Abstract
Objectives
To determine if ablation that targets patient-specific AF-sustaining substrates (rotors or focal sources) is more durable than trigger ablation alone at preventing late AF recurrences.
Background
Late recurrence substantially limits the efficacy of pulmonary vein (PV) isolation for AF, and is associated with PV reconnection and the emergence of new triggers.
Methods
We performed 3 year follow-up of the CONFIRM trial, in which 92 consecutive AF patients (70.7% persistent) underwent novel computational mapping to reveal a median of 2 (IQR 1–2) rotors or focal sources in 97.7% of patients during AF. Ablation comprised source (Focal Impulse and Rotor Modulation, FIRM) then conventional ablation in n=27 (FIRM-guided), and conventional ablation alone in n=65 (FIRM-blinded). Patients were followed with implanted ECG monitors when possible (85.2% FIRM guided, 23.1% FIRM-blinded).
Results
On 890 days follow-up (median; IQR 224–1563) compared FIRM-blinded therapy, patients receiving FIRM-guided ablation maintained higher freedom from AF after 1.2±0.4 procedures (median 1, IQR 1–1) (77.8% vs 38.5%; p=0.001) and a single procedure (p>0.001), and higher freedom from all atrial arrhythmias (p=0.003). Freedom from AF was higher when ablation directly or coincidentally passed through sources than when it missed sources (p>0.001).
CONCLUSIONS
FIRM-guided ablation is more durable than conventional trigger-based ablation at preventing 3 year AF recurrence. Future studies should investigate how ablation of patient-specific AF-sustaining rotors and focal sources alters the natural history of arrhythmia recurrence.
Keywords: Atrial Fibrillation, Ablation, Electrical Rotors, FIRM, clinical trial
Atrial fibrillation (AF) is the most common sustained arrhythmia in the world and a leading cause of hospitalization and death(1). Ablation promises to eliminate AF and has enjoyed increased attention(1) as trials question the efficacy of pharmacologic strategies to suppress AF(2) or limit ventricular rate(3). However, while ablation at AF triggers can be effective, its 1 year efficacy is ≈40–50% for a single procedure(1, 4) and 50–70% for multiple procedures(1, 5). Moreover, AF often recurs more than 1 year after conventional ablation(1, 4) (defined as ‘late’(1)).
We hypothesized that elimination of patient-specific AF sources (AF-sustaining substrates) would provide more durable AF elimination than conventional (trigger) ablation, in which unablated substrates can subsequently be engaged by reconnected pulmonary veins (PV)(6, 7) or non-PV triggers(8–10). The CONFIRM trial (CONventional ablation for AF with or without Focal Impulse and Rotor Modulation, FIRM) demonstrated that substrates for persistent and paroxysmal AF include electrical rotors (spiral waves) or focal sources in spatially reproducible locations for each patient which sustain AF. Targeted ablation at AF sources alone in bi-atrial locations greatly improved the 1–2 year success of conventional ablation in the CONFIRM trial(11), as shown by independent laboratories(12, 13).
We studied whether FIRM-guided ablation prevented late AF recurrence, and thus maintained its relative efficacy over conventional ablation in an extended follow-up of the CONFIRM trial.
Methods
Study Design and Enrollment
We studied the 92 unique subjects in CONFIRM undergoing index FIRM-mapping procedures, each referred for ablation of symptomatic AF for standard indications at three centers(1). Subjects were ≥21 years of age, with AF despite one or more class I or III anti-arrhythmic drugs. The only exclusion to enrollment was an inability/refusal to provide written informed consent. The population included paroxysmal AF (self-limiting episodes), persistent AF (requiring drugs or electrical shock to terminate, or continuous AF for > 7 days), and longstanding persistent AF (continuous AF for over 1 year)(1). Table 1 summarizes patient characteristics.
Table I.
Characteristics of Population
| Characteristic | FIRM-blind (Conventional) |
FIRM-guided | p | |
|---|---|---|---|---|
| Type of AF | 65 | 27 | 0.336 | |
| Paroxysmal AF | 32.3% (21) | 22.2% (6) | ||
| Persistent AF | 52.3% (34) | 63.0% (17) | ||
| Long standing persistent AF | 15.4% (10) | 14.8% (4) | ||
| Age (years) | 61.2±8.7 | 63.0±8.3 | 0.362 | |
| Gender (Male/Female) | N=63/2 | N=25/2 | 0.578 | |
| AF history, days (median, IQR) | 1090 (413–2712) | 1753 (1127- | 0.056 | |
| Left Atrial diameter (mm) | 44±7 | 50±8 | <0.001 | |
| Left Ventricular Ejection Fraction | 55±12 | 52±17 | 0.430 | |
| Prior Ablations | 20% (13) | 22.2% (6) | 0.811 | |
| Comorbid Conditions | ||||
| Hypertension | 45/64 | 25/27 | 0.049 | |
| Diabetes | 20/64 | 11/27 | 0.383 | |
| Body Mass Index, kg/m2 | 32.2±5.7 | 33.5±6.1 | 0.324 | |
| Obstructive sleep apnea | 28/63 | 21/27 | 0.007 | |
| Congestive Heart Failure | 26/65 | 11/27 | 0.947 | |
| Brain Natriuretic Peptide (BNP), | 199±179 | 169±131 | 0.518 | |
| GFR, ml/min (mean) | 77.1±19.9 | 70.0±17.7 | 0.156 | |
| Magnesium, mg/dl | 2 (IQR 1,9–2.2) | 2 (IQR 1.9–2.2) | 0.972 | |
Statistical comparisons were performed using the Chi-squared test (for incidences of AF type, hypertension, diabetes mellitus, obstructive sleep apnea), the t-test (for age, Left atrial diameter, LV ejection fraction, body mass index and laboratory data), and Fisher’s exact test (for gender).
We analyzed AF recorded at multipolar biatrial catheters using previously described computational methods that interpret electrograms in the context of repolarization and conduction dynamics, to map patient-specific sources(14). Consecutive patients were prospectively enrolled under specific Institutional Review Board approval in a 2-arm 1:2 case cohort design for FIRM-Guided ablation, after real-time FIRM-mapping had been developed, or the FIRM-Blinded group, in which sources were mapped offline. The FIRM-Guided group received targeted ablation of sources followed by conventional ablation, while the FIRM-Blinded group received conventional ablation alone.
Electrophysiology Study
Electrophysiology study was performed after discontinuing anti-arrhythmic medications for 5 half lives, or >60 days for amiodarone (median 203 days). Catheters were advanced transvenously to the right atrium, coronary sinus and trans-septally to the left atrium. A 64-pole basket catheter (Constellation, Boston Scientific, Natick, MA) was advanced through an 8.5Fr SL1 sheath (Daig Medical, Minnetonka, MN) to map AF in left atrium (n=92) and also right atrium (n=61). Digital electroanatomic atrial shells were created using NavX (St Jude, Minneapolis, MN) or Carto (Biosense-Webster, Diamond Bar, CA)(15). Intravenous heparin was infused to achieve an activated clotting time > 350 seconds. Unipolar and bipolar atrial electrograms from the basket catheter were filtered at 0.05 – 500 Hz and recorded at 1kHz sampling frequency for export from our electrophysiologic recording system (Bard, Lowell, MA).
