Abstract
A heightened sensitivity to unpredictable aversiveness is a key component of several anxiety disorders. Neuroimaging studies of unpredictable aversiveness have shown that the anterior region of the insula cortex (AIC) plays a central role in the anticipation of unpredictable aversiveness. The present study extended these findings by examining the role of the AIC in temporal unpredictability (i.e., not knowing when the stimulus will occur), a particularly critical aspect of unpredictability as it increases contextual anxiety and vigilance given that the danger could happen ‘at any time.’ Nineteen healthy participants underwent functional magnetic resonance imaging while anticipating either temporally unpredictable or predictable aversive (or neutral) images. Participants exhibited greater right AIC while anticipating unpredictable relative to predictable aversive images. Additionally, activation in this region was positively correlated with self-reported individual differences in a key facet of intolerance of uncertainty (inhibitory behavior). Taken together, the present study suggests that the AIC plays an important role in the anticipation of temporally unpredictable aversiveness and may mediate key deficits in anxiety disorders.
Keywords: unpredictability, intolerance of uncertainty, insula, OFC, fMRI
Introduction
Fear and anxiety are emotions that are often used synonymously, however there is a growing literature distinguishing them. For example, one feature that has been proposed to differentiate fear and anxiety is whether the emotion is elicited by a predictable (fear) or unpredictable (anxiety) aversive stimulus [1]. Studies have shown that animals and humans exhibit different responses to predictable and unpredictable aversive stimuli [2] and numerous species have shown a preference for predictable aversiveness [3]. This preference is believed to be largely adaptive as having information about potential threat allows the organism to prepare for the danger and/or avoid it [1,4].
Some individuals, however, have a heightened sensitivity to unpredictable danger. For example, certain anxiety disorders (e.g., panic disorder) have been characterized by a reduced tolerance to unpredictable danger [5]. This has led some to argue that a heightened sensitivity to unpredictable danger may be a core deficit in several anxiety disorders [6] and may differentiate these disorders from depression [7].
Neuroimaging studies have begun to identify neural circuits that underlie emotional responding to unpredictable threat. Specifically, animal and human studies have indicated that the bed nucleus of the stria terminalis (BNST), amygdala, and other limbic and paralimbic structures are involved in responses to unpredictable threat [4]. One structure that appears to be particularly involved in responding to unpredictable threat is the anterior region of the insula cortex (AIC), a structure in the extended limbic system with afferent and efferent connections throughout limbic and cortical regions [8]. Most notably, the AIC appears to play a critical role in the anticipation of unpredictable aversiveness [9,10]. This is consistent with studies showing deficits in risk assessment in patients with AIC lesions [11], as well as theoretical models on the role of the insula in predicting affective states [8].
There are several ways to manipulate the unpredictability of aversiveness. For example, one could manipulate the unpredictability of the stimulus duration, intensity or type of stimulus (e.g., making uncertain whether a pending stimulus is aversive or neutral), all of which may have different neural substrates. Temporal unpredictability (i.e., not knowing when the stimulus will occur) is a particularly important aspect of unpredictability as it increases contextual anxiety and vigilance given that the danger could happen ‘at any time’. To our knowledge, only two neuroimaging studies have attempted to isolate the neural correlates of temporally unpredictable aversiveness. However, particular methodological aspects of these studies prohibit broader implications regarding the role of the AIC in responses to this type of aversiveness. Simmons et al. [12] employed combat exposed veterans with and without post-traumatic stress disorder, prohibiting conclusions about the role of the AIC in healthy populations. Somerville et al. [13] used healthy subjects but their design confounded anticipation of temporally unpredictable aversiveness and the presentation of the aversive stimuli. Specifically, their analysis combined the period when participants anticipated aversive images with the period in which they viewed the images. Isolating the neural correlates of aversive anticipation is particularly critical as heightened anticipation of future danger has long been viewed as a key aspect of anxiety [1]. Thus, the primary aim of the present study was to examine the role of the AIC during the anticipation of temporally unpredictable aversiveness using functional magnetic resonance imaging (fMRI) in a sample of healthy controls.
