Abstract
Purpose of review
Acute kidney injury (AKI) is a major public health concern, and preexisting kidney disease may be one of the most important risk factors. We review recent epidemiologic evidence supporting baseline proteinuria and reduced glomerular filtration rate as risk factors for AKI.
Recent findings
In 2008, a case–control study of over 600 000 patients in an integrated healthcare system in California first quantified a graded association between reduced baseline estimated glomerular filtration rate (eGFR) and risk of dialysis-requiring AKI; it also showed proteinuria as an independent predictor for AKI. In 2010, a cohort study consisting of 1235 adults undergoing coronary artery bypass graft in Taiwan demonstrated that mild and heavy degrees of proteinuria detected by dipstick were associated with increasingly higher odds ratio of postoperative AKI, independent of chronic kidney disease stage. A US cohort study in 2010 of over 11 000 patients determined that elevated urine albumin-to-creatinine ratio (UACR) was an independent risk factor for hospitalizations with AKI; this association started with the submicroalbuminuric range (UACR 11–29 mg/g) and increased stepwise along severity of albuminuria, after adjustment for eGFR. A cohort study in 2010 of over 900 000 adults in Alberta demonstrated increased rates of hospital admissions with AKI for patients with mild and moderate dipstick proteinuria across all values of eGFR.
Summary
The presence of baseline proteinuria and reduced baseline eGFR are powerful independent predictors for AKI and should be taken into account in clinical practice to identify high-risk patients for receipt of aggressive preventive measures to reduce risk of AKI.
Keywords: acute renal failure, albuminuria, chronic kidney disease, chronic renal insufficiency, dialysis
Introduction
Acute kidney injury (AKI) is one of the most common and serious complications of hospitalized patients [1,2]. Although clinicians have long recognized reduced baseline glomerular filtration rate (GFR) to be a risk factor for AKI, this important relationship has not been quantified until recently. In addition, until 3 years ago, no study had investigated whether and how proteinuria – another cardinal manifestation of chronic kidney disease (CKD) – was related to risk of AKI.
We will review here one study published in 2008 [3] and three studies published in 2010 [4••–6••] that have advanced our knowledge regarding CKD as a risk factor for AKI.
Background
In 2008, Hsu et al. [3] conducted a nested case–control study utilizing a large cohort of patients receiving medical care at an integrated healthcare delivery system in Northern California (Kaiser Permanente) to define the risk of dialysis-requiring AKI among patients with varying levels of baseline GFR. The authors identified 1764 patients who developed hospital-acquired AKI treated with dialysis from 1996 to 2003 as cases, and 600 820 hospitalized patients who did not develop AKI requiring dialysis as controls. Baseline kidney function was defined by estimated GFR (eGFR) from the last prehospitalization serum creatinine using the abbreviated Modification of Diet in Renal Disease (MDRD) equation [7]. Cases of dialysis-requiring AKI were defined by an increase in creatinine by 50% (from prehospitalization to peak inpatient creatinine), and receipt of dialysis as identified by International Classification of Disease, Ninth Revision, Clinical Modification (ICD-9-CM) procedure codes 54.98 and 39.95 or Current Procedural Terminology (CPT) codes 90935, 90937, 90945, 90947, and 90999. Patients who were on maintenance dialysis or had received a kidney transplant were excluded. Controls included all hospitalized patients during the same study period who did not develop dialysis-requiring AKI and who also had outpatient serum creatinine measurements before admission. Logistic regression model was used with dialysis-requiring AKI as the outcome, and baseline eGFR categorized using the National Kidney Foundation (NKF) CKD classification [8] as the exposure of interest.