AF was observed in 88 patients (including all FIRM-guided cases) including AF induced by rapid pacing (n=26) or isoproterenol (n=2) when required. Induced and spontaneous AF have recently been shown to have similar dominant frequency(16) and spatial patterns(14) in a given patient. The remaining 4 cases without induced AF underwent conventional ablation in sinus rhythm in routine fashion.
FIRM Mapping of AF Sources
FIRM mapping has been described(11, 14). Briefly, AF was recorded using wide field-of-view basket catheters then analyzed with a mapping system (RhythmView™, Topera, Palo Alto, California) that generates three dimensional maps of AF propagation projected onto grids.
AF propagation (FIRM) maps were analyzed intra-procedurally to guide ablation in FIRM-Guided patients, and post-procedurally in FIRM-blinded patients. Electrical rotors (figure 1) were defined as rotational activation around a core, while focal impulses showed centrifugal activation from an origin. Rotors and focal impulses were considered AF sources only if they lay in reproducible spatial regions, with source precession (‘wobble’) (17), on repeated analysis over >30 minutes (i.e. thousands of cycles.
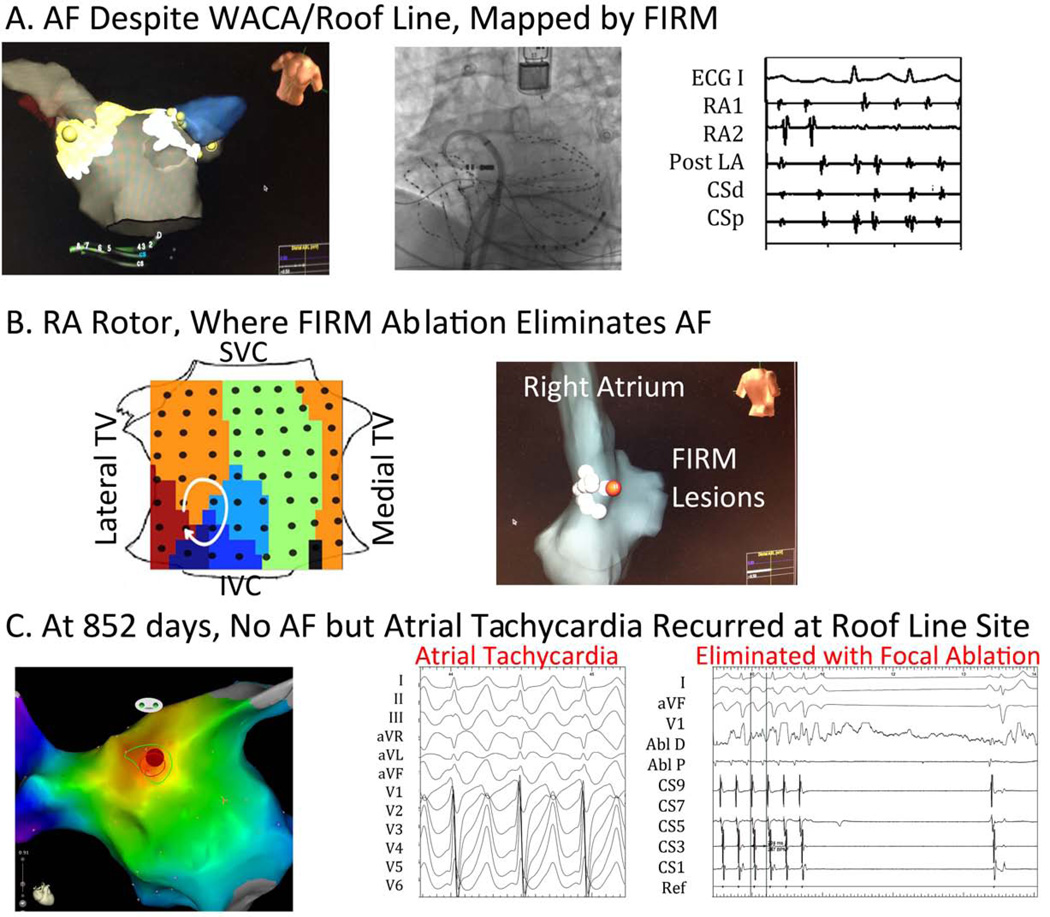
Figure 1. Patient Tailored Mapping. (A) Persistent Atrial Fibrillation Despite Extensive WACA and Roof line ablation.
FIRM mapping proceeded as shown fluoroscopically, using bi-atrial baskets, coronary sinus and ablation catheters, an implantable loop recorder and an esophageal temperature probe; (B) Detection of Right Atrial AF rotor, where FIRM eliminated AF with no other ablation; (C) At 852 days, Incessant Atrial Tachycardia Recurred, and was ablated at original roof line site. RA1,2=right atrial recordings; post LA=posterior left atrial recordings, CS =coronary sinus recordings; Abl=ablation catheter recordings.
Ablation Approach
Radiofrequency energy was delivered with a 3.5 mm tip irrigated catheter (Thermocool, Biosense-Webster, Diamond Bar, CA) at 25–35 W or, in heart failure subjects, an 8 mm tip catheter (Blazer, Boston Scientific, Natick, MA) at 40–50 W, target 52°C. Ablation commenced with FIRM in FIRM-guided subjects. Each lesion was applied for ≈30 seconds to cover the ≈2 cm2 area of phase mapped AF source precession(17). Remapping for rotor elimination was not possible in CONFIRM, since early software took hours to process, so that the endpoint was AF termination or 10 minutes’ energy delivery (typically <5 minutes), whichever came first. FIRM was repeated for ≤ 3 sources(11) guided by the single FIRM-map, followed by conventional ablation. In more recent studies, FIRM maps are generated fast enough to enable remapping for rotor elimination (13).
Conventional ablation(1), performed after FIRM in FIRM-guided patients and as sole therapy in FIRM-blinded patients, was standardized to comprise wide area circumferential ablation of left and right PV pairs with verification of PV isolation using a circular mapping catheter (Lasso, Biosense-Webster). In persistent AF patients, a left atrial roof line was also performed. Clinically relevant atrial tachycardias were ablated. If AF or atrial tachycardia persisted after the completion of ablation, cardioversion was performed.
