Abstract
Background
Amifostine is the most clinical used chemical radioprotector, but its effect in patients treated with radiation is not consistent.
Methods
By searching Medline, CENTRAL, EMBASE, ASCO, ESMO, and CNKI databases, the published randomized controlled trials (RCTs) about the efficacy of amifostine in HNSCC patients treated with radiotherapy were collected. The pooled efficacy and side effects of this drug were calculated by RevMan software.
Results
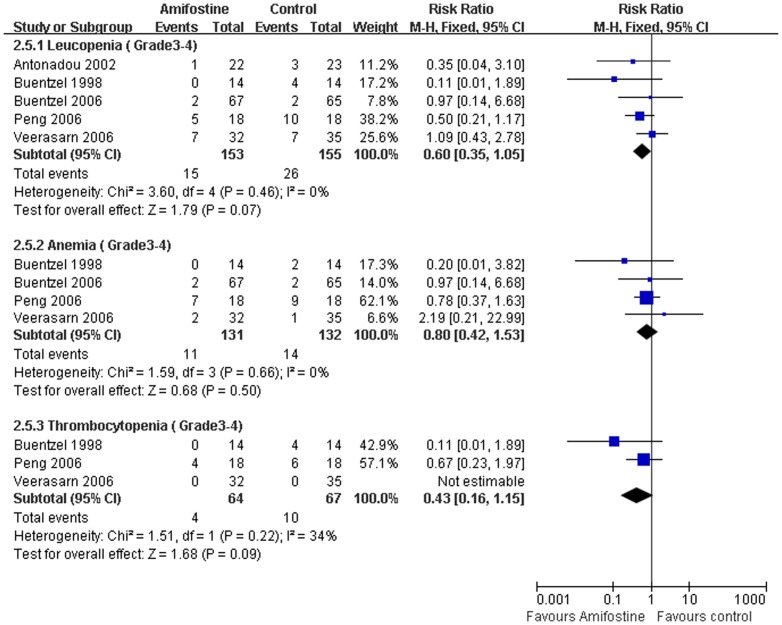
Seventeen trials including a total of 1167 patients (604 and 563 each arm) were analyzed in the meta-analysis. The pooled data showed that the use of amifostine significantly reduce the risk of developing Grade3–4 mucositis (relative risk [RR],0.72; 95% confidence interval [CI],0.54–0.95; p<0.00001), Grade 2–4 acute xerostomia (RR,0.70; 95%CI,0.52–0.96; p = 0.02), or late xerostomia (RR,0.60; 95%CI,0.49–0.74; p<0.00001) and Grade 3–4 dysphagia (RR,0.39; 95%CI,0.17–0.92; p = 0.03). However, subgroup analysis demonstrated that no statistically significant reduction of Grade3–4 mucositis (RR,0.97; 95% CI,0.74–1.26; p = 0.80), Grade 2–4 acute xerostomia (RR,0.35; 95%CI,0.02–5.44; p = 0.45), or late xerostomia (RR,0.40; 95%CI,0.13–1.24; p = 0.11) and Grade 3–4 dysphagia (RR,0.23; 95%CI,0.01–4.78; p = 0.35) was observed in patients treated with concomitant chemoradiotherapy. Compared with placebo or observation, amifostine does not show tumor protective effect in complete response (RR,1.02; 95%CI,0.89–1.17; p = 0.76) and partial response (RR,0.90; 95%CI, 0.56–1.44; p = 0.66). For the hematologic side effect, no statistical difference of Grade 3–4 leucopenia (RR,0.60; 95%CI,0.35–1.05; p = 0.07), anemia (RR,0.80; 95%CI, 0.42–1.53; p = 0.50) and thrombocytopenia (RR,0.43; 95%CI,0.16–1.15; p = 0.09) were found between amifostine and control groups. The most common amifostine related side effects were nausea, emesis, hypotension and allergic with an average incidence rate (Grade 3–4) of 5%, 6%, 4% and 4% respectively.
Conclusion
This systematic review showed that amifostine significantly reduce the serious mucositis, acute/late xerastomia and dysphagia without protection of the tumor in HNSCC patients treated with radiotherapy. And the toxicities of amifostine were generally acceptable.
Introduction
Radiotherapy plays a significant role in the management of head and neck squamous cell carcinoma (HNSCC), either for the definitive radiotherapy or for the post-surgical adjuvant radiation[1], [2]. The mucositis, acute or late xerostomia caused by radiation are the most common toxic effects, which usually interrupt the planned course of treatment[3]. Mucositis is an acute non-hematologic toxicity that occurs during the treatment; xerostomia usually develops acutely during chemoradiotherapy and persists for a long period of time. The late side effect always disrupts normal activities such as speaking, eating and may also cause sequelae, including tooth loss and dental caries, which could compromise the quality of patients' life.
For the past several decades, researchers have been investigating use of drugs to decrease the side effects during radiotherapy, so as to increase the amount of radiation that can be safely administered to the patients. The most clinical used radioprotective drug is amifostine that was initially developed as part of the nuclear warfare program[4]. Based on some randomized controlled rials (RCTs), it showed that amifostine could reduce acute and chronic xerostomia in HNSCC patients treated with radiation or concomitant chemoradiotherapy[3], [5]. Hower, some other trials did not demonstrate toxicities reduction effects in course of radiation by adding amifostine to the patients[6], [7]. And another controversy about the use of this drug is the tumor protective effect. Some researchers worried that amifostine may stop tumor tissue responding to radiation and therefore reduce treatment effectiveness[8]. Unfortunately, even after almost 30 years of its development and clinical use, there is still great controversy about its application. Although some reviews state that evidence is not enough to support the tumor protection efficacy of amifostine, the statistical confirmation of those facts has yet to be achieved. In order to answer this question we performed this systematic review and meta-analysis.
