-2), interleukin 6 (IL-6), and TNF-alpha (TNFalpha) gene polymorphisms with paranoid schizophrenia in a Polish population. J Neuropsychiatry Clin Neurosci 25: 72–82. [DOI] [PubMed] [Google Scholar]
- 10. Marsland AL, Gianaros PJ, Abramowitch SM, Manuck SB, Hariri AR (2008) Interleukin-6 covaries inversely with hippocampal grey matter volume in middle-aged adults. Biol Psychiatry 64: 484–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Behrens MM, Ali SS, Dugan LL (2008) Interleukin-6 mediates the increase in NADPH-oxidase in the ketamine model of schizophrenia. J Neurosci 28: 13957–13966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cole SW, Arevalo JM, Takahashi R, Sloan EK, Lutgendorf SK, et al. (2010) Computational identification of gene-social environment interaction at the human IL6 locus. Proceedings of the National Academy of Sciences of the United States of America 107: 5681–5686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Terry CF, Loukaci V, Green FR (2000) Cooperative influence of genetic polymorphisms on interleukin 6 transcriptional regulation. J Biol Chem 275: 18138–18144. [DOI] [PubMed] [Google Scholar]
- 14. Fishman D, Faulds G, Jeffery R, Mohamed-Ali V, Yudkin JS, et al. (1998) The effect of novel polymorphisms in the interleukin-6 (IL-6) gene on IL-6 transcription and plasma IL-6 levels, and an association with systemic-onset juvenile chronic arthritis. J Clin Invest 102: 1369–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pereira DS, Garcia DM, Narciso FM, Santos ML, Dias JM, et al. (2011) Effects of 174 G/C polymorphism in the promoter region of the interleukin-6 gene on plasma IL-6 levels and muscle strength in elderly women. Braz J Med Biol Res 44: 123–129. [DOI] [PubMed] [Google Scholar]
- 16. Giannitrapani L, Soresi M, Giacalone A, Campagna ME, Marasa M, et al. (2011) IL-6 -174G/C polymorphism and IL-6 serum levels in patients with liver cirrhosis and hepatocellular carcinoma. OMICS 15: 183–186. [DOI] [PubMed] [Google Scholar]
- 17. Hulkkonen J, Pertovaara M, Antonen J, Pasternack A, Hurme M (2001) Elevated interleukin-6 plasma levels are regulated by the promoter region polymorphism of the IL6 gene in primary Sjogren's syndrome and correlate with the clinical manifestations of the disease. Rheumatology (Oxford) 40: 656–661. [DOI] [PubMed] [Google Scholar]
- 18. Yang X, Jansson PA, Pellme F, Laakso M, Smith U (2005) Effect of the interleukin-6 (-174) g/c promoter polymorphism on adiponectin and insulin sensitivity. Obes Res 13: 813–817. [DOI] [PubMed] [Google Scholar]
- 19. Sanderson SC, Kumari M, Brunner EJ, Miller MA, Rumley A, et al. (2009) Association between IL6 gene variants -174G>C and -572G>C and serum IL-6 levels: interactions with social position in the Whitehall II cohort. Atherosclerosis 204: 459–464. [DOI] [PubMed] [Google Scholar]
- 20. Huang M, Wang L, Ma H, Wang J, Xiang M (2013) Lack of an Association Between Interleukin-6 -174G/C Polymorphism and Circulating Interleukin-6 Levels in Normal Population: A Meta-Analysis. DNA Cell Biol 32: 654–664. [DOI] [PubMed] [Google Scholar]
- 21. Kilpinen S, Hulkkonen J, Wang XY, Hurme M (2001) The promoter polymorphism of the interleukin-6 gene regulates interleukin-6 production in neonates but not in adults. Eur Cytokine Netw 12: 62–68. [PubMed] [Google Scholar]
- 22. Meyer U, Feldon J, Yee BK (2009) A review of the fetal brain cytokine imbalance hypothesis of schizophrenia. Schizophr Bull 35: 959–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Brown AS (2011) The environment and susceptibility to schizophrenia. Prog Neurobiol 93: 23–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nosarti C, Reichenberg A, Murray RM, Cnattingius S, Lambe MP, et al. (2012) Preterm birth and psychiatric disorders in young adult life. Archives of general psychiatry 69: E1–8. [DOI] [PubMed] [Google Scholar]