Post-Procedure Clinical Management
Follow up for arrhythmia recurrence exceeded guidelines(1). In a 3 month post-ablation ‘blanking period’, anti-arrhythmic medications were continued (except amiodarone) but repeat ablation was not permitted. Subjects were evaluated for recurrence using continuous implanted ECG monitors if possible (85.2%, 23/27 FIRM guided; 23.1%, 15/65 FIRM-blinded), using Reveal XT™ (Medtronic, Minneapolis, MN) after its U.S. approval for AF monitoring (2009) or clinical pacemaker/defibrillators. Subjects without implanted devices had external event monitors quarterly and at the time of symptoms for 2 years. After this time, we followed such patients at least annually and also reviewed ambulatory data from our electronic medical system reflecting periodic ECGs or ambulatory ECGs obtained for any clinic visit or hospitalization. In patients with arrhythmia recurrence after the blanking period, repeat ablation was offered per guidelines(1) and, if performed, detailed mapping was repeated if consent was granted.
Study Endpoints
The primary endpoint was freedom from AF, defined as <1% burden on continuous implanted ECGs monitors (recording 100% of the year)(11), or AF <30 seconds on quarterly monitors(1) (recording <28 days or 7.7% of the year). Secondary efficacy measures included freedom from all atrial arrhythmias, and arrhythmia freedom after a single procedure. Followup was facilitated by the comprehensive electronic medical records system in this largely Veterans Affairs population, and all patients lost to followup in the original CONFIRM trial(11) were recaptured. Followup continued for >3 years on all patients, with none lost to followup, for a median of 890 days (IQR 224–1563) when censored at recurrence.
On-Treatment Analysis: Did Ablation Pass Through Sources?
Electroanatomic and FIRM maps were analyzed in each patient blinded to demographics or outcomes, as described(11). Lesions were considered to pass through an AF source if 5 mm lesion markers on NavX lay within ± half inter-electrode spacing of the electrode marking the rotor core or focal origin. We assigned ‘source ablation’ if ≥1 source was ablated, whether directly by FIRM-guided ablation or coincidentally by anatomic lesion sets. Assignments were performed independently by SMN, DEK, KS, JMM and disputes were resolved by consensus. We compared long-term efficacy for patients in ‘source ablation’ and ‘non-source ablation’ groups.
Statistical Analysis