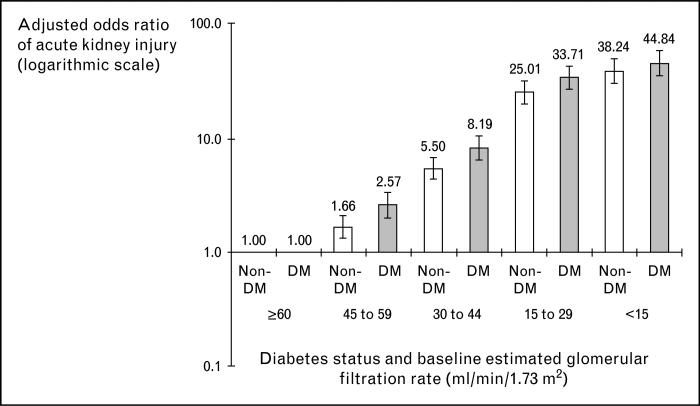
Of the 1746 cases of dialysis-requiring AKI, 1295 (74%) occurred among those with baseline eGFR less than 60 ml/min/1.73 m2. After adjusting for age, sex, race/ethnicity, and other baseline conditions, there was a strong graded relationship between risk of AKI and baseline eGFR. Patients with baseline eGFR 45–59 ml/min/1.73 m2 had nearly two-fold increase in adjusted odds ratio (OR) of dialysis-requiring AKI compared with referent patients with eGFR of 60 ml/min/1.73 m2 or above, while patients with baseline estimated GFR 15–29 ml/min/1.73 m2 had a 29-fold increase in adjusted OR compared with referent patients (Table 1). Within the same GFR category, patients with diabetes had higher OR of dialysis-requiring AKI than those without (Fig. 1).
Table 1.
Reduced eGFR and proteinuria as risk factors for acute kidney injury
| Author | Design | Population | Total study population | Outcome | Variables in multivariable analyses | Predictor | Adjusted measures of associationa (95% CI) |
|---|---|---|---|---|---|---|---|
| Hsu et al. [3] | Nested case–control | Kaiser-Permanente of Northern California | 602 566 | Dialysis-requiring AKI (n = 1764) | Age, sex, race/ethnicity, diabetes mellitus, diagnosed hypertension | eGFR ≥60 | Referent |
| eGFR 45–59 | OR 1.95 (1.66–2.30) | ||||||
| eGFR 30–44 | OR 6.54 (5.57–7.69) | ||||||
| eGFR 15–29 | OR 28.50 (24.50–33.14) | ||||||
| eGFR ≤15 | OR 40.07 (33.75–47.58) | ||||||
| No proteinuria | Referent | ||||||
| Proteinuria (dipstick 1+ or greater) | OR 2.79 (2.49–3.11) | ||||||
| Huang et al. [4••] | Cohort | National Taiwan University Hospital CABG surgery patients | 1235 | AKI defined using AKIN stage (n = 183) | Age, diabetes mellitus, recent MI, intra-aortic balloon pump, nonelective surgery | eGFR ≥60 | Referent |
| eGFR 30–59 | OR 1.68 (1.12–2.52) | ||||||
| eGFR 15–29 | OR 3.01 (1.57–6.03) | ||||||
| No proteinuriab | Referent | ||||||
| Mild proteinuriab | OR 1.6 (1.09–2.52) | ||||||
| Heavy proteinuriab | OR 2.30 (1.35–3.90) | ||||||
| Grams et al. [5••] | Cohort | Atherosclerosis Risk in Communities (ARIC) study | 11 200 | AKI defined as ICD-9-CM codes 584.5–584.9, or ICD-10-CM codes N17.0–N17.9 (n = 356) | Age, sex, race, cardiovascular risk factors | eGFR ≥105 | HR 1.1 (0.7–1.6) |
| eGFR 90–104 | Referent | ||||||
| eGFR 75–89 | HR 0.9 (0.7–1.3) | ||||||
| eGFR 60–74 | HR 1.5 (11–2.1) | ||||||
| eGFR 45–59 | HR 2.5 (17–3.7) | ||||||
| eGFR 30–44 | HR 7.0 (4.4–11.0) | ||||||
| eGFR 15–29 | HR 5.6 (2.6–12.1) | ||||||
| UACR ≤10 | Referent | ||||||
| UACR 10–20 | HR 1.9 (1.4–2.6) | ||||||
| UACR 30–299 | HR 2.2 (16–3.0) | ||||||
| UACR ≥300 | HR 4.8 (3.2–7.2) | ||||||
| James et al. [6••] | Cohort | Alberta Province adults | 920 985 | AKI defined as ICD-9-CM codes 584.5–584.9 (n = 6520) | Age, sex, aboriginal status, low income, social assistance, HIV/AIDS, history of cancer, cerebrovascular disease, congestive heart failure, chronic pulmonary disease, dementia, diabetes mellitus, hypertension, metastatic solid tumor, mild liver disease, moderate to severe liver disease, myocardial infarction, paralysis, peptic ulcer disease, peripheral vascular disease, rheumatic disease | eGFR ≥60 and no proteinuriab | Referent |
