Abstract
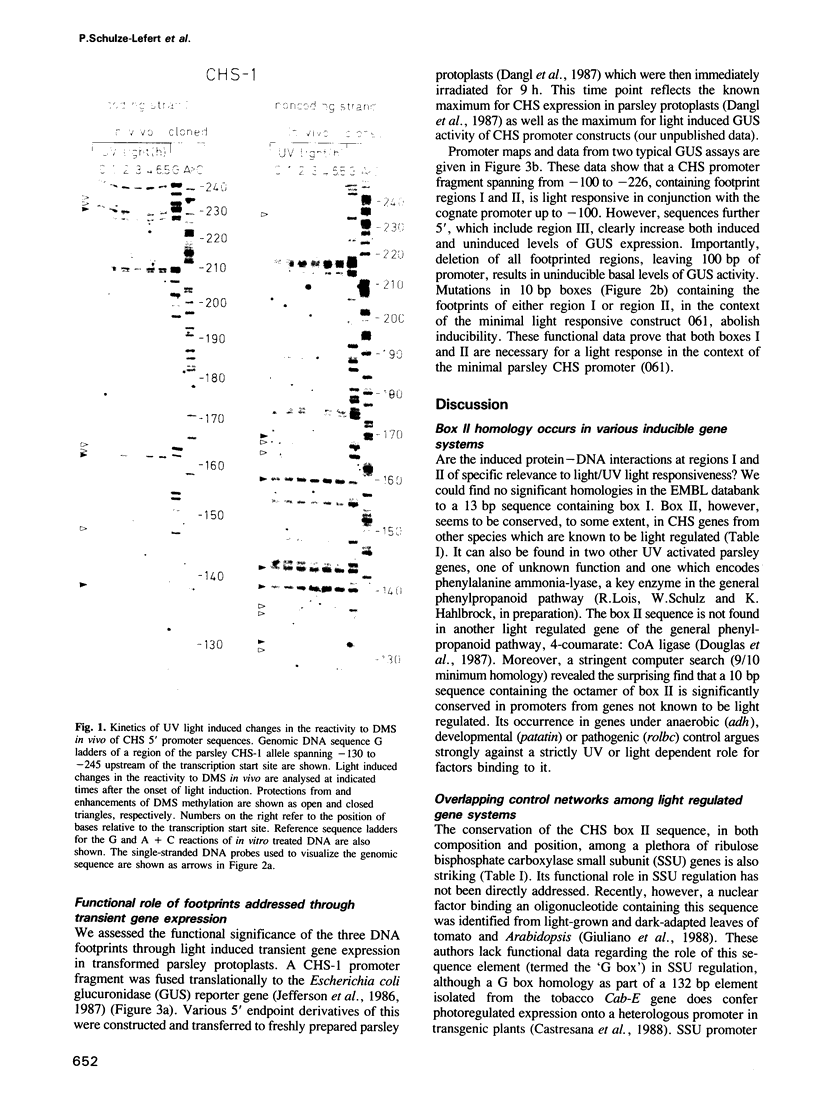
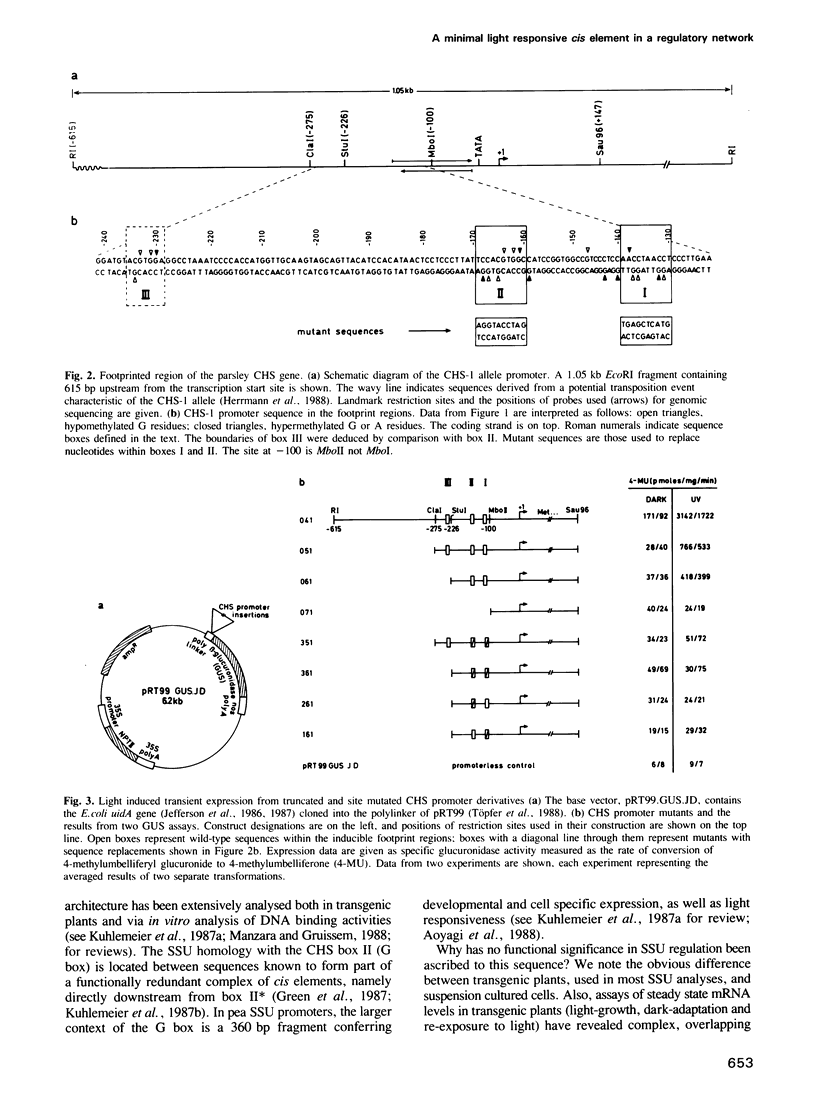
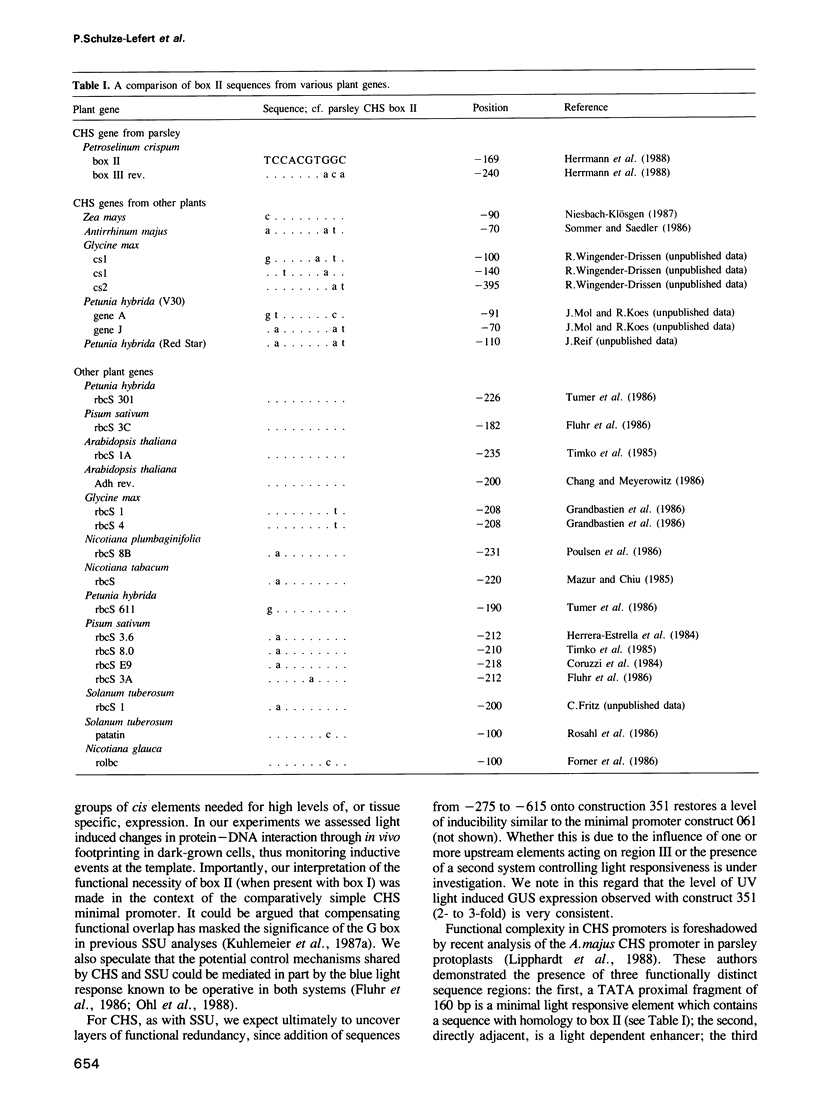
We began characterization of the protein--DNA interactions necessary for UV light induced transcriptional activation of the gene encoding chalcone synthase (CHS), a key plant defense enzyme. Three light dependent in vivo footprints appear on a 90 bp stretch of the CHS promoter with a time course correlated with the onset of CHS transcription. We define a minimal light responsive promoter by functional analysis of truncated CHS promoter fusions with a reporter gene in transient expression experiments in parsley protoplasts. Two of the three footprinted sequence 'boxes' reside within the minimal promoter. Replacement of 10 bp within either of these 'boxes' leads to complete loss of light responsiveness. We conclude that these sequences define the necessary cis elements of the minimal CHS promoter's light responsive element. One of the functionally defined 'boxes' is homologous to an element implicated in regulation of genes involved in photosynthesis. These data represent the first example in a plant defense gene of an induced change in protein--DNA contacts necessary for transcriptional activation. Also, our data argue strongly that divergent light induced biosynthetic pathways share common regulatory units.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Castresana C., Garcia-Luque I., Alonso E., Malik V. S., Cashmore A. R. Both positive and negative regulatory elements mediate expression of a photoregulated CAB gene from Nicotiana plumbaginifolia. EMBO J. 1988 Jul;7(7):1929–1936. doi: 10.1002/j.1460-2075.1988.tb03030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C., Meyerowitz E. M. Molecular cloning and DNA sequence of the Arabidopsis thaliana alcohol dehydrogenase gene. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1408–1412. doi: 10.1073/pnas.83.5.