Continuous variables are summarized with means and standard deviations and compared with independent samples t-tests if normally distributed as verified by the Kolmogorov-Smirnov test. Otherwise, they are summarized with medians and quartiles or ranges and compared with Mann-Whitney U tests. Nominal variables were compared with chi-square tests or Fisher exact tests if expected cell frequencies were less than 5. Long-term outcome was assessed and reported after a single procedure, and after multiple procedures of the same type (i.e. PVI to PVI, or FIRM-guided to FIRM-guided). Survival analyses were conducted using the Cox proportional hazards model. Survival curves are produced by the Kaplan Meier method and compared with log-rank tests. Cross-over repeat procedures (PVI at index procedure, crossing over to FIRM-guided) was censored from Kaplan-Meier analysis but described in the Results. The proportionality assumption was deemed satisfied upon inspection of log log plots. Covariates were added to this model if there were treatment group differences with p<0.10. In multivariable models AF history was transformed to the common log. A probability of < 0.05 was considered statistically significant.
Results
Table 1 summarizes characteristics of the study population.
Stable Localized Sources for Human AF
AF was observed intraprocedurally in 88/92 patients, in whom FIRM mapping revealed stable AF rotors or focal sources in 86 (97.7%) for 1.9 ± 1.1 concurrent stable sources per patient (median 2; IQR 1–2) of which 67% lay in left atrium (1.3±0.9 per patient; median 1, IQR 1–2) and 33% lay in right atrium (0.6±0.7 per patient; median 0, IQR 0–1).
Long term Efficacy
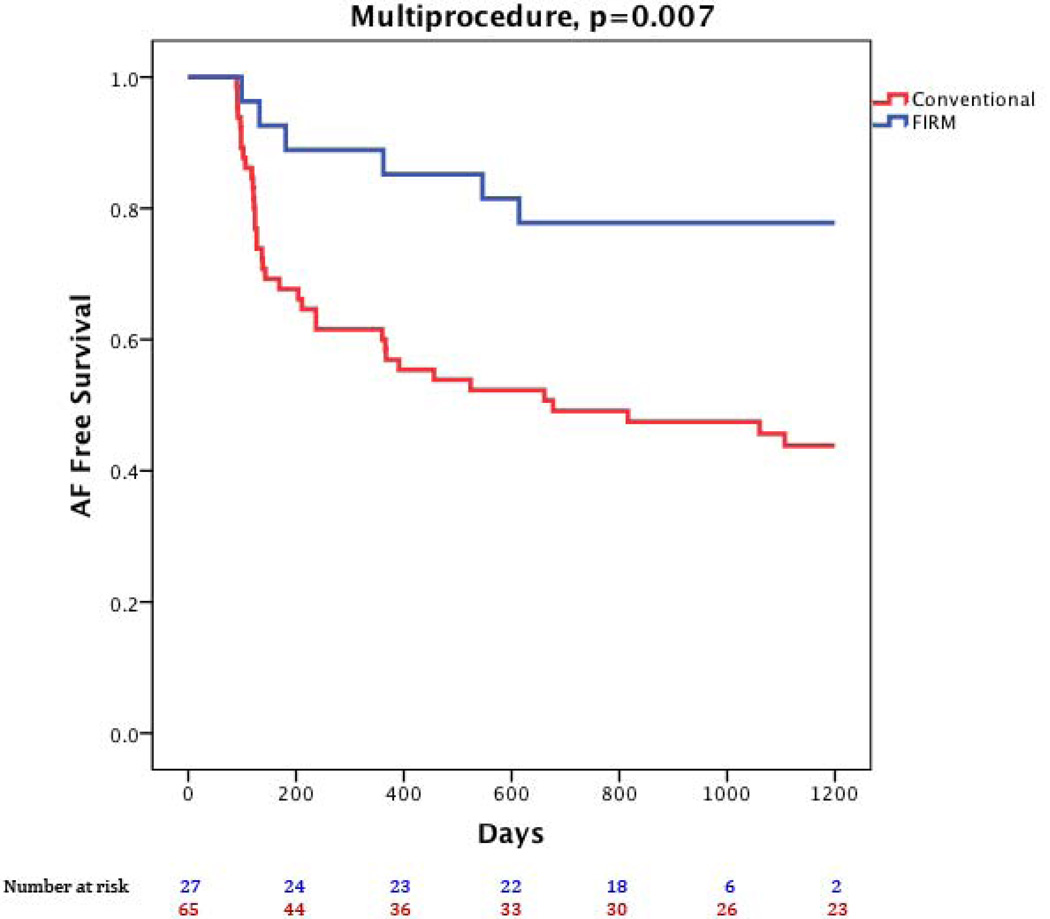
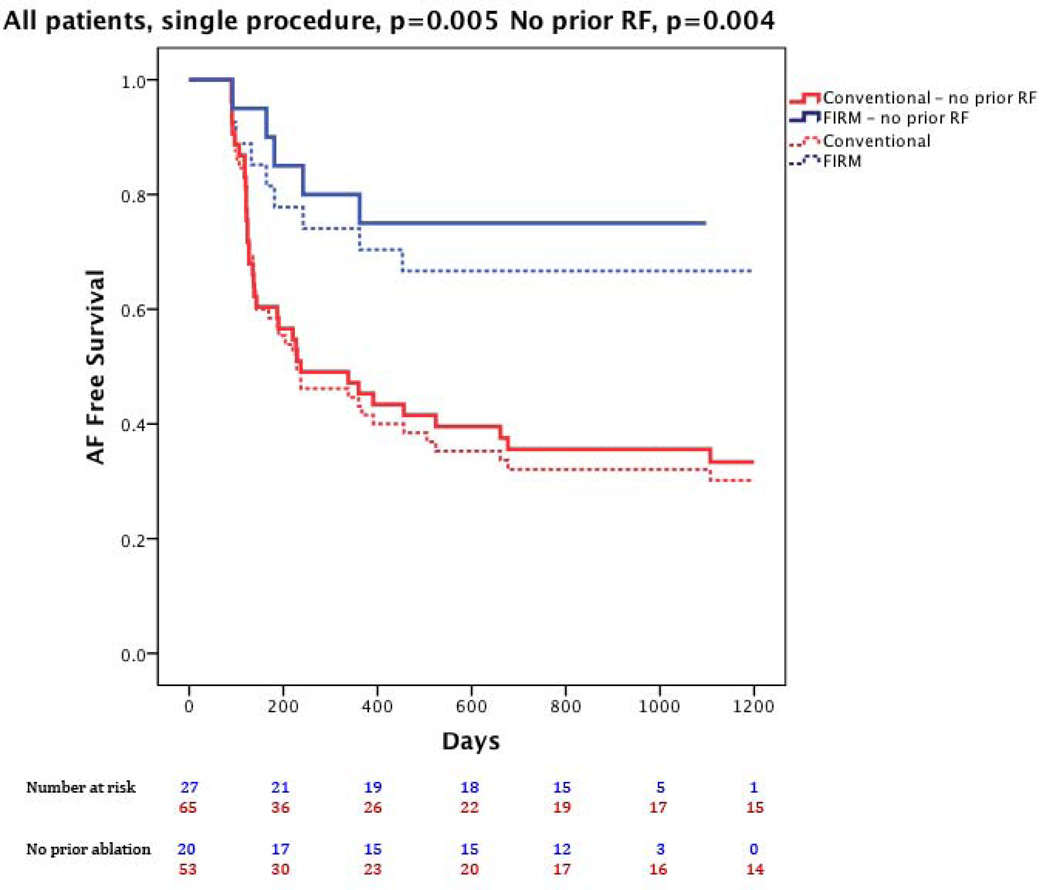
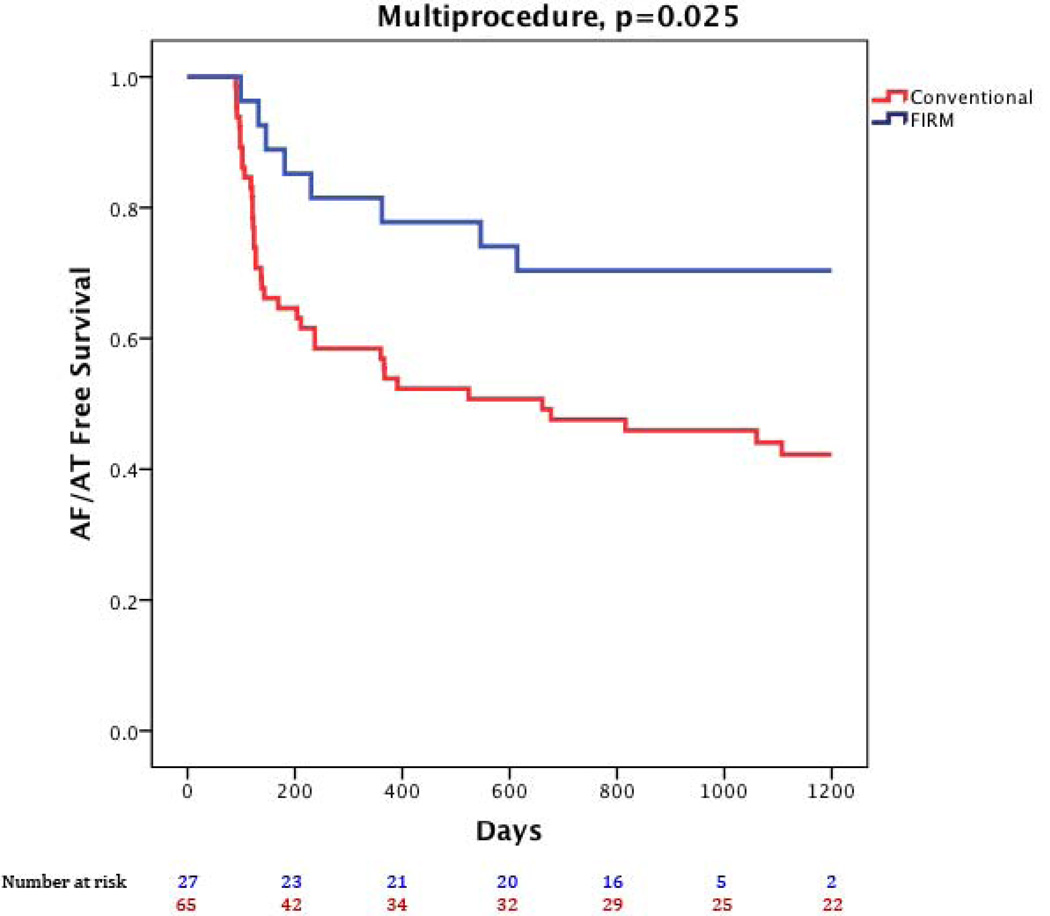
Figure 1 illustrates a patient with recurrent arrhythmia after FIRM-guided ablation. At 890 days (IQR 224–1563), freedom from AF was higher for FIRM-guided than conventional therapy (77.8%, 21/27 versus 38.5%, 25/65; p=0.001) after 1.2±0.4 procedures (median 1, IQR 1–1). After a single procedure alone, freedom from AF was also higher for FIRM-guided than conventional therapy for patients undergoing first ablation (75.0 %, 15/20 versus 30.2%, 16/53; p<0.001) and all patients (66.7%, 18/27 versus 27.7%, 18/65; p<0.001). Kaplan-Meier survival plots with p-values (logrank test) are illustrated in figures 2–4.
Figure 2. Freedom from the Primary End Point (atrial fibrillation).