A secondary aim was to examine whether individual differences in intolerance of uncertainty (IU) were associated with AIC response during the task. High IU individuals believe that uncertainty is unacceptable and leads to stress and the inability to take action. Thus, finding an association between IU and AIC activity would provide validation for the role of AIC in responsivity to unpredictable aversiveness. Additionally, as high IU individuals are at elevated risk for anxiety disorders [14], identification of neural markers of their response to unpredictability may aid in anxiety prevention treatments. Interestingly, several studies have shown that IU is not a unitary construct, but consists of two separable factors – inhibitory IU (freezing, or hindering behavior in response to uncertainty) and prospective IU (concerns/anxiety about future events [15]). Broadly, inhibitory IU captures behavioral symptoms whereas prospective IU captures cognitive perceptions. To date, no neuroimaging study has examined inhibitory IU and prospective IU separately. Therefore, the present study did not make specific hypotheses regarding which component is related to related to AIC’s role in unpredictable aversiveness responding.
Methods
Participants
The present study used 19 right-handed adults (68.4% female; 57.9% Caucasian; age: M = 30.14 years, SD = 12.76) from a larger study on emotional deficits in depression and anxiety [16]. Participants for the larger study were recruited from the community and were interviewed using the Structured Clinical Interview for DSM-IV (SCID; [17]). Participants were excluded if they had a lifetime Axis I diagnosis, were unable to read or write English, had a history of head trauma with loss of consciousness, or were left-handed. Interrater reliabilities of SCID diagnoses were conducted on a subset of participants and indicated perfect diagnostic agreement (all Kappas = 1.00). All methods were approved by the local Institutional Review Board.
Procedure
One week prior to the fMRI scan, participants were acclimated to the protocol by completing a mock scan and practice version of the experimental task. All scan sessions were between 7am and 12pm and participants were instructed to limit caffeine and tobacco intake for at least two hours prior to their scan. The task was a variant of that used by [18] and consisted of viewing a series of count-ups (CU; e.g., 1, 2, 3, etc.) that ended with the presentation of a negative or neutral image selected from the International Affective Picture System (IAPS; [19].1 The task design included two within-subjects factors – timing (Predictable [P] vs. Unpredictable [U]) and valence (Negative [Neg] vs. Neutral [Neut]). For each trial, text initially appeared at the bottom of the screen for 2-s indicating the timing and valence condition (i.e., P-Neut, P-Neg, U-Neut, or U-Neg). Next, the CU was presented above the text and ranged from 4 to 11-s. At the end of the CU the IAPS image appeared for 1.5-s. In the P condition, participants were explicitly told when count-up would end and when the image would appear (e.g., “Neutral image at 5”). In the U condition, participants did not know when the image would appear (e.g., “Unpleasant image can appear at anytime”). Importantly, across both P and U conditions, participants always knew the valence of the image that would appear, but only knew the timing of when the image would appear for the P (and not U) condition (thus, the timing was the only component that was unpredictable in U). The duration of the CU for both the P and U conditions ranged from 4 to 11-s and the mean CU duration was equivalent across conditions.
For each condition (i.e., P-Neut, P-Neg, U-Neut, U-Neg), trials were presented during 42-s blocks during which the CU was presented four times. In-between blocks, a fixation cross was presented for 10-s to allow the fMRI blood oxygenated level-dependent (BOLD) signal to return to baseline. Each condition block was presented twice and participants completed two separate versions of the task (each condition block viewed four times total) in counterbalanced orders.
After completing the task, participants were shown each IAPS image again in a random order and were asked to rate the image valence, on a scale ranging from 1 (Very unpleasant) to 9 (Very pleasant), and arousal, on a scale ranging from 1 (Not at all arousing) to 9 (Very arousing).