Methods
Search Strategy
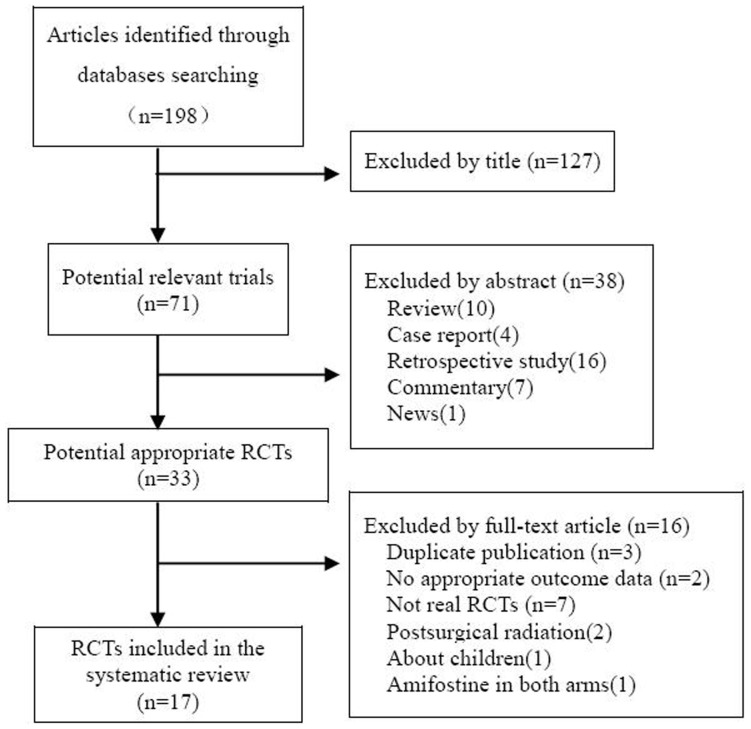
The selection procedure of studies was depicted in the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement flow chart (Figure 1). Randomized controlled trials comparing radiotherapy vs. radiotherapy plus amifostine for head and neck squamous cell carcinoma, published before January 2012, were identified through an electronic sensitive search of Medline, the Cochrane central register of controlled trials, EMBASE and CNKI databases. The European Society of Medical Oncology (ESMO) and the conference proceedings of the American Society of Clinical Oncology (ASCO) were also searched. The following search terms were used: “Head and neck cancer”, “head and neck carcinoma”, “amifostine”, “Ethyol” “WR-1065”, “WR2721”,“WR1065” or “WR33278”. Searches were limited to human trials, with the language restriction of English and Chinese. All references of relevant articles were scanned for additional analysis.
Figure 1. PRISMA Flowchart of the meta-analysis.
Selection Criteria
Details regarding the patients' eligibility criteria, treatment methods and outcomes of the relevant trials were extracted by two reviewer (JG and YW) and then checked by the third reviewer (FH) as described in the Cochrane Handbook for systematic reviews[9]. The patients were limited to locally advanced (Stage III–IV, or inoperable Stage II) head and neck squamous cell carcinoma. The intervention was radiotherapy or chemoradiotherapy plus amifostine and the control was radiotherapy or chemoradiotherapy with placebo or observation. The outcomes were restricted to about the non-hematological toxicity, response rate, hematological toxicity and side effects of amifostine. No surgical excision or neck dissection was performed before the radiation. The RCTs including patients with both HNSCC and other kinds of tumors were also included in the meta-analysis, but data were extracted only for the HNSCC patients.
Data Extraction and Quality assessment
Data were extracted by two reviewers (JG and HZ) independently from all included RCTs. Any disagreement was consulted to another investigator (YW) for consensus. The general characteristics (name of the first author, year of publication, number of patients, stages, chemoradiation regimens and amifostine dosage), outcomes (non-hematological toxicity: mucositis, acute and late xerostomia, dysphagia; hematological toxicity: leucopenia, anemia and thrombocytopenia; treatment response), and amifostine induced side effects were extracted. The methodological qualities of the trials were assessed by the same investigators (JG and HZ) according to the Cochrane Reviews Handbook 5.0. We pay special attention on the generation and concealment of the sequence of randomization, blinding, incomplete outcome date addresse and selective reports, which generally represent the quality of the RCT[9] in the procedure of trials inclusion.
Toxicity Assessment
The toxicities caused by radiation were graded according to “Radiation therapy Oncology Group (RTOG) Acute/Late Radiation Morbidity Scoring Criteria” [10]. Acute mucositis and xerostomia were defined as those that occurred up to 90 days after the start of protocol therapy, and late xerostomia was defined as those that occurred 90 days through 12 months after the start of protocol therapy. The treatment response (complete response and partial response) was assessed at about six weeks after the completion of the treatment regimen according to the World Health Organization guidelines.
Statistical Analysis
Review Manager(RevMan 5.0 provided by The Cochrane Collaboration) was used to do the statistical analysis. Dichotomous data are calculated as the risk ratio (RR) with the 95% confidence interval (CI). The null hypothesis test was considered as no association (RR = 1) between amifostine use and the incidence of radiation toxicity and the tumor response rate. A RR<1 indicates a positive effect of amifostine on the outcome. Statistical heterogeneity of the results across trials was assessed by chi-square (χ2) test [11], and the inconsistency was calculated by I2 [12]. If heterogeneity was found (χ2, p<0.05 or I2>50%), the random-effect method (Dersimonian-Laird method) was used to pool the data and subgroups analysis was done for further evaluation. Inversely, without significant heterogeneity, fixed-effect method was purchased. The Egger's tests were used for each effect size to evaluate possible publication bias as described by Egger[13].
Results
Included Trials and Characteristics
A total of one hundred twelve articles were initially identified by searching the electronic databases. 33 potential applicable studies, published between 1998 and 2010, were retrieved. Of those, sixteen publications were excluded for the reasons: duplicated publication[14]–[16], not real RCT[17]–[23], without appropriate outcome data[24], [25], postsurgical radiation[26]–[28], amifostine in both arms comparing different administration[29], about children[30]. Finally, seventeen trials that included a total of 1167 patients (604 and 563 in each arms) were analyzed in the meta-analysis[3], [5]–[7], [31]–[43]. Eight[6], [31], [32], [34]–[37], [41] of the 17 trials evaluated patients treated with concomitant chemoradiation and the other nine trials [3], [5], [7], [33], [38]–[40], [42], [43] focused on radiation only. Amifostine was delivered intravenously in 13 articles[3], [5]–[7], [31]–[33], [35], [37], [38], [40], [42], [43] and subcutaneously in 4[34], [36], [39], [41]. One trial was a three-arm study that compared different administration schedule of amifostine to observation[7]. The dose of daily amifostine delivered ranged from 200 mg/m2 to 400 mg/m2. The general characteristics of included trials were outlined in Table 1.