Freedom from the Primary End Point (atrial fibrillation) for FIRM-guided ablation (blue) and conventional ablation (red; p=0.003).
Figure 4. Single-Procedure freedom from the Primary End Point (atrial fibrillation) for FIRM-guided ablation (blue) and conventional ablation (red).
Data shows all cases (solid lines, p=0.002) and those undergoing their first ablation (dashed lines, p=0.002).
At 890 days (IQR 224–1563), freedom from any atrial tachyarrhythmias was also higher in FIRM-guided than conventional cases after 1.2±0.4 procedures (70.4%, 19/27 vs 36.9%, 24/65; p=0.003) and after a single procedure including typical cavotricuspid flutter (55.6%, 15/27 vs 26.2%, 17/65, p=0.001) and excluding typical cavotricuspid flutter (63.0%, 17/27 vs 26.2%, 17/65, p=0.001). Seven subjects (7.6%) remained on anti-arrhythmic medications due to patient/physician preference (3 FIRM-guided, 4 conventional).
Repeat procedures were performed in 28 patients (8 FIRM-guided, 20 conventional). Of FIRM patients who recurred: 3 had atrial tachycardias (2 cavotricuspid, 1 Left atrial) and were successfully ablated, while 5 AF recurrences were treated by FIRM. Six patients (75.0%) are free of recurrence at 950 (IQR 658–973) days. Of conventional ablation patients, 12 had repeat PV isolation (before availability of real-time FIRM) of whom 6 are recurrence free (50.0%) at 1435 (IQR 836–1767) days (PVI, then PVI). Eight patients who initially underwent conventional ablation and recurred crossed-over to FIRM ablation (and were excluded from multiprocedure Kaplan-Meier analysis), of whom six (75.0%) remain AF-free at 1939 (IQR 1109–2120) days (PVI, then FIRM-guided).
On-Treatment Analysis
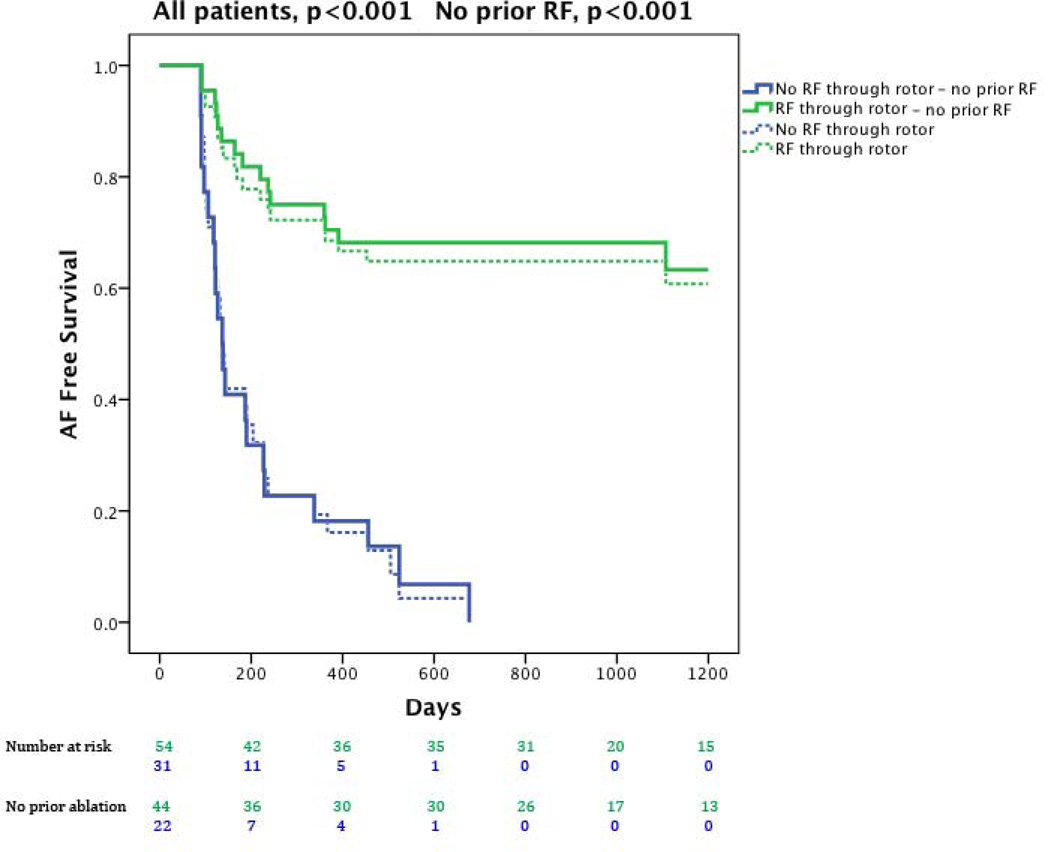
Examining only single-procedure results, ablation in which lesions passed through the sites of ≥1 rotors or focal sources (either directly, FIRM, or coincidentally by anatomically placed lesions) was more successful than ablation that missed all sources (figure 5; p-values reflect logrank test). Direct ablation through a source did not differ significantly from coincidental ablation of a source in this study (69.2% vs 57.1%, p=0.36), that may reflect patient numbers.
Figure 5. Cumulative freedom from the Primary End Point (atrial fibrillation) in patients based on whether the procedure directly or coincidentally ablated rotors or focal sources (red) or missed rotors/sources (blue).
Data shows all cases (solid lines, p<0.001) and those at first ablation (dashed lines, p<0.001).
Multivariate Predictors of Outcome
We studied the impact on freedom from AF of FIRM-guided versus conventional ablation and demographic factors that differed between treatment arms with P<0.10 (from table 1: the presence of hypertension and obstructive sleep apnea, and LA diameter and AF history as continuous variables). On multivariate analysis, single-procedure long-term success was associated with FIRM-guided ablation (p<0.001), and inversely with AF history (p=0.006), hypertension (p=0.014) and LA diameter (p=0.010). Multiple procedure long-term success was associated with FIRM-guided ablation (p=0.003) and trended inversely with LA diameter (p=0.052).
Discussion
Elimination of patient-specific electrical rotor and focal sources for AF significantly reduced late recurrence of atrial arrhythmias on rigorous >3 year follow-up compared to trigger ablation alone. On multivariate analysis, FIRM-guided ablation predicted procedural success independently of comorbidities that otherwise favor progression of AF substrates. These data strengthen the concept that ablation of defined patient-specific AF-sustaining mechanisms prevents recurrent AF even if pulmonary veins reconnect or alternative triggers become active, both of which are major causes of recurrent AF after conventional ablation.
Ablating Stable Rotors and Focal Sources Substantially Improves Arrhythmia Freedom
These data strengthen the concept that stable localized sources represent important AF-sustaining substrates for human AF after it is triggered, from the CONFIRM trial(11) and independent groups(12, 13, 18).