Prior to the fMRI scan, participants completed the Intolerance of Uncertainty Scale (IUS; [14], a 27-item questionnaire assessing the trait-like belief that uncertainty is unacceptable, reflects poorly on them, and leads to stress, and the inability to take action. Respondents rate each item on a scale ranging from 1 = ‘not at all characteristic of me’ to 5 = ‘entirely characteristic of me’, with higher scores representing a greater intolerance of uncertainty. Carleton et al. [15] conducted a factor analyses on the 27-item IUS and found a more parsimonious 12-item version that had better psychometric properties. In addition, a confirmatory factor analysis of the 12-item version revealed two sub-factors: a 5-item Inhibitory IU factor assessing the degree to which uncertainty inhibits action or experience and a 7-item Prospective IU factor assessing fear and anxiety of future events [15;18]. Thus, the present study utilized the 12-item total score (Total IU) and the two subscales (Inhibitory IU and Prospective IU).
fMRI Data Acquisition
Functional MRI was performed on a 3T GE magnetic resonance scanner at the University of Illinois Medical Center. Functional images were acquired using a gradient-echo echo-planar images (2s TR, 25ms TE, 82° flip, 64×64 matix, 200 mm FOV, 3mm slice thickness, 0mm gap, with 40 axial slices). A high-resolution, T1-weighted anatomical scan was also acquired in the same axial orientation (25° flip; 512×512 matrix, 220mm FOV; 1.5mm slice thickness; 120 axial slices).
fMRI Data Processing and Analyses
Data from all 19 participants met criteria for high quality and scan stability with minimum motion correction (i.e., 3 mm or less displacement in any one direction) and thus were included in subsequent analyses. Functional data were analyzed using Statistical Parametric Mapping software (SPM8; London, UK). Images were spatially realigned to correct for head motion, warped to standardized Montreal Neurological Institute (MNI) space using the participant’s T1 image, resampled to 2 mm3 voxels, and smoothed with an 8 mm3 kernel to minimize noise and residual differences in gyral anatomy. The general linear model was applied to the time series, convolved with the canonical hemodynamic response function and with a 128-s high-pass filter. Condition effects were modeled with box-car regressors representing the occurrence of each block type. Effects were estimated at each voxel and for each subject. Individual contrast maps (statistical parametric maps) for U-Neg > P-Neg, P-Neg > U-Neg, U-Neut > P-Neut, and P-Neut > U-Neut were generated for each participant. Next, we conducted two separate second-level, one-sample t-tests with our individual contrast maps (e.g., U-Neg > P-Neg, U-Neut > P-Neut). Because of our strong a priori hypothesis about the role of the AIC, we used the fMRI meta-analytic resource Neurosynth (http://neurosynth.org) to create a bilateral AIC mask that includes voxels likely to be activated during uncertainty. We applied a small-volume correction to both models using the mask. As an exploratory aim, we also conducted whole-brain second-level t-tests and considered activations that survived p < .005 (uncorrected), with a cluster extent threshold of greater than 20 contiguous voxels (volume > 160mm3), as significant to balance between Type I and Type II errors [20]. In-order to clarify the direction of condition effects, we extracted BOLD signal responses (i.e., parameter estimates, β weights [arbitrary units]) from 5 mm (radius) spheres surrounding significant peak activations.
Results
Behavioral Results
Negative images were rated as more unpleasant (valence: M=3.55, SD=1.22) and arousing (arousal: M=4.42, SD=1.77) relative to neutral images (valence: M=5.41, SD=0.90) (arousal: M=2.83, SD=1.72), F(1, 18) = 27.14, p < .001; F(1, 18) = 6.82, p < .05, respectively. Valence ratings did not differ between the P-Neg and U-Neg images, F(1, 18) = 1.01, ns, and P-Neut and U-Neut images, F(1, 18) = 0.61, ns. Similarly, arousal ratings did not differ between P-Neut and U-Neut images, F(1, 18) < 0.01, ns; however, participants rated the U-Neg images (M=4.56, SD=1.82) as slightly more arousing relative to the P-Neg images (M=4.27, SD=1.76), F(1, 18) = 5.45, p < .05.