Table 1. General characteristics of included randomized controlled trials.
| Trials | No.of patiens Ami/Control | Stages included | Daily ami (dose) | Administration | Concomitant Chemotherapy | Radiotherapy |
| Antonadou[32] (2002) | 22/23 | III, IV | 300 mg/m2 | IV, 30 minutes before RT, q.d | C (90 mg/m2) once per week before RT | 60–74Gy, 2 Gy/fraction, 5 fractions weekly |
| Bourhis[47] (2000) | 13/13 | IV | 300 mg/m2 | IV, 15–30 minutes before RT, b.i.d | — | 62–64 Gy, 2 Gy/fraction, b.i.d with a 8–9 hours interval |
| Brizel[3] (2000) | 143/153 | II, III | 200 mg/m2 | IV, 15–30 minutes before RT, q.d | — | 50–70Gy, 1.8–2.0 Gy/fraction. q.d |
| Koukourakis[39] (2000) | 19/20 | II–IV | 500 mg | IH, 20 minutes before RT, q.d | — | 44–70Gy,2 Gy/fraction, 5 fractions/week |
| Fan[38] (2011) | 30/26 | II–IV | 200 mg/m2 | IV, 15–30 minutes before RT, q.d | — | 70–76Gy, 2Gy/fraction, q.d |
| Peng[37] (2006) | 18/19 | III, IV | 400 mg | IV, 15 minutes before RT, q.d | 5-FU(750 mg/m2) days:1–3; DDP (50 mg/m2) day:5; D (75 mg/m2),day:6 | 74Gy,1.2 Gy/fraction, 2 fractions daily with a 6 hours interval |
| Yu[42] (2009) | 15/15 | III, IV | 200 mg/m2 | IV, 20 minutes before RT, q.d | — | 70Gy, 2Gy/fraction, q.d |
| Jiang[41] (2009) | 30/30 | III, IV | 400 mg | IH, 30 minutes before RT.qd | Nedaplatin (100 mg/m2) day:1,22 and 43 | 74Gy, 2Gy/fraction, 5 fractions weekly |
| He[43] (2004) | 17/15 | III, IV | 200 mg/m2 | IV, 15–30 minutes before RT, q.d | — | 65–74Gy, 2Gy/fraction, q.d |
| Zhang[40] (2010) | 40/40 | III, IV | 500 mg | IV, 30 minutes before RT, q.d | — | 70Gy, 2Gy/fraction, q.d 5 fractions weekly |
| Braaksma[34] (2005) | 27/27 | II–IV | 500 mg | IH, 15–30 minutes before RT, q.d | P (60 mg/m2) days: 1,8, 15, and 22 | 72Gy,12Gy/week |
| Buntzel[35] (1998) | 14/14 | II–IV | 500 mg | IV, 30 minutes before RT. qd | C (70 mg/m2) days:1–5 and days 21–26 | 60 Gy, 2 Gy/fraction, 5 fractions weekly |
| Veerasarn[5] (2006) | 32/35 | II–IV | 200 mg/m2 | IV, 30 minutes before RT, q.d | — | 66–70Gy, 2Gy/fraction, q.d |
| Buentzel[6] (2006) | 67/65 | II–IV | 300 mg/m2 | IV, 30 minutes before RT, q.d | C(70 mg/m2) Days: 1–5 and 21–25 | 60–66 Gy, 2 Gy/fraction, 5 fractions weekly |
| Amrein[31] (2005) | 28/8 | III, IV | 400 mg/m2 | IV, 30 minutes before RT, q.d | P (60 mg/m2) days: 1, 8, 15, and 22 | 70.4 Gy, 1.6 Gy/fraction,2 fractions daily with a 4–6 hour interval |
| Jellema[7] (2006) | 60/31 | III, IV | 200 mg/m2 | IV, 30 minutes before RT, 3/5 times weekly | — | 63.5–70Gy,2 Gy/fraction, 5 fractions weekly. |
| Haddad[36] (2009) | 29/29 | III,IV | 500 mg | IH, 30–60 minutes before RT, q.d | C(AUC 1.5) and P (45 mg/m2), weekly for the first 4 weeks of RT | 72 Gy, 1.5–1.8Gy/fraction over 6 weeks |
Abbreviations:Ami = amifostine; IV = intravenous injection; IH = Subcutaneously injection; RT = radiotherapy; C = carboplatin; 5-FU = 5-fluorouracil; DDP = Cisplatin; P = Paclitaxel;
D = Docetaxel; AUC = area under the curve; qd = daily; bid = twice daily.
The summary of methodological quality was evaluated with a five-question instrument described in the Cochrane Reviews Handbook 5.0. Generally, the qualities of seventeen included trials were considered to be of moderate risk of bias. Randomization was performed in all the 17 trials, but only 4 studies mentioned the allocation concealment[6], [7], [33], [36]. And the adequate sequence generation for randomization was delivered in 11 trials[3], [6], [7], [32], [33], [35], [36], [39]–[42]. For the blinding item, only two trials[3], [6] describe the blindness on evaluators but not on patients and physicians. The outcome of methodological quality for each trial was demonstrated in Figure 2.
Figure 2. Risk of bias summary.