These data also suggest that the success of FIRM-guided ablation is attributable more to FIRM than to PVI. Over a median follow-up of >3 years, the single procedure success of conventional ablation was <40% (figure 4), while FIRM-guided ablation produced 70–80% arrhythmia freedom. The single procedure success of conventionally treated patients in this study is in line with results from Weerasooriya et al. (1/3rd persistent AF)(4) and the <40–50% 1–2 year single-procedure success from multicenter trials of paroxysmal AF(5, 19, 20). The relatively small increment in success with multiple versus one procedure (figures 2,3 versus figure 4) in both limbs reflects fewer patients undergoing repeat procedure in this study (median 1, IQR 1–1; mean 1.2±0.4) than prior studies (for instance, median 2, mean ≈1.8 in reference(4)). Finally, ablation was more successful if sources were eliminated, as demonstrated by on-treatment analysis in this report (figure 5) and prospectively by the elimination of paroxysmal AF by FIRM-only ablation in the PRECISE trial(21). Although direct FIRM ablation appeared slightly better than coincidental rotor ablation in this long-term analysis, as may be expected, this was not statistically significant possibly due to patient numbers.
Figure 3. Freedom from the Secondary End Point (all Atrial Arrhythmias).
Freedom from the Secondary End Point (all Atrial Arrhythmias) for FIRM-guided ablation (blue) and conventional ablation (red; p=0.01).
Stable AF sources explain numerous observations that are difficult to reconcile by disorganized mechanisms(22), including AF termination by focused intervention at sites identified a priori hours or days earlier(11, 12, 23, 24), consistent and repetitive activation vectors in AF(25, 26), stable sites of rapid AF activity(27–31) and reproducible organized reentry at AF onset(32). Other groups have identified human AF rotors using methods including wavelet similarity(18), the inverse solution(33), intraoperative mapping plaques(26) and Shannon entropy(34, 35). Future work is required to define how electrical(36), structural(37) or neural(38) remodeling contribute to the formation and localization of AF sources.
Rotor and Source Ablation Prevents Late AF Recurrence
Recurrence and late recurrence of AF, occurring within 3–12 months and >12 months post ablation respectively(1), have unclear mechanisms. One hypothesis is that PV reconnection often accounts for recurrences(1, 6, 7) although, conversely, patients with reconnected PVs often do not have recurrent AF (39, 40). A second mechanism is that non-PV triggers may dominate after PVI(8–10). A third mechanism is that progressive electrical(36), structural(37) or neural(38) remodeling may facilitate the progression of new substrates.
These data support the concept that substrate ablation by FIRM may prevent AF recurrence by rendering reconnected PV or non-PV triggers impotent. It has been shown that patients after FIRM-only ablation may initially show triggers that no longer initiate sustained AF and may regress(21), a phenomenon also seen after ablation for supraventricular tachycardias. These results open the possibility that elimination of patient-specific AF substrates may attenuate deleterious remodeling, even in patients with clinical comorbidities associated with substrate progression such as hypertension and sleep apnea.
Efficacy of FIRM-Guided Ablation Across Patient Populations
FIRM-guided ablation retained its efficacy advantage in all patient subgroups. FIRM was more successful if performed as a first rather than redo procedure (figures 4–5), which may reflect signal degradation from prior ablation, pro-arrhythmia from prior ablation, or a biological resistance to therapy in such patients. Nonetheless, despite these issues, patients with previous ablation still achieved excellent long term outcomes with FIRM-guided therapy. FIRM also retained efficacy in patients with comorbidities that typically confer worse outcome from PV ablation, such as obesity and sleep apnea that associate with a higher number of AF rotors that may lie in locations readily targeted by FIRM but missed by PV ablation(41).
Limitations
The major limitation of CONFIRM is that it was non-randomized, although subjects were enrolled consecutively and treated prospectively for pre-specified endpoints. Differences between groups may have reduced the relative benefit of FIRM therapy. Compared to conventionally treated patients, FIRM-guided patients in this analysis had more persistent AF (77.8% vs 67.7%), a higher prevalence of implanted loop recorders to detect recurrence (85.2% vs 23.1%) and more co-morbidities. While multivariate analysis showed a benefit for FIRM-guided therapy, we accept that such analyses cannot take into account all potential differences. The CONFIRM protocol was not ideal for assessing atrial tachycardia recurrence since, for example, the protocol did not standardize ablation of the cavotricuspid isthmus (most atrial tachycardia recurrences in FIRM-guided patients were typical flutter). This should be improved in future FIRM-guided studies. Some of these limitations are balanced by the unique strengths of CONFIRM: implanted devices in >80% of active limb patients and the ‘captive audience’ of the largely VA population in whom the integrated electrical medical record system (CPRS) enabled us to capture events even for non-cardiology office visits and hospitalizations. The multiprocedure success of conventional (and FIRM-guided) ablation in this trial would be higher if more patients underwent repeat ablation, but due to patient preference the number of repeat ablations was low (mean 1.2±0.4). Multiprocedure success would also be higher if including cross-overs to FIRM ablation for the repeat procedure (6/8 being arrhythmia free). Statistically, the sample size imposed limits on the ability to conduction multivariate adjusted analyses. We limited the number of covariates but replication in larger samples would be useful. Finally, while CONFIRM predominantly recruited men, studies from other groups have since validated its findings in women(12, 13).
Conclusions
In this extended follow-up of the CONFIRM trial, FIRM-guided ablation was more effective than conventional ablation alone at preventing early and late arrhythmia recurrences. By removing patient-tailored AF substrates, FIRM may prevent recurrent AF from reconnected PVs or non-PV triggers. Future studies should further define how ablation of patient-specific AF-sustaining substrates alters the natural history of long-term arrhythmia recurrence.
Acknowledgements
Dr. Narayan is co-author of intellectual property owned by the University of California Regents and licensed to Topera Inc. Topera does not sponsor any research, including that presented here. Dr. Narayan holds equity in Topera, and reports having received honoraria from Medtronic, St. Jude Medical, and Biotronik. Dr. Miller reports having received honoraria from Medtronic, St. Jude Medical, Biotronik, Biosense-Webster, Boston Scientific and is a scientific advisor to Topera. Drs. Shivkumar, Krummen, Baykaner, Lalani, Schricker and Mr.
This work was supported by grants to SMN from the Doris Duke Foundation and National Institutes of Health (K23 HL70529, R01 HL83359, K24 HL103800), and to Dr. Shivkumar (NIH R01 HL084261). We thank Antonio Moyeda, RCVT, Kenneth Hopper, RCVT, Judy Hildreth, RN, Sherie Janes, RN, Stephanie Yoakum, RNP, Elizabeth Greer, RN, Donna Cooper, RN and Kathleen Mills, BA for helping to perform the clinical study and collecting followup data.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: Clopton report no conflicts.