- 25. Smith GN, Flynn SW, McCarthy N, Meistrich B, Ehmann TS, et al. (2001) Low birthweight in schizophrenia: prematurity or poor fetal growth? Schizophrenia research 47: 177–184. [DOI] [PubMed] [Google Scholar]
- 26. de Kieviet JF, Zoetebier L, van Elburg RM, Vermeulen RJ, Oosterlaan J (2012) Brain development of very preterm and very low-birthweight children in childhood and adolescence: a meta-analysis. Developmental medicine and child neurology 54: 313–323. [DOI] [PubMed] [Google Scholar]
- 27. Beauchamp MH, Thompson DK, Howard K, Doyle LW, Egan GF, et al. (2008) Preterm infant hippocampal volumes correlate with later working memory deficits. Brain: a journal of neurology 131: 2986–2994. [DOI] [PubMed] [Google Scholar]
- 28. Harrison PJ (2004) The hippocampus in schizophrenia: a review of the neuropathological evidence and its pathophysiological implications. Psychopharmacology (Berl) 174: 151–162. [DOI] [PubMed] [Google Scholar]
- 29. Heckers S (2001) Neuroimaging studies of the hippocampus in schizophrenia. Hippocampus 11: 520–528. [DOI] [PubMed] [Google Scholar]
- 30. Heckers S (2004) The hippocampus in schizophrenia. Am J Psychiatry 161: 2138–2139. [DOI] [PubMed] [Google Scholar]
- 31. Tamminga CA, Stan AD, Wagner AD (2010) The hippocampal formation in schizophrenia. Am J Psychiatry 167: 1178–1193. [DOI] [PubMed] [Google Scholar]
- 32. Ebner F, Tepest R, Dani I, Pfeiffer U, Schulze TG, et al. (2008) The hippocampus in families with schizophrenia in relation to obstetric complications. Schizophr Res 104: 71–78. [DOI] [PubMed] [Google Scholar]
- 33. Mondelli V, Cattaneo A, Belvederi Murri M, Di Forti M, Handley R, et al. (2011) Stress and inflammation reduce brain-derived neurotrophic factor expression in first-episode psychosis: a pathway to smaller hippocampal volume. The Journal of clinical psychiatry 72: 1677–1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, et al. (1998) The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 59 Suppl 20: 22–33;quiz 34–57. [PubMed]
- 35. Konrad C, Ukas T, Nebel C, Arolt V, Toga AW, et al. (2009) Defining the human hippocampus in cerebral magnetic resonance images—an overview of current segmentation protocols. Neuroimage 47: 1185–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. MacQueen GM, Campbell S, McEwen BS, Macdonald K, Amano S, et al. (2003) Course of illness, hippocampal function, and hippocampal volume in major depression. Proceedings of the National Academy of Sciences of the United States of America 100: 1387–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Neumeister A, Wood S, Bonne O, Nugent AC, Luckenbaugh DA, et al. (2005) Reduced hippocampal volume in unmedicated, remitted patients with major depression versus control subjects. Biological psychiatry 57: 935–937. [DOI] [PubMed] [Google Scholar]
- 38. Narr KL, Thompson PM, Szeszko P, Robinson D, Jang S, et al. (2004) Regional specificity of hippocampal volume reductions in first-episode schizophrenia. Neuroimage 21: 1563–1575. [DOI] [PubMed] [Google Scholar]
- 39. Ashburner J, Friston KJ (2005) Unified segmentation. Neuroimage 26: 839–851. [DOI] [PubMed] [Google Scholar]
- 40. Ashburner J (2007) A fast diffeomorphic image registration algorithm. Neuroimage 38: 95–113. [DOI] [PubMed] [Google Scholar]
- 41. Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH (2003) An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage 19: 1233–1239. [DOI] [PubMed] [Google Scholar]
- 42. Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, et al. (2004) Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23 Suppl 1 S208–219. [DOI] [PubMed] [Google Scholar]