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coruzzi G., Broglie R., Edwards C., Chua N. H. Tissue-specific and light-regulated expression of a pea nuclear gene encoding the small subunit of ribulose-1,5-bisphosphate carboxylase. EMBO J. 1984 Aug;3(8):1671–1679. doi: 10.1002/j.1460-2075.1984.tb02031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dangl J. L., Hauffe K. D., Lipphardt S., Hahlbrock K., Scheel D. Parsley protoplasts retain differential responsiveness to u.v. light and fungal elicitor. EMBO J. 1987 Sep;6(9):2551–2556. doi: 10.1002/j.1460-2075.1987.tb02543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Capua E., Müller B. The accessibility of DNA to dimethylsulfate in complexes with recA protein. EMBO J. 1987 Aug;6(8):2493–2498. doi: 10.1002/j.1460-2075.1987.tb02531.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Capua E., Müller B. The accessibility of DNA to dimethylsulfate in complexes with recA protein. EMBO J. 1987 Aug;6(8):2493–2498. doi: 10.1002/j.1460-2075.1987.tb02531.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas C., Hoffmann H., Schulz W., Hahlbrock K. Structure and elicitor or u.v.-light-stimulated expression of two 4-coumarate:CoA ligase genes in parsley. EMBO J. 1987 May;6(5):1189–1195. doi: 10.1002/j.1460-2075.1987.tb02353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fluhr R., Chua N. H. Developmental regulation of two genes encoding ribulose-bisphosphate carboxylase small subunit in pea and transgenic petunia plants: Phytochrome response and blue-light induction. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2358–2362. doi: 10.1073/pnas.83.8.2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fluhr Robert, Moses Phyllis, Morelli Giorgio, Coruzzi Gloria, Chua Nam-Hai. Expression dynamics of the pea rbcS multigene family and organ distribution of the transcripts. EMBO J. 1986 Sep;5(9):2063–2071. doi: 10.1002/j.1460-2075.1986.tb04467.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuliano G., Pichersky E., Malik V. S., Timko M. P., Scolnik P. A., Cashmore A. R. An evolutionarily conserved protein binding sequence upstream of a plant light-regulated gene. