Abstract
The mitochondrial NADH-ubiquinone reductase (complex I) is an assembly of approximately 26 different polypeptides. In vertebrates and invertebrates, seven of its subunits are the products of genes in the mitochondrial DNA, and homologues of these genes have been found previously in the chloroplast genomes of Marchantia polymorpha and Nicotiana tabacum, although their function in the chloroplast is unknown. The remainder of the subunits of the mitochondrial complex are nuclear gene products that are imported into the organelle, amongst them the 49 kd subunit, a component of the iron--sulphur subcomplex of the enzyme. In the present work, the N-terminal sequence of this protein has been determined, and this has been used to design two mixtures of synthetic oligonucleotides, each containing 32 different sequences 17 bases long. These mixtures have been used as hybridization probes to isolate cDNA clones from a bovine library. The DNA sequences of these clones have been determined and they encode the mature 49 kd protein, with the exception of amino acids 1 and 2. The protein sequence of 430 amino acids is closely related to those of proteins that are encoded in open reading frames (ORFs) present in the chloroplast genomes of M.polymorpha and N.tabacum. Only one cysteine is conserved and the sequences provide no indication that the 49 kd protein contains iron--sulphur centres. These ORFs are found in the single copy regions of chloroplast DNA in close proximity to four of the homologues of the mammalian mitochondrial genes that encode subunits of complex I.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S., Bankier A. T., Barrell B. G., de Bruijn M. H., Coulson A. R., Drouin J., Eperon I. C., Nierlich D. P., Roe B. A., Sanger F. Sequence and organization of the human mitochondrial genome. Nature. 1981 Apr 9;290(5806):457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- Anderson S., de Bruijn M. H., Coulson A. R., Eperon I. C., Sanger F., Young I. G. Complete sequence of bovine mitochondrial DNA. Conserved features of the mammalian mitochondrial genome. J Mol Biol. 1982 Apr 25;156(4):683–717. doi: 10.1016/0022-2836(82)90137-1. [DOI] [PubMed] [Google Scholar]
- Bennoun P. Evidence for a respiratory chain in the chloroplast. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4352–4356. doi: 10.1073/pnas.79.14.4352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibb M. J., Van Etten R. A., Wright C. T., Walberg M. W., Clayton D. A. Sequence and gene organization of mouse mitochondrial DNA. Cell. 1981 Oct;26(2 Pt 2):167–180. doi: 10.1016/0092-8674(81)90300-7. [DOI] [PubMed] [Google Scholar]