- 43. Rivera-Chavez FA, Peters-Hybki DL, Barber RC, O'Keefe GE (2003) Interleukin-6 promoter haplotypes and interleukin-6 cytokine responses. Shock 20: 218–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Maitra A, Shanker J, Dash D, John S, Sannappa PR, et al. (2008) Polymorphisms in the IL6 gene in Asian Indian families with premature coronary artery disease—the Indian Atherosclerosis Research Study. Thromb Haemost 99: 944–950. [DOI] [PubMed] [Google Scholar]
- 45. Flicek P, Amode MR, Barrell D, Beal K, Brent S, et al. (2012) Ensembl 2012. Nucleic Acids Res 40: D84–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Integrating common and rare genetic variation in diverse human populations. Nature 467: 52–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Qi HP, Qu ZY, Duan SR, Wei SQ, Wen SR, et al. (2012) IL-6-174 G/C and -572 C/G Polymorphisms and Risk of Alzheimer's Disease. PLoS One 7.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Freedman R, Goldowitz D (2010) Studies on the hippocampal formation: From basic development to clinical applications: Studies on schizophrenia. Prog Neurobiol 90: 263–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Keshavan MS, Dick E, Mankowski I, Harenski K, Montrose DM, et al. (2002) Decreased left amygdala and hippocampal volumes in young offspring at risk for schizophrenia. Schizophr Res 58: 173–183. [DOI] [PubMed] [Google Scholar]
- 50. Jayakumar PN, Venkatasubramanian G, Gangadhar BN, Janakiramaiah N, Keshavan MS (2005) Optimized voxel-based morphometry of gray matter volume in first-episode, antipsychotic-naive schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry 29: 587–591. [DOI] [PubMed] [Google Scholar]
- 51.Lodge DJ, Grace AA (2010) Developmental pathology, dopamine, stress and schizophrenia. Int J Dev Neurosci. [DOI] [PMC free article] [PubMed]
- 52. Phillips LJ, McGorry PD, Garner B, Thompson KN, Pantelis C, et al. (2006) Stress, the hippocampus and the hypothalamic-pituitary-adrenal axis: implications for the development of psychotic disorders. Aust N Z J Psychiatry 40: 725–741. [DOI] [PubMed] [Google Scholar]
- 53. McEwen BS (2008) Central effects of stress hormones in health and disease: Understanding the protective and damaging effects of stress and stress mediators. Eur J Pharmacol 583: 174–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Baune BT, Konrad C, Grotegerd D, Suslow T, Birosova E, et al. (2012) Interleukin-6 gene (IL-6): a possible role in brain morphology in the healthy adult brain. Journal of neuroinflammation 9: 125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Brune M (2012) Does the oxytocin receptor (OXTR) polymorphism (rs2254298) confer 'vulnerability' for psychopathology or 'differential susceptibility'? Insights from evolution. BMC medicine 10: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Belsky J, Pluess M (2009) Beyond diathesis stress: differential susceptibility to environmental influences. Psychological bulletin 135: 885–908. [DOI] [PubMed] [Google Scholar]
- 57. Kang HJ, Voleti B, Hajszan T, Rajkowska G, Stockmeier CA, et al. (2012) Decreased expression of synapse-related genes and loss of synapses in major depressive disorder. Nature medicine 18: 1413–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Muller N, Empl M, Riedel M, Schwarz M, Ackenheil M (1997) Neuroleptic treatment increases soluble IL-2 receptors and decreases soluble IL-6 receptors in schizophrenia. European archives of psychiatry and clinical neuroscience 247: 308–313. [DOI] [PubMed] [Google Scholar]
- 59. Zalcman S, Green-Johnson JM, Murray L, Nance DM, Dyck D, et al. (1994) Cytokine-specific central monoamine alterations induced by interleukin-1, -2 and -6. Brain research 643: 40–49. [DOI] [PubMed] [Google Scholar]