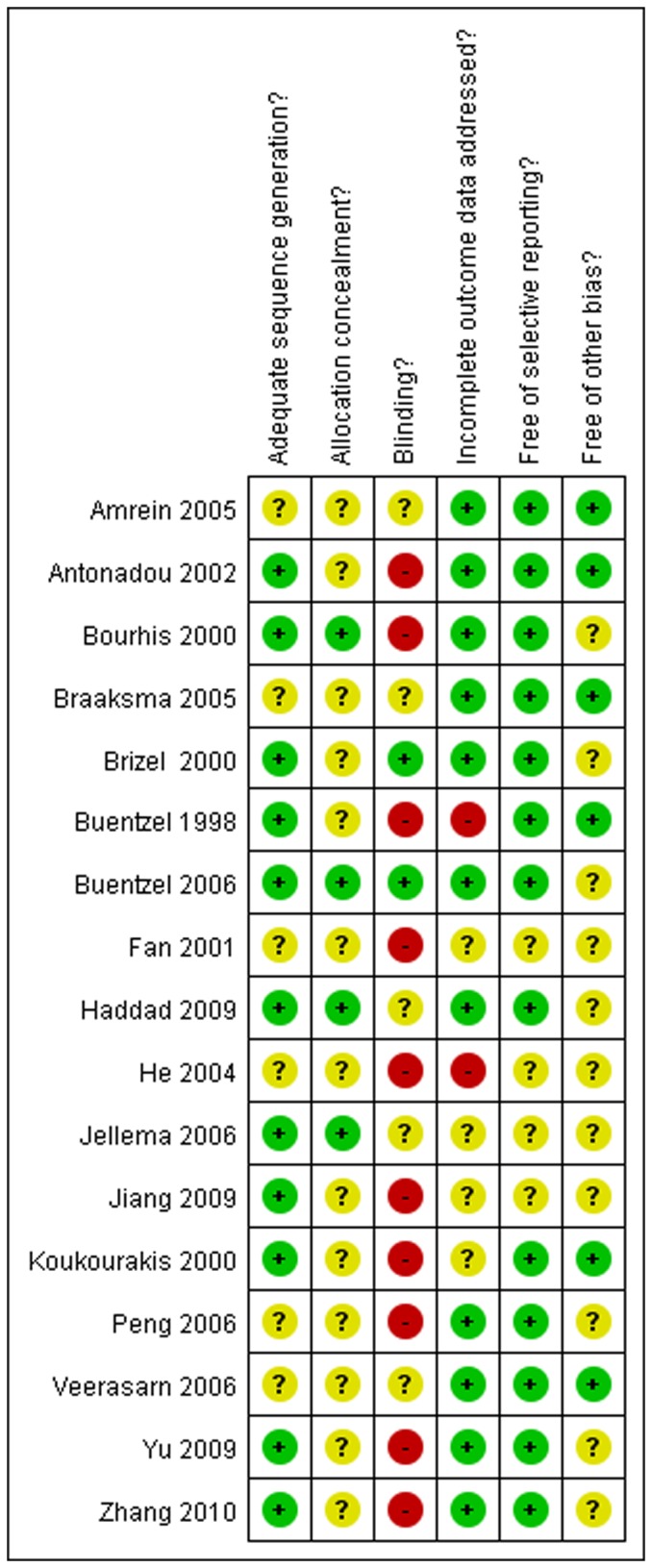
The authors' judgments for each risk of bias item. + is “low risk”; - is “high risk’’;? is “moderate risk.”
Mucositis
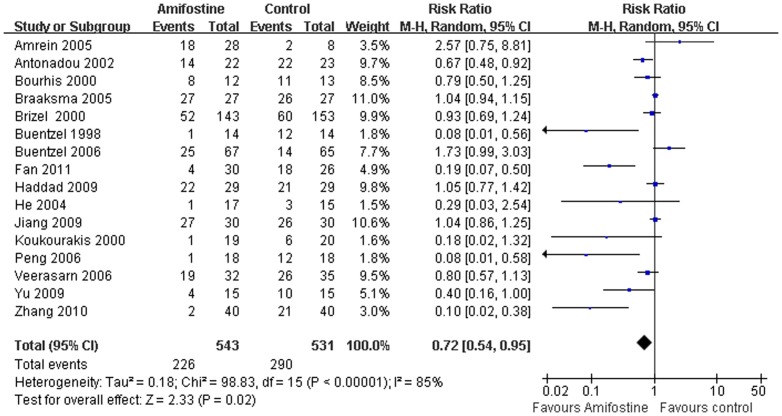
Of the seventeen trials, 16[3], [5], [6], [31]–[43] evaluated the mucositis with evident heterogeneity between studies(I2 = 85%) (Figure 3). The meta-analysis was performed in the random-effect model (Dersimonian-Laird method). Pooled analysis showed that amifostine reduced 28% of Grade 3–4 mucositis (RR,0.72; 95% CI,0.54–0.95; p<0.00001) indicating it can significantly decrease the risk of developing serious mucositis in patients treat with radiation. The publication bias was not detected in this subset analysis by Egger's test (p = 0.20). Subgroup analysis was performed cording to different treatment regimens (concomitant chemoradiotherapy or radiotherapy only) and admifostine administration (intravenously in or subcutaneously). In the subgroup analysis, statistically significant reduction of mucositis by using of amifostine was observed in patients treat with radiation only (RR,0.49; 95%CI,0.30–0.78; p = 0.03) and delivered intravenously(RR,0.52; 95%CI,0.34–0.78; p = 0.002) but not in patients treated with concurrent chemoradiation (RR,0.97; 95%CI,0.74–1.26; p = 0.80) and administered subcutaneously (RR,1.09; 95%CI,0.94–1.27; p = 0.24) (Table 2).
Figure 3. Forest plot of mucositis in HNSCC patients treated with radiotherapy/concomitant chemoradiation.
The squares and horizontal lines demonstrate the study-specific OR and 95% CI. The area of the squares reflects the study specific weight (inverse of the variance). The diamond represents the pooled OR and 95% CI.
Table 2. Subgroup analysis of radiation induced side effects according to treatment strategy.
| Subgroups | Mucositis | p | Acute xerostomia | p | Late xerostomia | p | Dysphagia | p | ||||
| RR | 95%CI | RR | 95%CI | RR | 95%CI | RR | 95%CI | |||||
| Treatment | ||||||||||||
| Chemradiation | 0.97 | 0.74–1.26 | 0.80 | 0.35 | 0.02–5.44 | 0.45 | 0.40 | 0.13–1.24 | 0.11 | 0.23 | 0.01–4.78 | 0.35 |
| Radiation only | 0.49 | 0.30–0.78 | 0.03 | 0.69 | 0.52–0.93 | 0.02 | 0.64 | 0.45–0.91 | 0.01 | 0.32 | 0.17–0.61 | 0.0004 |
| Administration | ||||||||||||
| IV | 0.52 | 0.34–0.78 | 0.002 | 0,73 | 0.54–0.97 | 0.03 | 0.60 | 0.49–0.74 | 0.00001 | 0.39 | 0.17–0.92 | 0.03 |
| IH | 1.09 | 0.94–1.27 | 0.24 | 0.08 | 0–1.34 | 0.08 | ||||||
Acute xerostomia
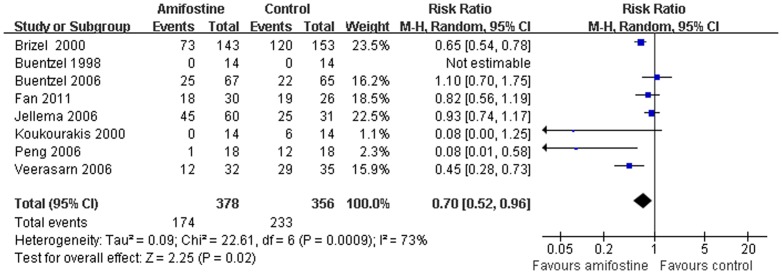
Eight trials[3], [5]–[7], [35], [37]–[39] report the number of patients developed acute xerostomia in both arms. The heterogeneity between the trials was obvious. Pooled analysis with the random-effect model demonstrated that significant reduction of Grade 2–4 acute xerostomia was achieved suing amifostine in patients with HNSCC (RR,0.71; 95%CI,0.52–0.96; p = 0.02) (Figure 4). No publication bias was found in the acute xerostomia analysis (Egger's test, p = 0.76). But in the subgroup analysis, use of amifostine does not reduce the risk of developing acute xerostomia in patients receiving concomitant radiation (RR,0.35; 95%CI,0.02–5.44; p = 0.45) (Table 2).