| eGFR ≥60 and mild proteinuriab | RR 2.5 (2.3–2.7) | ||||||
| eGFR ≥60 and heavy proteinuriab | RR 4.4 (3.7–5.2) | ||||||
| eGFR 45–59 and no proteinuriab | RR 2.3 (2.1–2.4) | ||||||
| eGFR 45–59 and mild proteinuriab | RR 4.3 (3.8–4.8) | ||||||
| eGFR 45–59 and heavy proteinuriab | RR 8.2 (7.0–9.6) | ||||||
| eGFR 30–44 and no proteinuriab | RR 5.6 (5.1–6.2) | ||||||
| eGFR 30–44 and mild proteinuriab | RR 8.2 (7.2–9.3) | ||||||
| eGFR 30–44 and heavy proteinuriab | RR 13 (12–15) | ||||||
| eGFR 15–29 and no proteinuriab | RR 13 (11–15) | ||||||
| eGFR 15–29 and mild proteinuriab | RR 16 (14–19) | ||||||
| eGFR 15–29 and heavy proteinuriab | RR 19 (16–23) |
AIDS, acquired immune deficiency syndrome; AKIN, Acute Kidney Injury Network; CABG, coronary artery bypass graft; CI, confidence interval; eGFR, estimated glomerular filtration rate (in ml/min/1.73 m2); HIV, human immunodeficiency virus; ICD-9-CM, International Classification of Diseases, 9th Revision, Clinical Modification; ICD-10-CM, International Classification of Diseases, 10th Revision, Clinical Modification; UACR, urine albumin-to-creatinine ratio (in mg/g).
OR, odds ratio; HR, hazard ratio; RR, rate ratio.
No proteinuria defined as dipstick negative; mild proteinuria defined as dipstick trace to 1+; heavy proteinuria defined as dipstick ≥2+.
Figure 1. Multivariable association of baseline estimated glomerular filtration rate and dialysis-requiring acute kidney injury stratified by the presence or absence of diabetes mellitus.
Each model adjusted for age, sex, race/ethnicity, diagnosed hypertension, and documented proteinuria. DM, diabetes mellitus. Reproduced with permission from [3].
The authors also evaluated the risk of AKI among patients with documented proteinuria independent of baseline estimated GFR. Proteinuria was identified as outpatient urine protein dipstick result of 1+ or greater and analyzed as a binary variable. The adjusted OR of dialysis-requiring AKI for patients with documented proteinuria was 2.79 (compared to referent patients without proteinuria).
This was the first study to quantify the graded relationship between baseline eGFR and risk of AKI. It was also the first to identify proteinuria as an important independent risk factor for AKI. Other strengths of the study include the diversity of the study population, chart validation of the outcome, and the authors’ careful attention in defining what constitutes ‘baseline’ kidney function (with several attendant sensitivity analyses). The latter point is an important methodological issue that has not received much attention in the past [9,10]. This study is limited by its inclusion of only dialysis-requiring AKI as cases and in defining proteinuria as only being present or absent based on dipstick urinalyses performed as part of routine clinical care at varying time points prior to the AKI episode.
Chronic kidney disease as a risk factor for acute kidney injury: more recent studies
We next review three studies published in 2010 investigating proteinuria and reduced GFR as risk factors for AKI.