- Biggin M. D., Gibson T. J., Hong G. F. Buffer gradient gels and 35S label as an aid to rapid DNA sequence determination. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3963–3965. doi: 10.1073/pnas.80.13.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown T. A., Waring R. B., Scazzocchio C., Davies R. W. The Aspergillus nidulans mitochondrial genome. Curr Genet. 1985;9(2):113–117. doi: 10.1007/BF00436957. [DOI] [PubMed] [Google Scholar]
- Burger G., Werner S. The mitochondrial URF1 gene in Neurospora crassa has an intron that contains a novel type of URF. J Mol Biol. 1985 Nov 20;186(2):231–242. doi: 10.1016/0022-2836(85)90100-7. [DOI] [PubMed] [Google Scholar]
- Chomyn A., Mariottini P., Cleeter M. W., Ragan C. I., Matsuno-Yagi A., Hatefi Y., Doolittle R. F., Attardi G. Six unidentified reading frames of human mitochondrial DNA encode components of the respiratory-chain NADH dehydrogenase. Nature. 1985 Apr 18;314(6012):592–597. doi: 10.1038/314592a0. [DOI] [PubMed] [Google Scholar]
- Cozens A. L., Walker J. E., Phillips A. L., Huttly A. K., Gray J. C. A sixth subunit of ATP synthase, an F(0) component, is encoded in the pea chloroplast genome. EMBO J. 1986 Feb;5(2):217–222. doi: 10.1002/j.1460-2075.1986.tb04201.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozens A. L., Walker J. E. The organization and sequence of the genes for ATP synthase subunits in the cyanobacterium Synechococcus 6301. Support for an endosymbiotic origin of chloroplasts. J Mol Biol. 1987 Apr 5;194(3):359–383. doi: 10.1016/0022-2836(87)90667-x. [DOI] [PubMed] [Google Scholar]
- Duckworth M. L., Gait M. J., Goelet P., Hong G. F., Singh M., Titmas R. C. Rapid synthesis of oligodeoxyribonucleotides VI. Efficient, mechanised synthesis of heptadecadeoxyribonucleotides by an improved solid phase phosphotriester route. Nucleic Acids Res. 1981 Apr 10;9(7):1691–1706. doi: 10.1093/nar/9.7.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galante Y. M., Hatefi Y. Purification and molecular and enzymic properties of mitochondrial NADH dehydrogenase. Arch Biochem Biophys. 1979 Feb;192(2):559–568. doi: 10.1016/0003-9861(79)90126-7. [DOI] [PubMed] [Google Scholar]
- Gay N. J., Walker J. E. Molecular cloning of a bovine cathepsin. Biochem J. 1985 Feb 1;225(3):707–712. doi: 10.1042/bj2250707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gay N. J., Walker J. E. Two genes encoding the bovine mitochondrial ATP synthase proteolipid specify precursors with different import sequences and are expressed in a tissue-specific manner. EMBO J. 1985 Dec 16;4(13A):3519–3524. doi: 10.1002/j.1460-2075.1985.tb04111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Halphen D., Lindorfer M. A., Capaldi R. A. Subunit arrangement in beef heart complex III. Biochemistry. 1988 Sep 6;27(18):7021–7031. doi: 10.1021/bi00418a053. [DOI] [PubMed] [Google Scholar]
- HATEFI Y., HAAVIK A. G., GRIFFITHS D. E. Studies on the electron transfer system. XL. Preparation and properties of mitochondrial DPNH-coenzyme Q reductase. J Biol Chem. 1962 May;237:1676–1680. [PubMed] [Google Scholar]