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7089–7093. doi: 10.1073/pnas.85.19.7089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green P. J., Kay S. A., Chua N. H. Sequence-specific interactions of a pea nuclear factor with light-responsive elements upstream of the rbcS-3A gene. EMBO J. 1987 Sep;6(9):2543–2549. doi: 10.1002/j.1460-2075.1987.tb02542.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera-Estrella L., Van den Broeck G., Maenhaut R., Van Montagu M., Schell J., Timko M., Cashmore A. Light-inducible and chloroplast-associated expression of a chimaeric gene introduced into Nicotiana tabacum using a Ti plasmid vector. Nature. 1984 Jul 12;310(5973):115–120. doi: 10.1038/310115a0. [DOI] [PubMed] [Google Scholar]
- Herrmann A., Schulz W., Hahlbrock K. Two alleles of the single-copy chalcone synthase gene in parsley differ by a transposon-like element. Mol Gen Genet. 1988 Apr;212(1):93–98. doi: 10.1007/BF00322449. [DOI] [PubMed] [Google Scholar]
- Jefferson R. A., Burgess S. M., Hirsh D. beta-Glucuronidase from Escherichia coli as a gene-fusion marker. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8447–8451. doi: 10.1073/pnas.83.22.8447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson R. A., Kavanagh T. A., Bevan M. W. GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 1987 Dec 20;6(13):3901–3907. doi: 10.1002/j.1460-2075.1987.tb02730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaulen Hildegard, Schell Jeff, Kreuzaler Fritz. Light-induced expression of the chimeric chalcone synthase-NPTII gene in tobacco cells. EMBO J. 1986 Jan;5(1):1–8. doi: 10.1002/j.1460-2075.1986.tb04169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer W., Drutsa V., Jansen H. W., Kramer B., Pflugfelder M., Fritz H. J. The gapped duplex DNA approach to oligonucleotide-directed mutation construction. Nucleic Acids Res. 1984 Dec 21;12(24):9441–9456. doi: 10.1093/nar/12.24.9441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlemeier C., Fluhr R., Green P. J., Chua N. H. Sequences in the pea rbcS-3A gene have homology to constitutive mammalian enhancers but function as negative regulatory elements. Genes Dev. 1987 May;1(3):247–255. doi: 10.1101/gad.1.3.247. [DOI] [PubMed] [Google Scholar]