Patients undergoing cardiac surgery
Huang et al. [4••] analyzed a cohort of patients undergoing coronary artery bypass graft (CABG) surgery at a large university hospital in Taiwan and its two branches from 2003 to 2007 to investigate the association between preoperative proteinuria and postoperative AKI. The authors included adult patients undergoing first-time CABG surgery, and excluded those with baseline eGFR less than 15 ml/min/1.73 m2 and those with history of preoperative renal replacement therapy with any modality. Preoperative proteinuria was measured using a dipstick within 2 days before surgery and was classified as ‘no proteinuria’ (=dipstick negative), ‘mild proteinuria’ (=dipstick trace to 1+), and ‘heavy proteinuria’ (=dipstick 2+ to 4+). Preoperative eGFR was calculated using the MDRD equation based on the creatinine at discharge from the previous hospitalization or the lowest creatinine during the index admission; patients were then separated into CKD stages 1–2 (eGFR ≥ 60 ml/min), stage 3 (eGFR 30–59 ml/min), and stage 4 (eGFR 15–29 ml/min). Postoperative AKI was defined using the Acute Kidney Injury Network (AKIN) criteria [11], and logistic regression analysis was performed to identify risk factors associated with postoperative AKI.
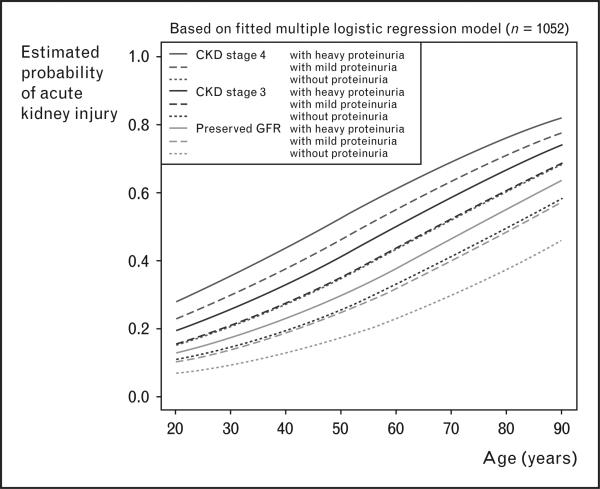
Among the 1051 patients included in the study analysis, 183 patients (17.4%) developed AKI. The unadjusted risk of AKI increased stepwise along each stage of CKD and severity of proteinuria. When stratified into CKD stages, a stepwise escalation in risk of AKI along increasing severity of proteinuria was seen in patients with preserved eGFR and CKD stage 3, but not for stage 4. In a multiple logistic regression analysis, mild and heavy proteinuria was each associated with an increased OR of postoperative AKI, independent of CKD stage and the presence of diabetes mellitus (Table 1). Heavy proteinuria was also associated with an increased OR of postoperative renal replacement therapy during the ICU stay (OR 7.29). The authors also performed a conditional effect plot of the estimated risk for postoperative AKI against patient's age, stratified by CKD stage and severity of proteinuria (Fig. 2). Notably, risk of postoperative AKI for patients with preserved eGFR and mild proteinuria was nearly equal to that for patients with stage 3 CKD without proteinuria. Similarly, patients with stage 3 CKD and mild proteinuria carried about the same risk of postoperative AKI as patients with stage 4 CKD without proteinuria (Fig. 2).
Figure 2. Conditional effect plot of baseline chronic kidney disease stage and severity or proteinuria on estimated probability of postoperative acute kidney injury.
Patients with mild or heavy proteinuria had a higher risk of postoperative acute kidney injury, even after adjusting for chronic kidney disease (CKD) stage. GFR, glomerular filtration rate. Reproduced with permission from [4••].
This study is thus an advance in that proteinuria was quantified in a graded manner and not simply classified as present or absent as before [3]. Another strength is that the outcomes included nondialysis-requiring AKI cases defined utilizing detailed clinical data such as changes in serum creatinine and urine output, which were well captured and validated in these cardiac surgery patients [12]. The study's limitations include its less diverse study population (only patients undergoing CABG) and smaller sample size. Misclassification of ‘baseline’ (usual) renal function is also possible as only inpatient creatinine measurements (either from prior hospitalization or index hospitalization) were used for this purpose; serum creatinine values may be higher than usual because of early AKI or lower than usual because of hemodilution or acute decrease in creatinine production [13].