- 60. Zaretsky MV, Alexander JM, Byrd W, Bawdon RE (2004) Transfer of inflammatory cytokines across the placenta. Obstetrics and gynecology 103: 546–550. [DOI] [PubMed] [Google Scholar]
- 61. Lowe GC, Luheshi GN, Williams S (2008) Maternal infection and fever during late gestation are associated with altered synaptic transmission in the hippocampus of juvenile offspring rats. American journal of physiology Regulatory, integrative and comparative physiology 295: R1563–1571. [DOI] [PubMed] [Google Scholar]
- 62. Marx CE, Jarskog LF, Lauder JM, Lieberman JA, Gilmore JH (2001) Cytokine effects on cortical neuron MAP-2 immunoreactivity: implications for schizophrenia. Biological Psychiatry 50: 743–749. [DOI] [PubMed] [Google Scholar]
- 63. Gilmore JH, Fredrik Jarskog L, Vadlamudi S, Lauder JM (2004) Prenatal infection and risk for schizophrenia: IL-1beta, IL-6, and TNFalpha inhibit cortical neuron dendrite development. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology 29: 1221–1229. [DOI] [PubMed] [Google Scholar]
- 64. Samuelsson AM, Jennische E, Hansson HA, Holmang A (2006) Prenatal exposure to interleukin-6 results in inflammatory neurodegeneration in hippocampus with NMDA/GABA(A) dysregulation and impaired spatial learning. American journal of physiology Regulatory, integrative and comparative physiology 290: R1345–1356. [DOI] [PubMed] [Google Scholar]
- 65. Hama T, Miyamoto M, Tsukui H, Nishio C, Hatanaka H (1989) Interleukin-6 as a neurotrophic factor for promoting the survival of cultured basal forebrain cholinergic neurons from postnatal rats. Neuroscience letters 104: 340–344. [DOI] [PubMed] [Google Scholar]
- 66. Mehler MF, Kessler JA (1998) Cytokines in brain development and function. Advances in protein chemistry 52: 223–251. [DOI] [PubMed] [Google Scholar]
- 67. Baier PC, May U, Scheller J, Rose-John S, Schiffelholz T (2009) Impaired hippocampus-dependent and -independent learning in IL-6 deficient mice. Behavioural brain research 200: 192–196. [DOI] [PubMed] [Google Scholar]
- 68. Vignozzi L, Cellai I, Santi R, Lombardelli L, Morelli A, et al. (2012) Antiinflammatory effect of androgen receptor activation in human benign prostatic hyperplasia cells. J Endocrinol 214: 31–43. [DOI] [PubMed] [Google Scholar]
- 69. Kovacs EJ, Plackett TP, Witte PL (2004) Estrogen replacement, aging, and cell-mediated immunity after injury. Journal of leukocyte biology 76: 36–41. [DOI] [PubMed] [Google Scholar]
- 70. Bonafe M, Olivieri F, Cavallone L, Giovagnetti S, Mayegiani F, et al. (2001) A gender—dependent genetic predisposition to produce high levels of IL-6 is detrimental for longevity. Eur J Immunol 31: 2357–2361. [DOI] [PubMed] [Google Scholar]
- 71. Bergouignan L, Chupin M, Czechowska Y, Kinkingnehun S, Lemogne C, et al. (2009) Can voxel based morphometry, manual segmentation and automated segmentation equally detect hippocampal volume differences in acute depression? Neuroimage 45: 29–37. [DOI] [PubMed] [Google Scholar]
- 72. Keller SS, Mackay CE, Barrick TR, Wieshmann UC, Howard MA, et al. (2002) Voxel-based morphometric comparison of hippocampal and extrahippocampal abnormalities in patients with left and right hippocampal atrophy. Neuroimage 16: 23–31. [DOI] [PubMed] [Google Scholar]