Figure 4. Forest plot of acute xerostomia in HNSCC patients treated with radiotherapy/concomitant chemoradiation.
The squares and horizontal lines demonstrate the study-specific OR and 95% CI. The area of the squares reflects the study specific weight (inverse of the variance). The diamond represents the pooled OR and 95% CI.
Late xerostomia
Late xerostomia were reported in six trials[3], [5]–[7], [32], [37] without the heterogeneity between the studies (I2 = 38%). Meta-analysis showed amifostine significant reduced 40% risk (RR,0.60; 95%CI,0.49–0.74; p<0.00001) of Grade 2–4 late xerostomia compared with placebo or observation (Figure 5). Subgroup analysis also showed that amifostine does not reduce the risk of developing late xerostomia in HNSCC patients treated with radiotherapy plus chemotherapy (p = 0.11) (Table2).
Figure 5. Forest plot of late xerostomia in HNSCC patients treated with radiotherapy/concomitant chemoradiation.
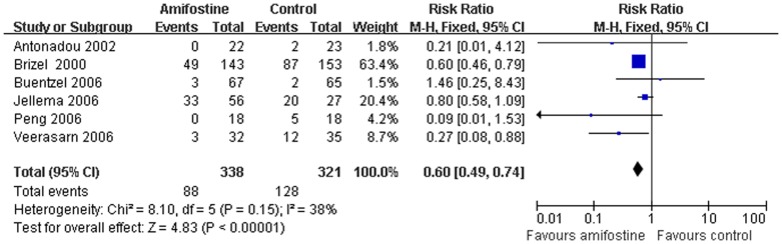
The squares and horizontal lines demonstrate the study-specific OR and 95% CI. The area of the squares reflects the study specific weight (inverse of the variance). The diamond represents the pooled OR and 95% CI.
Dysphagia
Data of dysphagia was pooled from five [5], [32]–[35] of the 17 included trials. Compared with the control, use of amifostine significant decrease the risk of developing Grade 3–4 dysphagia (RR,0.39; 95%CI,0.17–0.92; p = 0.03). Extreme heterogeneity between the studies was found (I2 = 94%). However, all the trials included in the analysis of dysphagia had an estimate point that favored amifostine, and three of them reached statistical significance. We preferred that amifostine significant decrease the risk of developing Grade 3–4 dysphagia.
Treatment response
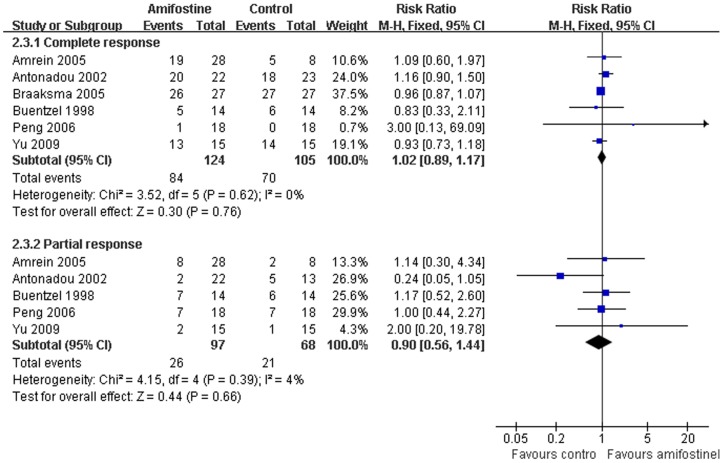
Response rates were available from six trials[31], [32], [34], [35], [37], [42]. No statistical heterogeneity between studies was found by Egger's test in both complete (I2 = 21%) and partial (I2 = 0%) response analysis. The pooled RR estimate for the complete and partial response was 1.02 (95%CI, 0.89–1.17; p = 0.76) and 0.90 (95%CI,0.56–1.44; p = 0.66) respectively (Figure 6). Neither of the them reached statistical significance (Figure 6).
Figure 6. Forest plot of dysphagia in HNSCC patients treated with radiotherapy/concomitant chemoradiation.
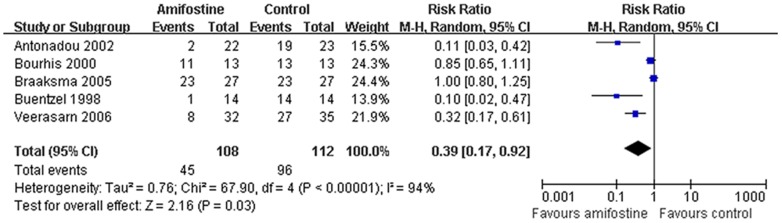
The squares and horizontal lines demonstrate the study-specific OR and 95% CI. The area of the squares reflects the study specific weight (inverse of the variance). The diamond represents the pooled OR and 95% CI.
Hematological toxicity
Hematological toxicity, including leucopenia, anemia and thrombocytopenia were extracted in five trials[5], [6], [32], [35], [37] without heterogeneity between the studies (I2 = 38%). Meta-analysis showed that no statistical difference of Grade 3–4 leucopenia (RR, 0.60; 95%CI,0.35–1.05; p = 0.07), anemia (RR,0.80; 95%CI,0.42–1.53; p = 0.50) and thrombocytopenia (RR, 0.43; 95%CI, 0.16–1.15; p = 0.09) were found between amifostine and control groups. Publication bias was not observed by Egger's test (p = 0.63) (Figure 7).
Figure 7. Forest plot of acute response rate in HNSCC patients treated with radiotherapy/concomitant chemoradiation.
The squares and horizontal lines demonstrate the study-specific OR and 95% CI. The area of the squares reflects the study specific weight (inverse of the variance). The diamond represents the pooled OR and 95% CI.