Community-based research cohort
Grams et al. [5••] performed a large cohort study using data from 11 200 patients in the Atherosclerosis Risk in Communities (ARIC) study to characterize the association between baseline proteinuria and hospitalizations for AKI. The ARIC cohort consisted of black and white adults between the ages of 45 and 64 years from four US enrollment sites (Forsyth County, NC; Jackson, MS; suburban Minneapolis, MN; and Washington County, MD). The authors included ARIC participants with a baseline urine albumin-to-creatinine ratio (UACR, in mg/g) and calibrated serum creatinine measures, and excluded patients with eGFR less than 15 ml/min/1.73 m2 (CKD stage 5). The primary outcome of hospitalization with AKI was abstracted from appropriate International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) codes 584.5–584.9, or 10th Revision, Clinical Modification (ICD-10-CM) codes N17.0–N17.9, listed in any position on the discharge diagnoses, and in the listed causes of death on the death certificate.
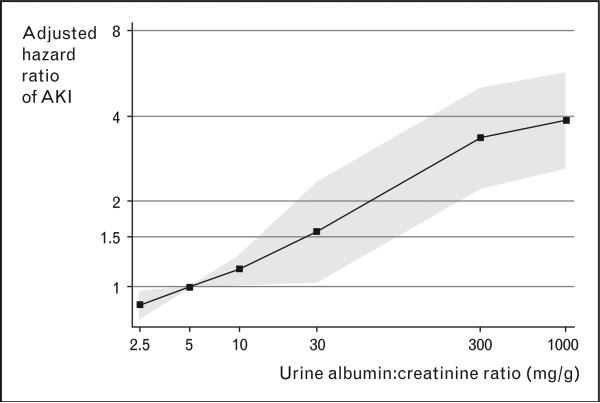
Among the 11 200 cohort participants, there were 356 patients hospitalized (3.2%) with AKI during an average follow-up period of 8.0 years (in 492 hospitalizations with AKI). When stratified into groups of UACR ≤10 mg/g (no albuminuria), 10–29 mg/g (subclinical albuminuria), 30 to 299 mg/g (microalbuminuria), and ≥300 mg/g (macroalbuminuria), there was a stepwise increase in incidence rate, from 2.6 events in the no albuminuria group to 41.2 events per 1000 person-years in the macroalbuminuria group. Using Cox proportional hazards regression and patients with no albuminuria as a reference, the adjusted relative hazards of AKI stratified by albuminuria categories were 1.9, 2.2, and 4.8 for subclinical albuminuria, microalbuminuria, and macroalbuminuria, respectively (Table 1). There was also a graded increase in the hazards of AKI along the worsening eGFR categories, adjusted for albuminuria (Table 1). The authors further modeled the relationship between albuminuria and risk of AKI by expressing UACR as a continuous variable and utilizing a spline approach to demonstrate a nearly linear relationship (Fig. 3).
Figure 3. Adjusted relative hazard ratio of acute kidney injury vs. urine albumin-to-creatinine ratio using a linear spline model.
Relative hazards of acute kidney injury (AKI) increases with increasing continuous urine albumin-to-creatinine ratio, adjusted for estimated glomerular filtration rate, age, sex, race, and cardiovascular risk factors. Reproduced with permission from [5••].
A distinguishing strength of this study is the rigorous quantification of proteinuria using UACR measurements taken as part of a research study to demonstrate a continuous association between baseline proteinuria and risk of AKI, independent of eGFR [14]. This is a community-based study that included many patients with eGFR in the normal range and showed that presence of albuminuria, even at a level below the microalbuminuria threshold, is associated with an increased risk of AKI among patients with eGFR greater than 60 ml/min/1.73 m2. The authors took pains to perform sensitivity analyses to account for possible confounders, such as cardiovascular procedures and angiotensin-converting enzyme inhibitor (ACEI) and angiotensin II receptor blocker (ARB) use [14]. A limitation is the ascertainment of AKI relying on ICD-9-CM or ICD-10-CM administrative codes; these codes have been shown to be insensitive compared with the gold standard definition of AKI based on the changes in serum creatinine [15], rendering the calculated AKI incidence rates unreliable. Another limitation is the few patients with advanced CKD, making the confidence intervals for the hazard ratio estimates at low eGFR levels very wide.
