Abstract
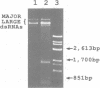
We have determined the organization within the terminal domains of the major large double-stranded RNA genetic elements associated with the hypovirulent strain EP713 of the chestnut blight pathogen Cryphonectria (Endothia) parasitica. Only the polyadenylated strand contained long open reading frames. Furthermore, only RNA of the same polarity as the polyadenylated strand was detectable in a single-stranded form, indicating that the polyadenylated strand is the coding or plus strand. The organization of the 5'-proximal portion of the plus strand consisted of a 495 nucleotide non-coding leader sequence followed by two overlapping open reading frames. The first, ORF1, extended 957 nucleotides while the second, ORF2, began 68 nucleotides upstream of the ORF1 termination codon and extended at least 1412 nucleotides. No open reading frames of significant size were detected within 0.8 kb of the poly(A) tail. In vitro translation of synthetic transcripts containing ORF1 yielded a polypeptide of Mr 29 kd. The ORF1 product was also detected in lysates of the hypovirulent strain but was absent in lysates of the isogenic virulent strain. It represents the first protein to be identified as a gene product encoded by a hypovirulence-associated double-stranded RNA genetic element.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anagnostakis S. L. Biological control of chestnut blight. Science. 1982 Jan 29;215(4532):466–471. doi: 10.1126/science.215.4532.466. [DOI] [PubMed] [Google Scholar]
- Anzola J. V., Xu Z. K., Asamizu T., Nuss D. L. Segment-specific inverted repeats found adjacent to conserved terminal sequences in wound tumor virus genome and defective interfering RNAs. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8301–8305. doi: 10.1073/pnas.84.23.8301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asamizu T., Summers D., Motika M. B., Anzola J. V., Nuss D. L. Molecular cloning and characterization of the genome of wound tumor virus: a tumor-inducing plant reovirus. Virology. 1985 Jul 30;144(2):398–409. doi: 10.1016/0042-6822(85)90281-8. [DOI] [PubMed] [Google Scholar]
- Bassel-Duby R., Spriggs D. R., Tyler K. L., Fields B. N. Identification of attenuating mutations on the reovirus type 3 S1 double-stranded RNA segment with a rapid sequencing technique. J Virol. 1986 Oct;60(1):64–67. doi: 10.1128/jvi.60.1.64-67.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Geller A. I., Rich A. A UGA termination suppression tRNATrp active in rabbit reticulocytes. Nature. 1980 Jan 3;283(5742):41–46. doi: 10.1038/283041a0. [DOI] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D., Meselson M. Plasmid screening at high colony density. Methods Enzymol. 1983;100:333–342. doi: 10.1016/0076-6879(83)00066-x. [DOI] [PubMed] [Google Scholar]
- Hatfield D., Diamond A., Dudock B. Opal suppressor serine tRNAs from bovine liver form phosphoseryl-tRNA. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6215–6219. doi: 10.1073/pnas.79.20.6215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiremath S., L'Hostis B., Ghabrial S. A., Rhoads R. E. Terminal structure of hypovirulence-associated dsRNAs in the chestnut blight fungus Endothia parasitica. Nucleic Acids Res. 1986 Dec 22;14(24):9877–9896. doi: 10.1093/nar/14.24.9877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Kozak M. Selection of initiation sites by eucaryotic ribosomes: effect of inserting AUG triplets upstream from the coding sequence for preproinsulin. Nucleic Acids Res. 1984 May 11;12(9):3873–3893. doi: 10.1093/nar/12.9.3873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller P. P., Hinnebusch A. G. Multiple upstream AUG codons mediate translational control of GCN4. Cell. 1986 Apr 25;45(2):201–207. doi: 10.1016/0092-8674(86)90384-3. [DOI] [PubMed] [Google Scholar]
- Rüther U., Müller-Hill B. Easy identification of cDNA clones. EMBO J. 1983;2(10):1791–1794. doi: 10.1002/j.1460-2075.1983.tb01659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartaglia J., Paul C. P., Fulbright D. W., Nuss D. L. Structural properties of double-stranded RNAs associated with biological control of chestnut blight fungus. Proc Natl Acad Sci U S A. 1986 Dec;83(23):9109–9113. doi: 10.1073/pnas.83.23.9109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z. K., Anzola J. V., Nuss D. L. Tailored removal of flanking homopolymer sequences from cDNA clones. DNA. 1987 Oct;6(5):505–513. doi: 10.1089/dna.1987.6.505. [DOI] [PubMed] [Google Scholar]