Side effects of Amifostine
Eight trials[3], [5]–[7], [32]–[35] describe the amifostine toxicity. The most common amifostine related side effects were nausea, emesis, transient hypotension and allergic. The average incidence rate of them (Grade 3–4) was 5%, 6%, 4% and 4% respectively (Figure 8). Of the eight trials reported the amifostine-induced toxic effects, seven studies[3], [5]–[7], [32], [33], [35] delivered the this drug by intravenous injection and only one [34] by subcutaneous injection, indicating that hypodermic administration of the drug may substantially reduce these side-effects[44].
Figure 8. Forest plot of hematological toxicity in HNSCC patients treated with radiotherapy/concomitant chemoradiation.
The squares and horizontal lines demonstrate the study-specific OR and 95% CI. The area of the squares reflects the study specific weight (inverse of the variance). The diamond represents the pooled OR and 95% CI.
Discussion
About half a million cases of head and neck squamous cell carcinomas are diagnosed yearly around the world[45]. The majority of the new cases of head and neck cancer have locally advanced disease. Management of these patients is complex because the vital structures in this area. Radiotherapy or chemoradiation is the most common used strategy at this stage. However, the radiation-related toxicities are inevitable and sometimes vital with the increase of radiotherapy dose. In the past several decades, researchers have been investigating use of drugs to decrease the side effects during radiotherapy, in order to increase the amount of radiation that can be safely administered to the patients. Amifostine is one of the radioprotector initially developed as part of the nuclear warfare program. Some randomized controlled trials demonstrated that this drug reduced some of severity radiation-related toxic effects (acute or late xerostomia) without tumor protection. However, some other trials do not support this. So, we performed this systematic review and meta-analysis to compile the inconsistent evidence together to evaluate the true clinical efficacy of this drug.
In this meta-analysis, we generally find that use of amifostine significantly reduced the radiation-induced toxicities of serious mucositis(p<0.00001), acute or late xerostomia(p = 0.02), and dysphagia(p<0.00001). However, the subgroup analysis, according to the treatment regimen (radiation only or concurrent chemoradiotherapy), demonstrate that patients receiving concomitant chemoradiation can not benefit reduction of side effects from use of amifostine. Heterogeneity between trials in this subgroup analysis set was found. And the trials included in this subset had inconsistent estimate points that statistically significant favored the amifostine or placebo/observation. So, the definitive efficacy of this drug in HNSCC patients receiving concomitant chemoradiation is conservative.
Two major controversy for clinical use of amifostine is tumor protection and its toxicity. Some oncologists argued that amifostine may reduce treatment effectiveness by stopping tumor tissue responding to radiation[8]. Morever, some opponents point out that there are not enough evidence to justify the conservative tumor protective effects of this drug[46]. However, considering the realities of randomized controlled trials and practice, absolute rejection the tumor protective effects compromised by amifostine is difficult. To demonstrate a hypothetical 40%–45% reduction of survival odds (α = 0.05; 80% power), 2492 patients would require in the trial, which was highly impractical[46].Under this situation, meta-analysis was conducted where possible to gain an objective consensus from repeatedly inconsistent trials. In this systematic review, we did not found statistical significant difference in complete response (RR,1.02; 95%CI,0.89–1.17; p = 0.76) and partial response (RR,0.90; 95%CI,0.56–1.44; p = 0.66) between the groups. However, without enough individual data, the disease-free survival (DFS) and overall survival (OS) were not pooled in this meta-analysis. However, Bourish et al [47] published a meta-analysis about effect of amifostine on survival among patients treated by radiotherapy with individual patients data recently. In that meta-analysis, twelve randomized trials consisted of 1119 patients were analyzed in the study and the result showed that amifostine did not reduce OS and DFS in patients treated with radiotherapy or concomitant chemoradiotherapy. So, we concluded that amifostine does not protect the tumor during radiation.
Another controversial issue about amifostine is toxicities induced by itself. The most common side effects of this drug were nausea, vomiting or transient hypotension, ranging from 5% to 30% according to the literatures[7], [32], [33]. Our study showed that the average incidence of Grade 3–4 nausea, emesis, hypotension and allergic were 5%, 6%, 4% and 4% respectively. Of the nine trials that reported the amifostine-induced toxic effects, 8 delivered the drug by intravenous injection and only one by subcutaneously, indicating that subcutaneous administration of this drug may substantially reduce these side-effects. However, we also find, in the subgroup analysis, that significant reduction of Grade 3–4 mucositis can not be reached in patients receive amifostine subcutaneously(p = 0.24).
The results of this meta-analysis were based on published randomized controlled trials not on individual patients' data. The results should therefore be interpreted cautiously. Although no evident of publication bias was found in this study by Egger's test, the small number of trials and possible existence of unpublished studies are inevitable and completely ruling out this possibility in all aspects is difficult[48].
In conclusion, this systematic review demonstrates that amifostine concurrently with RT significantly reduced the side effects of serious mucositis, acute/late xerastomia and dysphagia without protection of the tumor in HNSCC patients. The reduction of radiation-induced toxicities by amifostine should be weighed against the toxicities of amifostine itself according to the individual treatment strategy. Therefore, amifostine does have a role during radiation, a role that is still evolving. Well-designed RCTs are the necessary investments that will further explore the potential benefits of amifostine.
Supporting Information
PRISMA Checklist.