Imaging Results
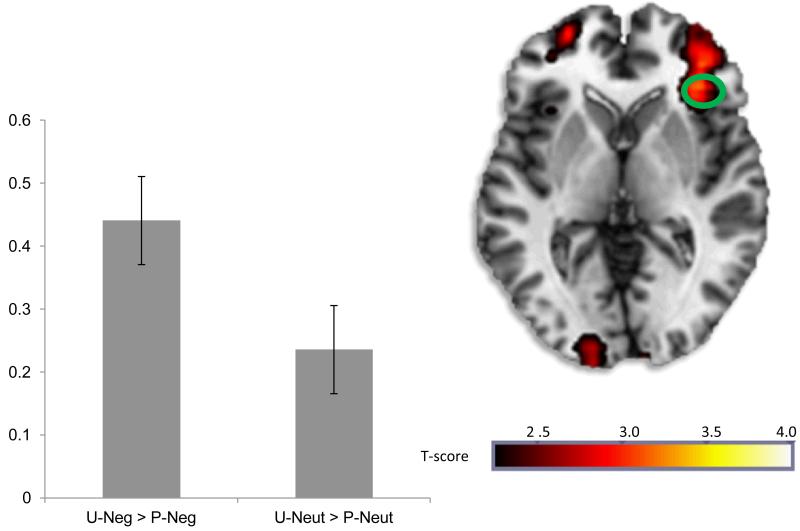
During anticipation of negative images, there was greater right AIC activation (MNI peak: [34, 28, −2], Z = 3.02, p < .001) for U-Neg images relative to P-Neg images (see Figure 1). In contrast, there were no differences in AIC activation during U-Neut compared to P-Nuet, suggesting that the effects of unpredictability were specific to negative images. All significant whole-brain results are presented in Table 1 for completeness.
Figure 1. AIC activation to unpredictable vs. predictable negative images.
Table 1. Significant Condition Effects from Whole-Brain Analyses.
| Contrast | Region Name | L/R | MNI Coordinates | Voxels | Z-score | p-value | ||
|---|---|---|---|---|---|---|---|---|
| X | Y | Z | ||||||
| UNeg > PNeg | Caudal Superior Temporal Sulcus |
L | −60 | −54 | 36 | 466 | 3.87 | .000 |
| Inferior Precentral Sulcus | R | 44 | 8 | 42 | 300 | 3.75 | .000 | |
| Caudal Superior Temporal Sulcus – First Segment |
R | 56 | −48 | 38 | 361 | 3.53 | .000 | |
| Lateral Orbital Gyrus/ Anterior Insula |
R | 40 | 46 | −4 | 395 | 3.37 | .000 | |
| Posterior Middle Frontal Sulcus- Anterior Segment |
L | −42 | 40 | 28 | 27 | 3.06 | .001 | |
| Posterior Calcarine Sulcus | L | −12 | −100 | −6 | 273 | 2.94 | .002 | |
| Intermediate Frontomarginal Sulcus |
L | −26 | 56 | 2 | 27 | 2.91 | .002 | |
| PNeg > UNeg | Central Sulcus | L | −58 | −8 | 44 | 23 | 3.48 | .000 |
| Uncal Sulcus | L | −18 | −8 | −26 | 64 | 3.22 | .001 | |
| Amygdala | R | 24 | 6 | −18 | 27 | 2.86 | .002 | |
| UNeut > PNeut | Sulcus of Brissaud | L | −32 | −66 | 60 | 32 | 3.57 | .000 |
| PNeut > UNeut | Posterior Middle Frontal Sulcus – Intermediate Segment |
L | −44 | 34 | 36 | 49 | 3.43 | .000 |
| Caudal Superior Temporal Sulcus – First Segment |
R | 62 | −54 | 30 | 29 | 3.02 | .001 | |
| Intraparietal Sulcus – Paroccipital Segment |
R | 16 | −68 | 40 | 37 | 3.00 | .001 | |
Note. Region locations were determined using a human MRI atlas [25].