References
- 1.Calkins CH. 2012 HRS/EHRA/ECAS Expert Consensus Statement on Catheter and Surgical Ablation of Atrial Fibrillation: Recommendations for Patient Selection, Procedural Techniques, Patient Management and Follow-up, Definitions, Endpoints, and Research Trial Design. Heart Rhythm. 2012;9:632–696. [Google Scholar]
- 2.Roy D, Talajic M, Nattel S, et al. Rhythm control versus rate control for atrial fibrillation and heart failure. N Engl J Med. 2008;358:2667–2677. doi: 10.1056/NEJMoa0708789. [DOI] [PubMed] [Google Scholar]
- 3.Van Gelder IC, Groenveld HF, Crijns HJ, et al. Lenient versus strict rate control in patients with atrial fibrillation. N Engl J Med. 2010;362:1363–1373. doi: 10.1056/NEJMoa1001337. [DOI] [PubMed] [Google Scholar]
- 4.Weerasooriya R, Khairy P, Litalien J, et al. Catheter ablation for atrial fibrillation: Are results maintained at 5 years of follow-up? J Am Coll Cardiol. 2011;57:160–166. doi: 10.1016/j.jacc.2010.05.061. [DOI] [PubMed] [Google Scholar]
- 5.Wilber DJ, Pappone C, Neuzil P, et al. Comparison of antiarrhythmic drug therapy and radiofrequency catheter ablation in patients with paroxysmal atrial fibrillation: a randomized controlled trial. JAMA. 2010;303:333–340. doi: 10.1001/jama.2009.2029. [DOI] [PubMed] [Google Scholar]
- 6.Ouyang F, Antz M, Ernst S, et al. Recovered Pulmonary Vein Conduction as a Dominant Factor for Recurrent Atrial Tachyarrhythmias After Complete Circular Isolation of the Pulmonary Veins: Lessons From Double Lasso Technique. Circulation. 2005;111:127–135. doi: 10.1161/01.CIR.0000151289.73085.36. [DOI] [PubMed] [Google Scholar]
- 7.Verma A, Kilicaslan F, Pisano E, et al. Response of atrial fibrillation to pulmonary vein antrum isolation is directly related to resumption and delay of pulmonary vein conduction. Circulation. 2005;112:627–635. doi: 10.1161/CIRCULATIONAHA.104.533190. [DOI] [PubMed] [Google Scholar]
- 8.Schmitt C, Ndrepepa G, Weber S, et al. Biatrial multisite mapping of atrial premature complexes triggering onset of atrial fibrillation. Am J Cardiol. 2002;89:1381–1387. doi: 10.1016/s0002-9149(02)02350-0. [DOI] [PubMed] [Google Scholar]
- 9.Elayi CS, Di Biase L, Barrett C, et al. Atrial fibrillation termination as a procedural endpoint during ablation in long-standing persistent atrial fibrillation. Heart Rhythm. 2010;7:1216–1223. doi: 10.1016/j.hrthm.2010.01.038. [DOI] [PubMed] [Google Scholar]
- 10.Dixit S, Marchlinski FE, Lin D, et al. Randomized ablation strategies for the treatment of persistent atrial fibrillation: RASTA study. Circulation Arrhythmia and electrophysiology. 2012;5:287–294. doi: 10.1161/CIRCEP.111.966226. [DOI] [PubMed] [Google Scholar]
- 11.Narayan SM, Krummen DE, Shivkumar K, Clopton P, Rappel W-J, Miller J. Treatment of Atrial Fibrillation by the Ablation of Localized Sources: The Conventional Ablation for Atrial Fibrillation With or Without Focal Impulse and Rotor Modulation: CONFIRM Trial. J Am Coll Cardiol. 2012;60:628–636. doi: 10.1016/j.jacc.2012.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shivkumar K, Ellenbogen KA, Hummel JD, Miller JM, Steinberg JS. Acute Termination of Human Atrial Fibrillation by Identification and Catheter Ablation of Localized Rotors and Sources: First Multicenter Experience of Focal Impulse and Rotor Modulation (FIRM) Ablation. J Cardiovasc Electrophysiol. 2012;23:1277–1285. doi: 10.1111/jce.12000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miller JM, Daubert J, Day J, et al. Long-Term Results of Patients Receiving Focal Impulse and Rotor Modulation (FIRM) for Atrial Fibrillation: Extended Multi-center Experience (abstract) 2013 in review. [Google Scholar]
- 14.Narayan SM, Krummen DE, Rappel W-J. Clinical Mapping Approach to Identify Rotors and Focal Beats in Human Atrial Fibrillation. J Cardiovascular Electrophysiology. 2012;23:447–454. doi: 10.1111/j.1540-8167.2012.02332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Narayan SM, Clopton P, Krummen DE, Shivkumar K, Miller J. Direct or Concidental Ablation of Localized Sources May Explain the Success of Atrial Fibrillation Ablation. On Treatment Analysis from the CONFIRM Trial. J Am Coll Cardiol. 2013a;62:138–147. doi: 10.1016/j.jacc.2013.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Calvo D, Atienza F, Jalife J, et al. High-rate pacing-induced atrial fibrillation effectively reveals properties of spontaneously occurring paroxysmal atrial fibrillation in humans. Europace : European pacing, arrhythmias, and cardiac electrophysiology : journal of the working groups on cardiac pacing, arrhythmias, and cardiac cellular electrophysiology of the European Society of Cardiology. 2012;14:1560–1566. doi: 10.1093/europace/eus180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Narayan SM, Shivkumar K, Krummen DE, Miller JM, Rappel W-J. Panoramic Electrophysiological Mapping But Not Individual Electrogram Morphology Identifies Sustaining Sites for Human Atrial Fibrillation (AF Rotors and Focal Sources Relate Poorly to Fractionated Electrograms) Circulation Arrhythmia and electrophysiology. 2013;6:58–67. doi: 10.1161/CIRCEP.111.977264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin YJ, Lo MT, Lin C, et al. Prevalence, characteristics, mapping, and catheter ablation of potential rotors in nonparoxysmal atrial fibrillation. Circulation Arrhythmia and electrophysiology. 2013;6:851–858. doi: 10.1161/CIRCEP.113.000318. [DOI] [PubMed] [Google Scholar]
- 19.Morillo C, Verma A, Kuck KH, et al. Radiofrequency Ablation vs Antiarrhythmic Drugs as First-Line Treatment of Symptomatic Atrial Fibrillation: (RAAFT 2): a Randomized Trial (abstract) Heart Rhythm. 2012;9:1580. [Google Scholar]