- Lipphardt S., Brettschneider R., Kreuzaler F., Schell J., Dangl J. L. UV-inducible transient expression in parsley protoplasts identifies regulatory cis-elements of a chimeric Antirrhinum majus chalcone synthase gene. EMBO J. 1988 Dec 20;7(13):4027–4033. doi: 10.1002/j.1460-2075.1988.tb03296.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazur B. J., Chui C. F. Sequence of a genomic DNA clone for the small subunit of ribulose bis-phosphate carboxylase-oxygenase from tobacco. Nucleic Acids Res. 1985 Apr 11;13(7):2373–2386. doi: 10.1093/nar/13.7.2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmelzer E., Jahnen W., Hahlbrock K. In situ localization of light-induced chalcone synthase mRNA, chalcone synthase, and flavonoid end products in epidermal cells of parsley leaves. Proc Natl Acad Sci U S A. 1988 May;85(9):2989–2993. doi: 10.1073/pnas.85.9.2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebenlist U., Gilbert W. Contacts between Escherichia coli RNA polymerase and an early promoter of phage T7. Proc Natl Acad Sci U S A. 1980 Jan;77(1):122–126. doi: 10.1073/pnas.77.1.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumer N. E., Clark W. G., Tabor G. J., Hironaka C. M., Fraley R. T., Shah D. M. The genes encoding the small subunit of ribulose-1,5-bisphosphate carboxylase are expressed differentially in petunia leaves. Nucleic Acids Res. 1986 Apr 25;14(8):3325–3342. doi: 10.1093/nar/14.8.3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Töpfer R., Schell J., Steinbiss H. H. Versatile cloning vectors for transient gene expression and direct gene transfer in plant cells. Nucleic Acids Res. 1988 Sep 12;16(17):8725–8725. doi: 10.1093/nar/16.17.8725. [DOI] [PMC free article] [PubMed] [Google Scholar]