To examine whether AIC activation was correlated with IUS scores, we extracted BOLD parameter estimates from a 5mm radius sphere surrounding the peak activation. Results indicated that greater right AIC (r = .58, p = .02) activation was associated with greater scores on the Inhibitory IU subscale. This effect was specific to Inhibitory IU as AIC activation was not associated with Total IU or Prospective IU subscale (both p’s > .34).
Discussion
The present study examined the neural correlates of temporally unpredictable aversiveness in a sample of healthy controls. Results indicated that the right AIC was more activated during anticipation of temporally unpredictable aversive images relative to predictable aversive images. Further validating these results, activation in the AIC was positively correlated with individual differences in self-reported inhibitory IU.
Converging lines of evidence indicate that the insula plays an essential role in affective processing. The insula has been shown to be particularly involved in interoception, such that the insula integrates emotionally salient environmental information with representations of bodily states to form a subjective evaluation of a given moment in time [21]. These processes specifically occur in the anterior portion of the insula and are integral for awareness of both current and future affective states. Thus, in response to temporally unpredictable threat (and perhaps other types of unpredictability [4]), the AIC may engage in an ‘anxious risk assessment’ where information about environmental ambiguity is combined with perceptions of bodily states to generate immediate and future-oriented affective responses. Although clearly evolutionarily adaptive, studies indicate that in individuals with anxiety disorders, the AIC is chronically hyperactive leading to repeated false predictions of future bodily states [22]. As such, AIC reactivity may be an important target for anxiety disorder treatments – particularly for anxiety disorders characterized by heightened responsiveness to unpredictable threat (e.g., panic disorder; [6]).
It is also important to note that the AIC uses information about internal bodily states, more broadly, to perceive the passage of time [21]. Time perception is critical in order to anticipate aversive events/affective states, and may be particularly relevant to the present study which manipulated the temporal unpredictability of aversive stimuli. In other words, although studies suggest that the AIC may be involved in processing unpredictable aversiveness across a variety of contexts, it is possible that the AIC may play an especially key role in temporal unpredictability given the salience of time.
Further validating the role of AIC in response to unpredictable threat, the present study found that AIC activation was positively correlated with individual differences in inhibitory IU. This is consistent with one study that found a relation between insula activation and IU while viewing affectively ambiguous stimuli [10] and another that found an association between insula activation and IU during a similar task to ours [13]. The present study extends this work by noting that the association may be specific to inhibitory IU, as there was no significant correlation between AIC activation and prospective IU. This suggests that the behavioral symptoms of IU (i.e., apprehension, inhibition) may be mediated by the AIC, as it signals to other areas of the brain to allocate attentional resources and execute behavioral responses.
In addition to the AIC, our exploratory whole brain results suggest that the orbital frontal cortex (OFC) is also associated with processing temporally unpredictable threat, a finding consistent with several prior studies [23]. Animal and human evidence indicates that the OFC is involved in determining the affective salience of threat stimuli and guiding goal-directed behavior [24]. Similarly, the OFC is involved in behavioral inhibition and emotion regulation processes via the modulation of limbic structures associated with affective reactivity to unpredictability (e.g., BNST). As activation in the AIC appeared to extend to portions of the OFC, the OFC and AIC may play different, yet complimentary roles in responsiveness to unpredictable aversiveness.
Briefly, it is worth noting that the current study found greater right amygdala activation during anticipation of predictable negative images relative to unpredictable negative images. The amygdala has long been identified as a key region for threat perception and fear learning in animals and humans [1]. Several studies have found increased amygdala activation during the anticipation of unpredictable threat [9]; however, no prior study has directly compared anticipation of temporally predictable versus unpredictable threat. As previously mentioned, several theorists have made a distinction between fear (associated with predictable threat) and anxiety (associated with unpredictable threat), and research has indicated that the amygdala is implicated in both emotional states. However, fear has been shown to be more related to the central nucleus of the amygdala and anxiety has been more related to the BNST, which could account for the present pattern of results [1].