Province-wide study population
James et al. [6••] utilized a province-wide sample of 920 985 adults in Alberta, Canada, to examine the associations of eGFR and proteinuria with risk of AKI. This cohort study included adults with at least one outpatient measurement of serum creatinine and one measurement of proteinuria between 2002 and 2007, and excluded those with end-stage renal disease at study entry (eGFR <15 ml/min/1.73 m2, chronic dialysis, or previous kidney transplant). The baseline eGFR was calculated using the MDRD equation after averaging all outpatient serum creatinine measurements taken within 6 months of the first creatinine measurement within the cohort study period, while proteinuria was measured by urine dipstick for the primary analysis. Similar to the Grams study, this article utilized administrative codes to identify hospital admissions with AKI as the primary outcome of interest. Poisson regression was used to evaluate the hypothesized associations, and incidence rates and rate ratios for AKI and AKI requiring dialysis were calculated.
During median follow-up of 35 months, 6520 (0.7%) participants in the cohort were admitted with AKI and 516 (<0.01%) with AKI requiring dialysis. The authors presented the incidence rates of AKI and rate ratios adjusted for age, sex, socioeconomic variables, and comorbidities such as diabetes and hypertension, stratified by baseline eGFR. Within each eGFR stratum, the rate ratios for both AKI and AKI requiring dialysis increased with higher levels of proteinuria, categorized as normal (dipstick negative), mild (dipstick trace to 1+), and heavy dipstick (≥2+). For example, patients with normal eGFR (≥60 ml/min/1.73 m2) and mild proteinuria developed AKI at an adjusted rate of 2.5 times higher than patients with normal eGFR and no proteinuria; the rate ratio increased further to 4.4 in patients with normal eGFR and heavy proteinuria. In sensitivity analysis, among the subset of 102 701 patients who had urinary UACR measurements – stratified into normal (<3.4 mg/mmol), mild (3.4–33.9 mg/mmol), or heavy (>33.9 mg/mmol) categories – the investigators found consistent trends for risk for AKI and AKI requiring dialysis.
The major strength of the study by James et al. is the very large sample of nearly 1 million persons, which represent about 40% of the entire adult population in Alberta, Canada. Importantly, the study went on to provide adjusted rates of death and composite renal outcomes (end-stage renal disease or doubling of serum creatinine concentration) during the follow-up period, adding to a growing body of literature demonstrating deleterious long-term renal and nonrenal sequelae of AKI [16–19]. Their analysis addresses the significant issue of confounding by the effects of preexisting kidney disease when examining associations between acute kidney injury and subsequent adverse outcomes.
However, as in the Hsu study [3], this study had to rely on serum creatinine and proteinuria measurements obtained as part of clinical care, which may have selected preferentially for patients who were older and have more comorbidities. Similar to the Grams study [5••], the reliance of administrative codes to identify hospitalizations with AKI likely resulted in underestimation of the true event rate because of the validated algorithm's high specificity, yet low sensitivity [15]. In contrast, administrative codes for dialysis-requiring AKI have been shown to be highly sensitive and specific [15], making the corresponding adjusted event rates more likely to be accurate.
Conclusion
Taken together, these four studies have clearly established preinsult proteinuria as an independent risk factor for AKI. They have also rigorously quantified the graded association between severity of reduction in baseline renal function (eGFR) and progressively higher risk of AKI.
AKI is a growing epidemic [20] with few treatment options, so identifying those at highest risk can lead to better targeted efforts at prevention. Current risk stratification tools to help predict AKI in high-risk settings such as during cardiac surgery [21,22] and iodinated contrast administration [23] utilize only preprocedure eGFR to assess ‘baseline’ renal status with regard to risk of AKI. The finding that even a small amount of protein-uria from a simple urine dipstick may serve as a ‘low eGFR equivalent’ to identify a hitherto neglected subset of at-risk individuals has therapeutic implications. For example, a very cogent argument can be made that patients with normal eGFR but abnormal proteinuria should receive ample hydration and N-acetylcysteine prior to iodinated contrast, just like patients with low eGFR.