- Himeno H., Masaki H., Kawai T., Ohta T., Kumagai I., Miura K., Watanabe K. Unusual genetic codes and a novel gene structure for tRNA(AGYSer) in starfish mitochondrial DNA. Gene. 1987;56(2-3):219–230. doi: 10.1016/0378-1119(87)90139-9. [DOI] [PubMed] [Google Scholar]
- Høj P. B., Svendsen I., Scheller H. V., Møller B. L. Identification of a chloroplast-encoded 9-kDa polypeptide as a 2[4Fe-4S] protein carrying centers A and B of photosystem I. J Biol Chem. 1987 Sep 15;262(26):12676–12684. [PubMed] [Google Scholar]
- Jacobs H. T., Elliott D. J., Math V. B., Farquharson A. Nucleotide sequence and gene organization of sea urchin mitochondrial DNA. J Mol Biol. 1988 Jul 20;202(2):185–217. doi: 10.1016/0022-2836(88)90452-4. [DOI] [PubMed] [Google Scholar]
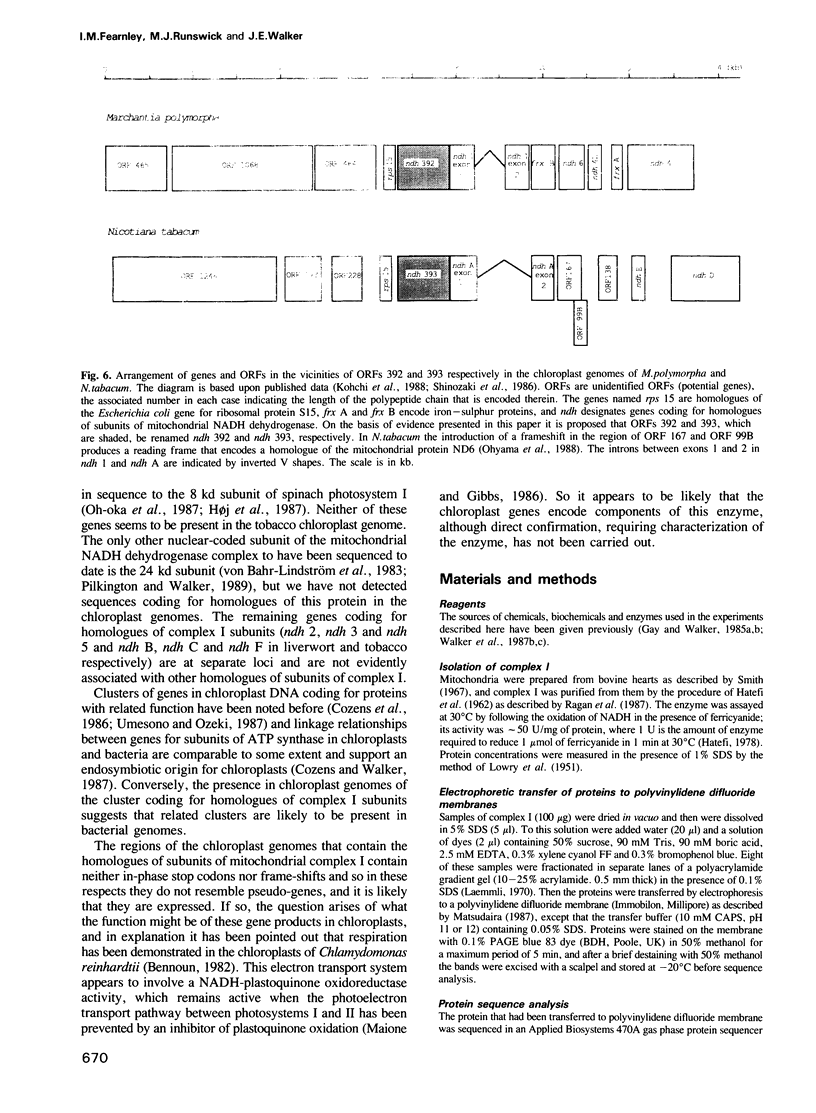
- Kohchi T., Shirai H., Fukuzawa H., Sano T., Komano T., Umesono K., Inokuchi H., Ozeki H., Ohyama K. Structure and organization of Marchantia polymorpha chloroplast genome. IV. Inverted repeat and small single copy regions. J Mol Biol. 1988 Sep 20;203(2):353–372. doi: 10.1016/0022-2836(88)90004-6. [DOI] [PubMed] [Google Scholar]
- Koike K., Kobayashi M., Yaginuma K., Taira M., Yoshida E., Imai M. Nucleotide sequence and evolution of the rat mitochondrial cytochrome b gene containing the ochre termination codon. Gene. 1982 Dec;20(2):177–185. doi: 10.1016/0378-1119(82)90036-1. [DOI] [PubMed] [Google Scholar]
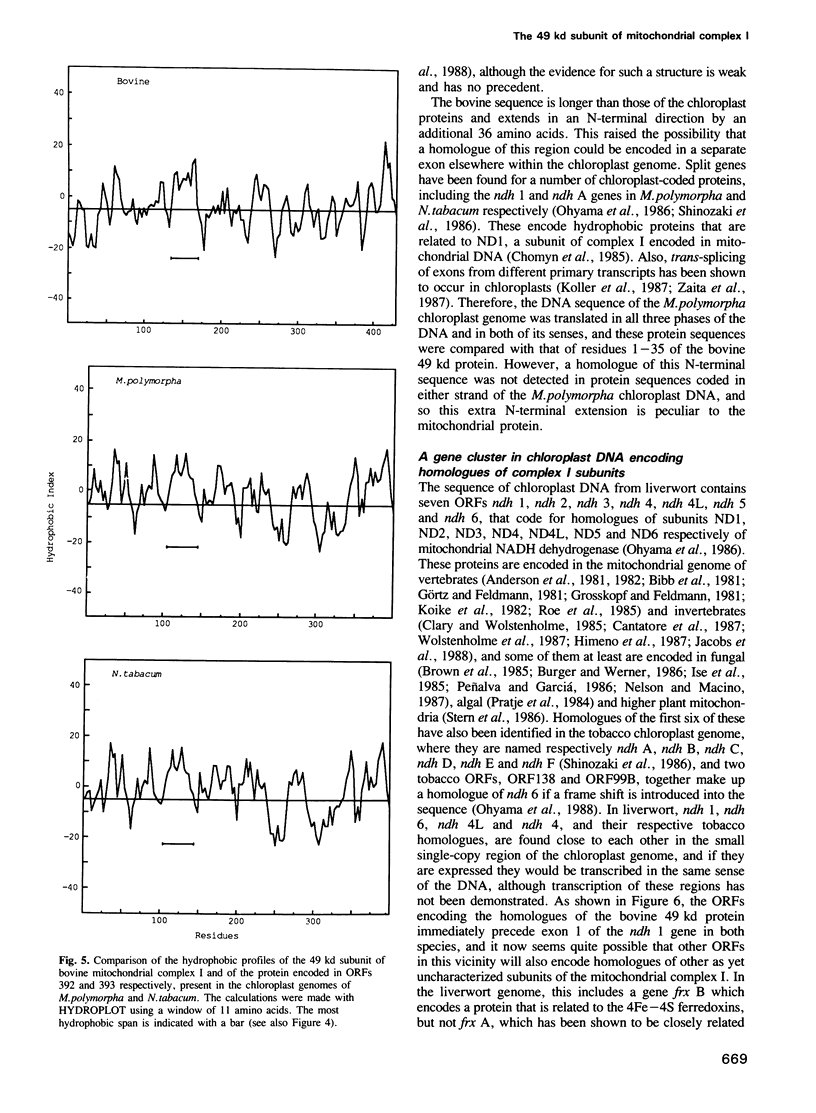
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lipman D. J., Pearson W. R. Rapid and sensitive protein similarity searches. Science. 1985 Mar 22;227(4693):1435–1441. doi: 10.1126/science.2983426. [DOI] [PubMed] [Google Scholar]
- Nelson M. A., Macino G. Structure and expression of the overlapping ND4L and ND5 genes of Neurospora crassa mitochondria. Mol Gen Genet. 1987 Feb;206(2):307–317. doi: 10.1007/BF00333589. [DOI] [PubMed] [Google Scholar]
- Ohyama K., Fukuzawa H., Kohchi T., Sano T., Sano S., Shirai H., Umesono K., Shiki Y., Takeuchi M., Chang Z. Structure and organization of Marchantia polymorpha chloroplast genome. I. Cloning and gene identification. J Mol Biol. 1988 Sep 20;203(2):281–298. doi: 10.1016/0022-2836(88)90001-0. [DOI] [PubMed] [Google Scholar]
- Peñalva M. A., García J. L. The subunit I of the respiratory-chain NADH dehydrogenase from Cephalosporium acremonium: the evolution of a mitochondrial gene. Curr Genet. 1986;10(11):797–801. doi: 10.1007/BF00418525. [DOI] [PubMed] [Google Scholar]
- Proudfoot N. J., Brownlee G. G. 3' non-coding region sequences in eukaryotic messenger RNA. Nature. 1976 Sep 16;263(5574):211–214. doi: 10.1038/263211a0. [DOI] [PubMed] [Google Scholar]