Despite several strengths, including a study design which isolated temporal unpredictability, the present study also had several limitations. First, the sample size was relatively small (N=19), which likely reduced statistical power and limited our ability to conduct sub-analyses (e.g., sex differences). Second, as the sample had no lifetime history of any psychiatric disorders, there was a potentially restricted range of IUS scores and our findings may not generalize to a sample with a broader range of IU. Third, unpredictable negative images were rated as more arousing compared with predictable negative images. As such, although there were no differences in valence, it is possible that differences in arousal influenced the present results.
In sum, the present study indicated that the right AIC was associated with anticipation of temporally unpredictable aversive stimuli and therefore may be a key region underlying affective responses to unpredictable danger. Given that numerous anxiety disorders are characterized by heightened responsiveness to unpredictable danger, particularly temporal unpredictability, the AIC may play an important role in the onset and/or maintenance of these debilitating disorders.
Acknowledgments
Source of Funding
This study was supported by a grant from Brain and Behavior Research Foundation and an R21 from the National Institute of Mental Health (PI: Shankman).
Footnotes
The following images from the IAPS library were used in the present study: neutral (2038, 2102, 2104, 2210, 2499, 2635, 2840, 2880, 5395, 5520, 7002, 7009, 7010, 7020, 7034, 7040, 7055, 7100, 7130, 7150, 7160, 7161, 7179, 7183, 7190, 7192, 7237, 7248, 7249, 7491, 7504, 7546) and negative (2590, 2700, 2716, 2750, 3060, 3071, 3080, 3160, 3250, 3261, 3280, 3350, 6260, 6530, 6570, 7380, 9090, 9180, 9182, 9270, 9280, 9290, 9330, 9340, 9428, 9520, 9560, 9584, 9630, 9810, 9830, 9920).
Conflicts of Interest
The authors have no conflicts of interest to report.
References
- 1.Davis M, Walker DL, Miles L, Grillon C. Phasic vs sustained fear in rats and humans: role of the extended amygdala in fear vs anxiety. Neuropsychopharmacol. 2010;35:105–135. doi: 10.1038/npp.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grillon C, Baas JP, Lissek S, Smith K, Milstein J. Anxious responses to predictable and unpredictable aversive events. Behav Neurosci. 2004;118:916–924. doi: 10.1037/0735-7044.118.5.916. [DOI] [PubMed] [Google Scholar]
- 3.Abbott BB, Schoen LS, Badia P. Predictable and unpredictable shock: behavioral measures of aversion and physiological measures of stress. Psychol Bull. 1984;96:45–71. [PubMed] [Google Scholar]
- 4.Grupe DW, Nitschke JB. Uncertainty and anticipation in anxiety: an integrated neurobiological and psychological perspective. Nat Rev Neurosci. 2013;14:488–501. doi: 10.1038/nrn3524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holaway RM, Heimberg RG, Coles ME. A comparison of intolerance of uncertainty in analogue obsessive-compulsive disorder and generalized anxiety disorder. J Anxiety Disord. 2006;20:158–174. doi: 10.1016/j.janxdis.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 6.Grillon C, Lissek S, Rabin S, McDowell D, Dvir S, Pine DS. Increased anxiety during anticipation of unpredictable but not predictable aversive stimuli as a psychophysiologic marker of panic disorder. Am J Psychiatry. 2008;165:898–904. doi: 10.1176/appi.ajp.2007.07101581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nelson BD, McGowan SK, Sarapas C, Robison-Andrew EJ, Altman SE, Campbell ML, et al. Biomarkers for threat and reward sensitivity demonstrate unique associations with risk for psychopathology. J Abnorm Psychol. 2013;122:662–671. doi: 10.1037/a0033982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paulus MP, Stein MB. An insular view of anxiety. Biol Psychiatry. 2006;60:383–387. doi: 10.1016/j.biopsych.2006.03.042. [DOI] [PubMed] [Google Scholar]