(DOC)
Funding Statement
This project was supported by the grants from National Natural Science Foundation of China (to Xuebing Li) (No 81302002). Science and technology foundation of Tianjin Municipal Health Bureau(to Jundong Gu) (2010KZ040). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Toledano I, Graff P, Serre A, Boisselier P, Bensadoun RJ, et al. (2012) Intensity-modulated radiotherapy in head and neck cancer: results of the prospective study GORTEC 2004-03. Radiother Oncol 103(1): 57–62. [DOI] [PubMed] [Google Scholar]
- 2. Cvek J, Kubes J, Skacelikova E, Otahal B, Kominek P, et al. (2012) Hyperfractionated accelerated radiotherapy with concomitant integrated boost of 70–75 Gy in 5 weeks for advanced head and neck cancer: A phase I does escalation study. Strahlenther Onkol 188(8): 666–670. [DOI] [PubMed] [Google Scholar]
- 3. Brizel DM, Wasserman TH, Henke M, Strnad V, Rudat V, et al. (2000) Phase III randomized trial of amifostine as a radioprotector in head and neck cancer. J Clin Oncol 18(19): 3339–3345. [DOI] [PubMed] [Google Scholar]
- 4. Koukourakis MI (2002) Amifostine in clinical oncology: current use and future applications. Anticancer Drugs 13(3): 181–209. [DOI] [PubMed] [Google Scholar]
- 5. Veerasarn V, Phromratanapongse P, Suntornpong N, Lorvidhaya V, Sukthomya V, et al. (2006) Effect of Amifostine to prevent radiotherapy-induced acute and late toxicity in head and neck cancer patients who had normal or mild impaired salivary gland function. J Med Assoc Thai 89(12): 2056–2067. [PubMed] [Google Scholar]
- 6. Buentzel J, Micke O, Adamietz IA, Monnier A, Glatzel M, et al. (2006) Intravenous amifostine during chemoradiotherapy for head-and-neck cancer: Arandomized placebo-controlled phase III study. Int J Radiat Oncol Biol Phys 64(3): 684–691. [DOI] [PubMed] [Google Scholar]
- 7. Jellema AP, Slotman BJ, Muller MJ, Leemans CR, Smeele LE, et al. (2006) Radiotherapy alone, versus radiotherapy with amifostine 3 times weekly, versus radiotherapy with amifostine 5 times weekly: A prospective randomized study in squamous cell head and neck cancer. Cancer 107(3): 544–553. [DOI] [PubMed] [Google Scholar]
- 8. Koukourakis MI (2003) Amifostine: is there evidence of tumor protection? Semin Oncol 30 6 Suppl 1818–30. [DOI] [PubMed] [Google Scholar]
- 9.Higgins JP, Green S (2008) Cochrane Handbook for Systematic Reviews of Interventions updated March 2011 John Wiley & Sons, Available: http://www.cochrane-handbook.org/.Accessed: 2011 DEC 12.
- 10. Cox JD, Stetz J, Pajak TF (1995) Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the EuropeanOrganization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys 31(5): 1341–1346. [DOI] [PubMed] [Google Scholar]
- 11. DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7(3): 177–188. [DOI] [PubMed] [Google Scholar]
- 12. Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327(7414): 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Egger M, Davey SG, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315(7109): 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wasserman TH, Brizel DM, Henke M, Monnier A, Eschwege F, et al. (2005) Influence of intravenous amifostine on xerostomia, tumor control, and survival after radiotherapy for head-and- neck cancer: 2-year follow-up of a prospective, randomized, phase III trial. Int J Radiat Oncol Biol Phys 63(4): 985–990. [DOI] [PubMed] [Google Scholar]
- 15. Bennett CL, Lane D, Stinson T, Glatzel M, Buntzel J (2001) Economic analysis of amifostine as adjunctive support for patients with advanced head and neck cancer: preliminary results from a randomized phase II clinical trial from Germany. Cancer Invest 19(2): 107–113. [DOI] [PubMed] [Google Scholar]
- 16. Buntzel J, Schuth J, Kuttner K, Glatzel M (1998) Radiochemotherapy with amifostine cytoprotection for head and neck cancer. Support Care Cancer 6(2): 155–160. [DOI] [PubMed] [Google Scholar]
- 17. Anne PR, Machtay M, Rosenthal DI, Brizel DM, Morrison WH, et al. (2007) A Phase II trial of subcutaneous amifostine and radiation therapy in patients with head-and-neck cancer. Int J Radiat Oncol Biol Phys 67(2): 445–452. [DOI] [PubMed] [Google Scholar]
- 18. Abitbol A, Abdel-Wahab M, Harvey M, Lewin A, Troner M, et al. (2005) Phase II study of tolerance and efficacy of hyperfractionated radiation therapy and 5-fluorouracil, cisplatin, and paclitaxel (taxol) and amifostine (ethyol) in head and neck squamous cell carcinomas: A-3 protocol. Am J Clin Oncol 28(5): 449–455. [DOI] [PubMed] [Google Scholar]
- 19. Trog D, Bank P, Wendt TG, Koscielny S, Beleites E (1999) Daily amifostine given concomitantly to chemoradiation in head and neck cancer. Apilot study. Strahlenther Onkol 175(9): 444–449. [DOI] [PubMed] [Google Scholar]
- 20. Rosenthal DI, Chambers MS, Weber RS, Eisbruch A (2004) A phase II study to assess the efficacy of amifostine for submandibular/sublingual salivary sparing during the treatment of head and neck cancer with intensity modulated radiation therapy for parotid salivary sparing. Semin Oncol 31 6 Suppl 1825–28. [DOI] [PubMed] [Google Scholar]
- 21. Zoberi I, Wasserman TH, Chao KS (2002) A prospective, nonrandomized study of the impact of amifostine on subsequent hypothyroidism in irradiated patients with head and neck cancers. Semin Radiat Oncol 12 1 Suppl 114–17. [DOI] [PubMed] [Google Scholar]
- 22. Suntharalingam M, Jaboin J, Taylor R, Wolf J, Banglore M, et al. (2004) The evaluation of amifostine for mucosal protection in patients with advanced loco-regional squamous cell carcinomas of the head and neck (SCCHN) treated with concurrent weekly carboplatin, paclitaxel, and daily radiotherapy (RT). Semin Oncol 31 6 Suppl 182–7. [DOI] [PubMed] [Google Scholar]
- 23. Thorstad WL, Haughey B, Chao KS (2003) Pilot study of subcutaneous amifostine in patients undergoing postoperative intensity modulated radiation therapy for head and neck cancer: preliminary data. Semin Oncol 30 6 Suppl 1896–100. [DOI] [PubMed] [Google Scholar]