The findings also underscore that the current staging system of chronic kidney disease based primarily on the eGFR without fully incorporating the severity of concomitant proteinuria can be improved [8]. Aside from the papers reviewed here demonstrating the independent link between proteinuria and AKI, there is a burgeoning body of work to suggest proteinuria's association with mortality and other adverse renal and cardiovascular outcomes [24–26]. Incorporating information on baseline proteinuria into future classification schemes of chronic kidney disease would provide richer prognostic information for providers.
Key points.
The presence of baseline proteinuria and reduced glomerular filtration rate are both powerful independent risk factors for AKI.
The risk of acute kidney injury increases along severity of baseline proteinuria.
Even a very mild degree of proteinuria – that is, trace to 1+ proteinuria on urine dipstick, or submicroalbuminuric range on urine albumin-to-creatinine ratio (11–29 mg/g) – predicts increased risk of acute kidney injury.
There is a graded association between reduced eGFR at baseline and risk of AKI, independent of proteinuria.
Evidence of proteinuria before potential acute renal insult should prompt clinical identification of an individual at increased risk of AKI to receive aggressive preventive measures.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
•of special interest
••of outstanding interest
Additional references related to this topic can also be found in the Current World Literature section in this issue (p. 318).
- 1.Uchino S, Kellum J, Bellomo R, et al. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005;294:813–818. doi: 10.1001/jama.294.7.813. [DOI] [PubMed] [Google Scholar]
- 2.Hoste E, Clermont G, Kersten A, et al. RIFLE criteria for acute kidney injury are associated with hospital mortality in critically ill patients: a cohort analysis. Crit Care. 2006;10:R73. doi: 10.1186/cc4915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hsu CY, Ordoñez JD, Chertow GM, et al. The risk of acute renal failure in patients with chronic kidney disease. Kidney Int. 2008;74:101–107. doi: 10.1038/ki.2008.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4••.Huang TM, Wu VC, Young GH, et al. Preoperative proteinuria predicts adverse renal outcomes after coronary artery bypass grafting. J Am Soc Nephrol. 2010;22:156–163. doi: 10.1681/ASN.2010050553. [This study of 1235 adult patients undergoing CABG in Taiwan demonstrated that mild and heavy proteinuria were each associated with an increased odds ratio of postoperative AKI and AKI requiring dialysis, independent of CKD stage and presence of diabetes.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5••.Grams ME, Astor BC, Bash LD, et al. Albuminuria and estimated glomerular filtration rate independently associate with acute kidney injury. J Am Soc Nephrol. 2010;21:1757–1764. doi: 10.1681/ASN.2010010128. [This study of over 11 000 patients and 8 years of follow-up determined that elevated urine albumin-to-creatinine ratio was an independent risk factor for hospitalizations with AKI. This association was evident starting with the submicroalbuminuric range (UACR 11–29 mg/g) and persisted stepwise along the severity of albuminuria, after adjustment for eGFR and cardiovascular risk factors.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6••.James MT, Hemmelgarn BR, Wiebe N, et al. Glomerular filtration rate, proteinuria, and the incidence and consequences of acute kidney injury: a cohort study. Lancet. 2010;376:2096–2103. doi: 10.1016/S0140-6736(10)61271-8. [This large study consisting of over 900 000 adults in Alberta, Canada, demonstrated increased rates of hospital admissions with AKI and AKI requiring dialysis for patients with heavy proteinuria across all values of eGFR.] [DOI] [PubMed] [Google Scholar]
- 7.Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 8.Foundation NK. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(2 Suppl. 1):S1–S266. [PubMed] [Google Scholar]