- 9.Sarinopoulos I, Grupe DW, Mackiewicz KL, Herrington JD, Lor M, Steege EE, et al. Uncertainty during anticipation modulates neural responses to aversion in human insula and amygdala. Cereb Cortex. 2010;20:929–940. doi: 10.1093/cercor/bhp155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simmons A, Matthews SC, Paulus MP, Stein MB. Intolerance of uncertainty correlates with insula activation during affective ambiguity. Neurosci Lett. 2008;430:92–97. doi: 10.1016/j.neulet.2007.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clark L, Bechara A, Damasio H, Aitken MRF, Sahakian BJ, Robbins TW. Differential effects of insular and ventromedial prefrontal cortex lesions on risky decision-making. Brain. 2008;131:1311–1322. doi: 10.1093/brain/awn066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simmons AN, Flagan TM, Wittmann M, Strigo IA, Matthews SC, Donovan H, et al. The effects of temporal unpredictability in anticipation of negative events in combat veterans with PTSD. J Affect Disorders. 2013;146:426–432. doi: 10.1016/j.jad.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 13.Somerville LH, Wagner DD, Wig GS, Moran JM, Whalen PJ, Kelley WM. Interactions between transient and sustained neural signals support the generation and regulation of anxious emotion. Cereb Cortex. 2013;23:49–60. doi: 10.1093/cercor/bhr373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Freeston MH, Rhéaume J, Letarte H, Dugas MJ. Why do people worry? Pers Individ Dif. 1994;17:791–802. [Google Scholar]
- 15.Carleton RN, Norton MAPJ, Asmundson GJG. Fearing the unknown: a short version of the intolerance of uncertainty scale. J Anxiety Disord. 2007;21:105–117. doi: 10.1016/j.janxdis.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 16.Shankman SA, Nelson BD, Sarapas C, Robison-Andrew EJ, Campbell ML, Altman SE, et al. A psychophysiological investigation of threat and reward sensitivity in individuals with panic disorder and/or major depressive disorder. J Abnorm Psychol. 2013;122:322–338. doi: 10.1037/a0030747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV-TR axis I disorders, clinician version (SCID-CV) American Psychiatric Press; Washington D.C.: 2002. [Google Scholar]
- 18.Nelson BD, Shankman SA. Does intolerance of uncertainty predict anticipatory startle responses to uncertain threat? Int J Psychophysiol. 2011;81:107–115. doi: 10.1016/j.ijpsycho.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lang PJ, Bradley MM, Cuthbert BN. Technical Report A-8. University of Florida; Gainesville, FL: 2008. International affective picture system (IAPS): affective ratings of pictures and instruction manual. [Google Scholar]
- 20.Lieberman MD, Cunningham WA. Type I and Type II error concerns in fMRI research: Re-balancing the scale. Soc Cogn Affect Neurosci. 2009;4:423–428. doi: 10.1093/scan/nsp052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Craig AD. How do you feel—now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- 22.Lorberbaum JP, Kose S, Johnson MR, Arana GW, Sullivan LK, Hamner MB, et al. Neural correlates of speech anticipatory anxiety in generalized social phobia. Neuroreport. 2004;15:2701–2705. [PubMed] [Google Scholar]
- 23.Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- 24.Rolls ET. The functions of the orbitofrontal cortex. Brain Cognition. 2004;55:11–29. doi: 10.1016/S0278-2626(03)00277-X. [DOI] [PubMed] [Google Scholar]
- 25.Petrides M. An MRI atlas of the sulci and gyri in MNI stereotaxic space. Academic Press; New York: 2012. The human cerebral cortex. [Google Scholar]