- 73. Maguire EA, Gadian DG, Johnsrude IS, Good CD, Ashburner J, et al. (2000) Navigation-related structural change in the hippocampi of taxi drivers. Proceedings of the National Academy of Sciences 97: 4398–4403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Kubicki M, Shenton ME, Salisbury DF, Hirayasu Y, Kasai K, et al. (2002) Voxel-based morphometric analysis of gray matter in first episode schizophrenia. Neuroimage 17: 1711–1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Good CD, Scahill RI, Fox NC, Ashburner J, Friston KJ, et al. (2002) Automatic Differentiation of Anatomical Patterns in the Human Brain: Validation with Studies of Degenerative Dementias. Neuroimage 17: 29–46. [DOI] [PubMed] [Google Scholar]
- 76. Lindberg O, Manzouri A, Westman E, Wahlund LO (2012) A comparison between volumetric data generated by voxel-based morphometry and manual parcellation of multimodal regions of the frontal lobe. AJNR Am J Neuroradiol 33: 1957–1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Uchida RR, Del-Ben CM, Araujo D, Busatto-Filho G, Duran FL, et al. (2008) Correlation between voxel based morphometry and manual volumetry in magnetic resonance images of the human brain. An Acad Bras Cienc 80: 149–156. [DOI] [PubMed] [Google Scholar]
- 78. Job DE, Whalley HC, McConnell S, Glabus M, Johnstone EC, et al. (2002) Structural Gray Matter Differences between First-Episode Schizophrenics and Normal Controls Using Voxel-Based Morphometry. Neuroimage 17: 880–889. [PubMed] [Google Scholar]
- 79. Rajarethinam R, DeQuardo JR, Miedler J, Arndt S, Kirbat R, et al. (2001) Hippocampus and amygdala in schizophrenia: assessment of the relationship of neuroanatomy to psychopathology. Psychiatry Res 108: 79–87. [DOI] [PubMed] [Google Scholar]
- 80. Csernansky JG, Wang L, Jones D, Rastogi-Cruz D, Posener JA, et al. (2002) Hippocampal deformities in schizophrenia characterized by high dimensional brain mapping. Am J Psychiatry 159: 2000–2006. [DOI] [PubMed] [Google Scholar]
- 81. Tepest R, Wang L, Miller MI, Falkai P, Csernansky JG (2003) Hippocampal deformities in the unaffected siblings of schizophrenia subjects. Biol Psychiatry 54: 1234–1240. [DOI] [PubMed] [Google Scholar]
- 82. McDonald C, Marshall N, Sham PC, Bullmore ET, Schulze K, et al. (2006) Regional brain morphometry in patients with schizophrenia or bipolar disorder and their unaffected relatives. Am J Psychiatry 163: 478–487. [DOI] [PubMed] [Google Scholar]
- 83. Testa C, Laakso MP, Sabattoli F, Rossi R, Beltramello A, et al. (2004) A comparison between the accuracy of voxel-based morphometry and hippocampal volumetry in Alzheimer's disease. Journal of Magnetic Resonance Imaging 19: 274–282. [DOI] [PubMed] [Google Scholar]
- 84. Ebdrup BH, Glenthoj B, Rasmussen H, Aggernaes B, Langkilde AR, et al. (2010) Hippocampal and caudate volume reductions in antipsychotic-naive first-episode schizophrenia. J Psychiatry Neurosci 35: 95–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Hu M, Li J, Eyler L, Guo X, Wei Q, et al. (2013) Decreased left middle temporal gyrus volume in antipsychotic drug-naive, first-episode schizophrenia patients and their healthy unaffected siblings. Schizophr Res 144: 37–42. [DOI] [PubMed] [Google Scholar]
- 86. Rizos EN, Papathanasiou M, Michalopoulou PG, Mazioti A, Douzenis A, et al. (2011) Association of serum BDNF levels with hippocampal volumes in first psychotic episode drug-naive schizophrenic patients. Schizophr Res 129: 201–204. [DOI] [PubMed] [Google Scholar]
- 87. Chua SE, Cheung C, Cheung V, Tsang JT, Chen EY, et al. (2007) Cerebral grey, white matter and csf in never-medicated, first-episode schizophrenia. Schizophr Res 89: 12–21. [DOI] [PubMed] [Google Scholar]
- 88. Salgado-Pineda P, Baeza I, Perez-Gomez M, Vendrell P, Junque C, et al. (2003) Sustained attention impairment correlates to gray matter decreases in first episode neuroleptic-naive schizophrenic patients. Neuroimage 19: 365–375. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Comparative analysis of one-to-on matched samples of patients and controls.
(DOCX)