- 9.Liu KD, Lo L, Hsu CY. Some methodological issues in studying the long-term renal sequelae of acute kidney injury. Curr Opin Nephrol Hypertens. 2009;18:241–245. doi: 10.1097/MNH.0b013e328329d0a3. [DOI] [PubMed] [Google Scholar]
- 10.Lo L, Liu K, Hsu C. Long-term outcomes after acute kidney injury: where we stand and how we can move forward. Am J Kidney Dis. 2009;53:928–931. doi: 10.1053/j.ajkd.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 11.Mehta RL, Kellum JA, Shah SV, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haase M, Bellomo R, Matalanis G, et al. A comparison of the RIFLE and Acute Kidney Injury Network classifications for cardiac surgery-associated acute kidney injury: a prospective cohort study. J Thorac Cardiovasc Surg. 2009;138:1370–1376. doi: 10.1016/j.jtcvs.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 13.Doi K, Yuen PS, Eisner C, et al. Reduced production of creatinine limits its use as marker of kidney injury in sepsis. J Am Soc Nephrol. 2009;20:1217–1221. doi: 10.1681/ASN.2008060617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cho K, Hsu CY. Quantifying severity of chronic kidney disease as a risk factor for acute kidney injury. J Am Soc Nephrol. 2010;21:1602–1604. doi: 10.1681/ASN.2010080816. [DOI] [PubMed] [Google Scholar]
- 15.Waikar S, Wald R, Chertow G, et al. Validity of International Classification of Diseases, ninth revision, clinical modification codes for acute renal failure. J Am Soc Nephrol. 2006;17:1688–1694. doi: 10.1681/ASN.2006010073. [DOI] [PubMed] [Google Scholar]
- 16.Bihorac A, Yavas S, Subbiah S, et al. Long-term risk of mortality and acute kidney injury during hospitalization after major surgery. Ann Surg. 2009;249:851–858. doi: 10.1097/SLA.0b013e3181a40a0b. [DOI] [PubMed] [Google Scholar]
- 17.Hobson CE, Yavas S, Segal MS, et al. Acute kidney injury is associated with increased long-term mortality after cardiothoracic surgery. Circulation. 2009;119:2444–2453. doi: 10.1161/CIRCULATIONAHA.108.800011. [DOI] [PubMed] [Google Scholar]
- 18.Lo L, Go A, Chertow G, et al. Dialysis-requiring acute renal failure increases the risk of progressive chronic kidney disease. Kidney Int. 2009;76:893–899. doi: 10.1038/ki.2009.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coca S, Yusuf B, Shlipak M, et al. Long-term risk of mortality and other adverse outcomes after acute kidney injury: a systematic review and meta-analysis. Am J Kidney Dis. 2009;53:961–973. doi: 10.1053/j.ajkd.2008.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hsu CY. Where is the epidemic in kidney disease? J Am Soc Nephrol. 2010;21:1607–1611. doi: 10.1681/ASN.2010050546. [DOI] [PubMed] [Google Scholar]
- 21.Mehta RH, Grab JD, O'Brien SM, et al. Bedside tool for predicting the risk of postoperative dialysis in patients undergoing cardiac surgery. Circulation. 2006;114:2208–2216. doi: 10.1161/CIRCULATIONAHA.106.635573. quiz. [DOI] [PubMed] [Google Scholar]
- 22.Thakar CV, Arrigain S, Worley S, et al. A clinical score to predict acute renal failure after cardiac surgery. J Am Soc Nephrol. 2005;16:162–168. doi: 10.1681/ASN.2004040331. [DOI] [PubMed] [Google Scholar]
- 23.Mehran R, Aymong ED, Nikolsky E, et al. A simple risk score for prediction of contrast-induced nephropathy after percutaneous coronary intervention: development and initial validation. J Am Coll Cardiol. 2004;44:1393–1399. doi: 10.1016/j.jacc.2004.06.068. [DOI] [PubMed] [Google Scholar]
- 24.Ninomiya T, Perkovic V, de Galan BE, et al. Albuminuria and kidney function independently predict cardiovascular and renal outcomes in diabetes. J Am Soc Nephrol. 2009;20:1813–1821. doi: 10.1681/ASN.2008121270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hemmelgarn BR, Manns BJ, Lloyd A, et al. Relation between kidney function, proteinuria, and adverse outcomes. JAMA. 2010;303:423–429. doi: 10.1001/jama.2010.39. [DOI] [PubMed] [Google Scholar]
- 26.Matsushita K, van der Velde M, Astor BC, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375:2073–2081. doi: 10.1016/S0140-6736(10)60674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]