- 20.Nielsen JC, Johannessen A, Raatikainen P, et al. Radiofrequency Ablation as Initial Therapy in Paroxysmal Atrial Fibrillation. New Engl J Med. 2012;367:1587–1595. doi: 10.1056/NEJMoa1113566. [DOI] [PubMed] [Google Scholar]
- 21.Narayan SM, Krummen DE, Donsky A, Swarup V, Miller JM. Precise Rotor Elimination without Concomitant pulmonary vein Isolation for the Successful Elimination of Paroxysmal Atrial Fibrillation. PRECISE-PAF. Heart Rhythm. 2013;10:LBCT4. [Google Scholar]
- 22.Allessie MA, de Groot NM, Houben RP, et al. The ElectroPathological Substrate of Longstanding Persistent Atrial Fibrillation in Patients with Structural Heart Disease: Longitudinal Dissociation. Circulation Arrhythmia and electrophysiology. 2010;122:1674–1682. doi: 10.1161/CIRCEP.109.910125. [DOI] [PubMed] [Google Scholar]
- 23.Herweg B, Kowalski M, Steinberg JS. Termination of persistent atrial fibrillation resistant to cardioversion by a single radiofrequency application. Pacing and clinical electrophysiology : PACE. 2003;26:1420–1423. doi: 10.1046/j.1460-9592.2003.t01-1-00203.x. [DOI] [PubMed] [Google Scholar]
- 24.Tzou WS, Saghy L, Lin D. Termination of persistent atrial fibrillation during left atrial mapping. J Cardiovasc Electrophysiol. 2011;22:1171–1173. doi: 10.1111/j.1540-8167.2011.02079.x. [DOI] [PubMed] [Google Scholar]
- 25.Gerstenfeld E, Sahakian A, Swiryn S. Evidence for transient linking of atrial excitation during atrial fibrillation in humans. Circulation. 1992;86:375–382. doi: 10.1161/01.cir.86.2.375. [DOI] [PubMed] [Google Scholar]
- 26.Lee G, Kumar S, Teh A, et al. Epicardial Wave Mapping in Human Long-lasting Persistent AF. Rotors, complex wavefronts and disorganized activity. European Heart Journal. 2013 doi: 10.1093/eurheartj/eht267. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 27.Wu T-J, Doshi RN, Huang H-LA, et al. Simultaneous Biatrial Computerized Mapping During Permanent Atrial Fibrillation in Patients with Organic Heart Disease. J Cardiovasc Electrophysiol. 2002;13:571–577. doi: 10.1046/j.1540-8167.2002.00571.x. [DOI] [PubMed] [Google Scholar]
- 28.Sahadevan J, Ryu K, Peltz L, et al. Epicardial Mapping of Chronic Atrial Fibrillation in Patients: Preliminary Observations. Circulation. 2004;110:3293–3299. doi: 10.1161/01.CIR.0000147781.02738.13. [DOI] [PubMed] [Google Scholar]
- 29.Sanders P, Berenfeld O, Hocini M, et al. Spectral Analysis Identifies Sites of High-Frequency Activity Maintaining Atrial Fibrillation in Humans. Circulation. 2005a;112:789–797. doi: 10.1161/CIRCULATIONAHA.104.517011. [DOI] [PubMed] [Google Scholar]
- 30.Lemola K, Ting M, Gupta P, et al. Effects of Two Different Catheter Ablation Techniques on Spectral Characteristics of Atrial Fibrillation. Journal of the American College of Cardiology. 2006;48:340–348. doi: 10.1016/j.jacc.2006.04.053. [DOI] [PubMed] [Google Scholar]
- 31.Atienza F, Almendral J, Jalife J, et al. Real-time dominant frequency mapping and ablation of dominant frequency sites in atrial fibrillation with left-to-right frequency gradients predicts long-term maintenance of sinus rhythm. Heart Rhythm. 2009;6:33–40. doi: 10.1016/j.hrthm.2008.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin Y-J, Tai C-T, Kao T, et al. Electrophysiological Characteristics and Catheter Ablation in Patients With Paroxysmal Right Atrial Fibrillation. Circulation. 2005;112:1692–1700. doi: 10.1161/CIRCULATIONAHA.104.512731. [DOI] [PubMed] [Google Scholar]
- 33.Haissaguerre M, Hocini M, Shah AJ, et al. Noninvasive Panoramic Mapping of Human Atrial Fibrillation Mechanisms: A Feasibility Report. J Cardiovasc Electrophysiol. 2013;24:711–717. doi: 10.1111/jce.12075. [DOI] [PubMed] [Google Scholar]
- 34.Ganesan AN, Kuklik P, Lau DH, et al. Bipolar Electrogram Shannon Entropy at Sites of Rotational Activation: Implications for Ablation of Atrial Fibrillation. Circulation Arrhythmia and electrophysiology. 2013;6:48–57. doi: 10.1161/CIRCEP.112.976654. [DOI] [PubMed] [Google Scholar]
- 35.Ganesan AN, Kuklik P, Lau DH, et al. Bipolar Electrogram Shannon Entropy at Sites of Rotational Activation: Implications for Ablation of Atrial Fibrillation (abstract) Heart Rhythm. 2012;(suppl.) doi: 10.1161/CIRCEP.112.976654. [DOI] [PubMed] [Google Scholar]
- 36.Nattel S. New ideas about atrial fibrillation 50 years on. Nature. 2002;415:219–226. doi: 10.1038/415219a. [DOI] [PubMed] [Google Scholar]
- 37.Oakes RS, Badger TJ, Kholmovski EG, et al. Detection and quantification of left atrial structural remodeling with delayed-enhancement magnetic resonance imaging in patients with atrial fibrillation. Circulation. 2009;119:1758–1767. doi: 10.1161/CIRCULATIONAHA.108.811877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scherlag BJ, Nakagawa H, Jackman WM, et al. Electrical Stimulation to Identify Neural Elements on the Heart: Their Role in Atrial Fibrillation. Journal of Interventional Cardiac Electrophysiology. 2005;13:37–42. doi: 10.1007/s10840-005-2492-2. [DOI] [PubMed] [Google Scholar]
- 39.Pratola C, Baldo E, Notarstefano P, Toselli T, Ferrari R. Radiofrequency ablation of atrial fibrillation: is the persistence of all intraprocedural targets necessary for long-term maintenance of sinus rhythm? Circulation. 2008;117:136–143. doi: 10.1161/CIRCULATIONAHA.106.678789. [DOI] [PubMed] [Google Scholar]
- 40.Kuck K-H, Willems S, Breithardt G. GAP-AF Trial - AFNET1: (Late Breaking Clinical Trial Abstract) Europace. 2013 [Google Scholar]
- 41.Baykaner T, Clopton P, Schricker AA, Lalani G, Krummen DE, Narayan SM. Targeted Ablation at Stable Atrial Fibrillation Sources Improves Success Over Conventional Ablation In High Risk Patients: A Substudy of the CONFIRM Trial. Canadian J Cardiology. 2013 doi: 10.1016/j.cjca.2013.07.672. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]