- 24. Vacha P, Fehlauer F, Mahlmann B, Marx M, Hinke A, et al. (2003) Randomized phase III trial of postoperative radiochemotherapy +/− amifostine in head and neck cancer. Is there evidence for radioprotection. Strahlenther Onkol 179(6): 385–389. [DOI] [PubMed] [Google Scholar]
- 25. Wasserman T, Mackowiak JI, Brizel DM, Oster W, Zhang J, et al. (2000) Effect of amifostine on patient assessed clinical benefit in irradiated head and neck cancer. Int J Radiat Oncol Biol Phys 48(4): 1035–1039. [DOI] [PubMed] [Google Scholar]
- 26. Thorstad WL, Chao KS, Haughey B (2004) Toxicity and compliance of subcutaneous amifostine in patients undergoing postoperative intensity-modulated radiation therapy for head and neck cancer. Semin Oncol 31 6 Suppl 188–12. [DOI] [PubMed] [Google Scholar]
- 27. Chao KS, Ozyigit G, Thorsdad WL (2003) Toxicity profile of intensity-modulated radiation therapy for head and neck carcinoma and potential role of amifostine. Semin Oncol 30 6 Suppl 18101–108. [DOI] [PubMed] [Google Scholar]
- 28. Machtay M, Rosenthal DI, Chalian AA, Lustig R, Hershock D, et al. (2004) Pilot study of postoperative reirradiation, chemotherapy, and amifostine after surgical salvage for recurrent head-and-neck cancer. Int J Radiat Oncol Biol Phys 59(1): 72–77. [DOI] [PubMed] [Google Scholar]
- 29. Bardet E, Martin L, Calais G, Tuchais C, Bourhis J, et al. (2002) Preliminary data of the GORTEC 2000-02 phase III trial comparing intravenous and subcutaneous administration of amifostine for head and neck tumors treated by external radiotherapy. Semin Oncol 29 6 Suppl 1957–60. [DOI] [PubMed] [Google Scholar]
- 30. Anacak Y, Kamer S, Haydaroglu A (2005) Daily subcutaneous amifostine administration during irradiation of pediatric head and neck cancers. Pediatr Blood Cancer 48(5): 579–581. [DOI] [PubMed] [Google Scholar]
- 31. Amrein PC, Clark JR, Supko JG, Fabian RL, Wang CC, et al. (2005) Phase I trial and pharmacokinetics of escalating doses of paclitaxel and concurrent hyperfractionated radiotherapy with or without amifostine in patients with advanced head and neck carcinoma. Cancer 104(7): 1418–1827. [DOI] [PubMed] [Google Scholar]
- 32. Antonadou D, Pepelassi M, Synodinou M, Puglisi M, Throuvalas N (2002) Prophylactic use of amifostine to prevent radiochemotherapy-induced mucositis and xerostomia in head-and-neck cancer. Int J Radiat Oncol Biol Phys 52(3): 739–747. [DOI] [PubMed] [Google Scholar]
- 33. Bourhis J, De Crevoisier R, Abdulkarim B, Deutsch E, Lusinchi A, et al. (2000) A randomized study of very accelerated radiotherapy with and without amifostine in head and neck squamous cell carcinoma. Int J Radiat Oncol Biol Phys 46(5): 1105–1108. [DOI] [PubMed] [Google Scholar]
- 34. Braaksma M, van Agthoven M, Nijdam W, Uyl-de GC, Levendag P (2005) Costs of treatment intensification for head and neck cancer: concomitant chemoradiation randomised for radioprotection with amifostine. Eur J Cancer 41(14): 2102–2111. [DOI] [PubMed] [Google Scholar]
- 35. Buntzel J, Kuttner K, Frohlich D, Glatzel M (1998) Selective cytoprotection with amifostine in concurrent radiochemotherapy for head and neck cancer. Ann Oncol 9(5): 505–509. [DOI] [PubMed] [Google Scholar]
- 36. Haddad R, Sonis S, Posner M, Wirth L, Costello R, et al. (2009) Randomized phase 2 study of concomitant chemoradiotherapy using weeklycarboplatin/paclitaxel carboplatin/paclitaxel with or without daily subcutaneous amifostine in patients with locally advanced head and neck cancer. Cancer 115(19): 4514–4523. [DOI] [PubMed] [Google Scholar]
- 37. Peng LH, Sun Y, Zhang XL, Zhang Q, Fu S (2006) Efficacy of Amifostine on Patients with Head and Neck Squamous Cell Carcinomas Undergoing Radiation Therapy and Chemotherapy. China Pharmacy 17(5): 1166–1168. [Google Scholar]
- 38. Fan ZG, Lin HX, Liu ZQ, He QH, Zhang SL, et al. (2011) Protective effect of amifostine for mucositis and xerostomia in nasopharyngeal carcinoma patients treated with irradiation. Military Medical Journal of Southeast China 13(2): 146–148. [Google Scholar]
- 39. Koukourakis MI, Kyrias G, Kakolyris S, Kouroussis C, Frangiadaki C (2000) Subcutaneous administration of amifostine during fractionated radiotherapy: a randomized phase II study. J Clin Oncol 18(11): 2226–2233. [DOI] [PubMed] [Google Scholar]
- 40. Zhang N, Xu LJ, Ding JY (2010) Protection effect of amifostine on oral mucosa in patients treated with concurrent chemoradiation. Chin J Prim Med Pharm 17(5): 668–669. [Google Scholar]
- 41. Jiang F, Chen XZ, Wang FZ (2009) Effects of amifostine on nasopharyngeal patients treated with chemoradiotherapy. Chin J Radiol Med Prot 29(6): 612–613. [Google Scholar]
- 42.Yu DS, Zhao W, Wu XL, Huang ZQ, Huang RB (2009) Amifostine in Prevention of Radiation induced Xerostomia. Journal of Sun Yatsen University(Medical Sciences) 30(4S): : 86–87;95. [Google Scholar]
- 43. He XY, Hu CS, Wu YR (2004) Radioprotective Effect of Amifostine in Nasopharyngeal Carcinoma. Fudan Univ J Med Sci 31(1): 53–58. [Google Scholar]
- 44. Anne PR, Curran WJ Jr (2002) A phase II trial of subcutaneous amifostine and radiation therapy in patients with head and neck cancer. Semin Radiat Oncol 12 1 Suppl 118–19. [DOI] [PubMed] [Google Scholar]
- 45. Jemal A, Bray F (2011) Center MM, Ferlay J, Ward E, et al (2011) Global cancer statistics. CA Cancer J Clin 61(2): 69–90. [DOI] [PubMed] [Google Scholar]
- 46. Brizel DM, Overgaard J (2003) Does amifostine have a role in chemoradiation treatment. Lancet Oncol 4(6): 378–381. [DOI] [PubMed] [Google Scholar]
- 47. Bourhis J, Blanchard P, Maillard E, Brizel DM, Movsas B, et al. (2011) Effect of amifostine on survival among patients treated with radiotherapy: meta-analysis of individual patient data. J Clin Oncol 29(18): 2590–2597. [DOI] [PubMed] [Google Scholar]
- 48. Trinquart L, Abbe A, Ravaud P (2012) Impact of reporting bias in network meta-analysis of antidepressant placebo-controlled trials. PLOS ONE 7(4): e35219. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PRISMA Checklist.
(DOC)