- Roe B. A., Ma D. P., Wilson R. K., Wong J. F. The complete nucleotide sequence of the Xenopus laevis mitochondrial genome. J Biol Chem. 1985 Aug 15;260(17):9759–9774. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinozaki K., Ohme M., Tanaka M., Wakasugi T., Hayashida N., Matsubayashi T., Zaita N., Chunwongse J., Obokata J., Yamaguchi-Shinozaki K. The complete nucleotide sequence of the tobacco chloroplast genome: its gene organization and expression. EMBO J. 1986 Sep;5(9):2043–2049. doi: 10.1002/j.1460-2075.1986.tb04464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S., Ragan C. I. The organization of NADH dehydrogenase polypeptides in the inner mitochondrial membrane. Biochem J. 1980 Feb 1;185(2):315–326. doi: 10.1042/bj1850315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staden R. Automation of the computer handling of gel reading data produced by the shotgun method of DNA sequencing. Nucleic Acids Res. 1982 Aug 11;10(15):4731–4751. doi: 10.1093/nar/10.15.4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern D. B., Bang A. G., Thompson W. F. The watermelon mitochondrial URF-1 gene: evidence for a complex structure. Curr Genet. 1986;10(11):857–869. doi: 10.1007/BF00418532. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Walker J. E., Fearnley I. M., Gay N. J., Gibson B. W., Northrop F. D., Powell S. J., Runswick M. J., Saraste M., Tybulewicz V. L. Primary structure and subunit stoichiometry of F1-ATPase from bovine mitochondria. J Mol Biol. 1985 Aug 20;184(4):677–701. doi: 10.1016/0022-2836(85)90313-4. [DOI] [PubMed] [Google Scholar]
- Walker J. E., Gay N. J., Powell S. J., Kostina M., Dyer M. R. ATP synthase from bovine mitochondria: sequences of imported precursors of oligomycin sensitivity conferral protein, factor 6, and adenosinetriphosphatase inhibitor protein. Biochemistry. 1987 Dec 29;26(26):8613–8619. doi: 10.1021/bi00400a018. [DOI] [PubMed] [Google Scholar]
- Walker J. E., Runswick M. J., Poulter L. ATP synthase from bovine mitochondria. The characterization and sequence analysis of two membrane-associated sub-units and of the corresponding cDNAs. J Mol Biol. 1987 Sep 5;197(1):89–100. doi: 10.1016/0022-2836(87)90611-5. [DOI] [PubMed] [Google Scholar]
- Wikström M. Two protons are pumped from the mitochondrial matrix per electron transferred between NADH and ubiquinone. FEBS Lett. 1984 Apr 24;169(2):300–304. doi: 10.1016/0014-5793(84)80338-5. [DOI] [PubMed] [Google Scholar]
- Wolstenholme D. R., Macfarlane J. L., Okimoto R., Clary D. O., Wahleithner J. A. Bizarre tRNAs inferred from DNA sequences of mitochondrial genomes of nematode worms. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1324–1328. doi: 10.1073/pnas.84.5.1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Bahr-Lindström H., Galante Y. M., Persson M., Jörnvall H. The primary structure of subunit II of NADH dehydrogenase from bovine-heart mitochondria. Eur J Biochem. 1983 Jul 15;134(1):145–150. doi: 10.1111/j.1432-1033.1983.tb07543.x. [DOI] [PubMed] [Google Scholar]