Abstract
Advances in protein and metabolic engineering have led to wider use of enzymes to synthesize important molecules. However, many desirable transformations are not catalyzed by any known enzyme, driving interest in understanding how new enzymes can be created. The cytochrome P450 enzyme family, whose members participate in xenobiotic metabolism and natural products biosynthesis, catalyzes an impressive range of difficult chemical reactions that continues to grow as new enzymes are characterized. Recent work has revealed that P450-derived enzymes can also catalyze useful reactions previously accessible only to synthetic chemistry. The evolution and engineering of these enzymes provides an excellent case study for how to genetically encode new chemistry and expand biology’s reaction space.
Introduction
Impressive demonstrations of the use of engineered microbes to produce fuels and chemicals in recent years have led some to predict a future in which microbes can produce nearly all of the organic molecules upon which society depends from renewable resources [1]. This future may be desirable from the standpoint of energy efficiency and environmental sustainability, but it is also a ways off. Successful metabolic engineering efforts have for the most part depended on reassembling natural enzymes into biosynthetic pathways. Many desired products unfortunately fall outside the reach of the rather limited set of known enzyme-catalyzed transformations. Eventually, progress in biological production will depend on our ability to genetically encode new catalysts for known and novel chemical reactions.
Generating new enzymes de novo is difficult, although progress is being made with some relatively simple transformations—for example, computationally designed enzymes that catalyze the Kemp elimination and Diels-Alder reactions have been reported [2,3]. Nature, it seems, agrees with this assessment, preferring to repurpose existing enzyme scaffolds rather than create whole new enzymes [4]. Some scaffolds appear to be used more frequently than others: for example, the enolase and crotonase superfamilies (and many others) support several different reactions [5], whereas the dihydrofolate reductase family is only known to carry out a single reaction [6]. Thus a biomimetic alternative to de novo protein design might exploit enzymes that nature has already used for chemical innovations. But can nature’s past successes with catalytic diversification guide future efforts to generate new enzyme catalysts? Recent work suggests that the versatility of cytochrome P450 enzymes—which catalyze a multitude of reactions in nature—can indeed be replicated and even expanded upon by enzyme engineers to genetically encode new biosynthetic capabilities.
Cytochrome P450 enzymes are most commonly associated with the hydroxylation and dealkylation of xenobiotic molecules in mammals, and in this case the substrate scope is vast. But their natural roles far exceed this one niche. Biosynthetic pathways to many natural products, such as terpenes (including steroids), alkaloids, and polyketides involve P450-mediated oxidations, which add functional groups to simpler hydrophobic skeletons. P450s also occur in primary catabolic pathways for degradation of alkanes and other recalcitrant molecules. Beyond their large substrate scope, many different reaction types have been characterized for naturally occurring and engineered P450s [7–9•], including hydroxylation, epoxidation, sulfoxidation, aryl-aryl coupling, nitration, oxidative and reductive dehalogenations, and recently several synthetically important non-natural reactions (vide infra). Given future challenges in synthetic biology, the ability of P450 enzymes to assume new catalytic functions in natural and artificial contexts merits close inspection for insights into how we might discover or create new biocatalysts.
In this review, we present examples of the broad catalytic range of P450 enzymes from papers published during the last two years, with an emphasis on newly characterized reactions, both naturally occurring and artificially conceived. To help distinguish between the many natural P450 reactions and newly discovered non-natural reactions, we first review key aspects of P450 catalysis and describe how these characteristics allow access to a diverse set of reactions. Next, we describe recently published non-natural P450 reactions and contrast features of natural and non-natural chemical reactivity. Finally, we discuss the broader relevance of P450 reaction diversity to the goal of engineering new enzymes.
Cytochrome P450: a platform for powerful C-H oxidation chemistry
Here we introduce the P450 catalytic cycle and the key reactive intermediates that are responsible for much of the natural reactivity of P450 enzymes. Additionally, we make note of some of the key conserved residues involved in oxygen activation; mutations to some of these key residues lead to increased activity in non-natural reactions as we describe below. For a more detailed treatment of the P450 mechanism, we refer the reader to more specialized reviews [7,10•–13].
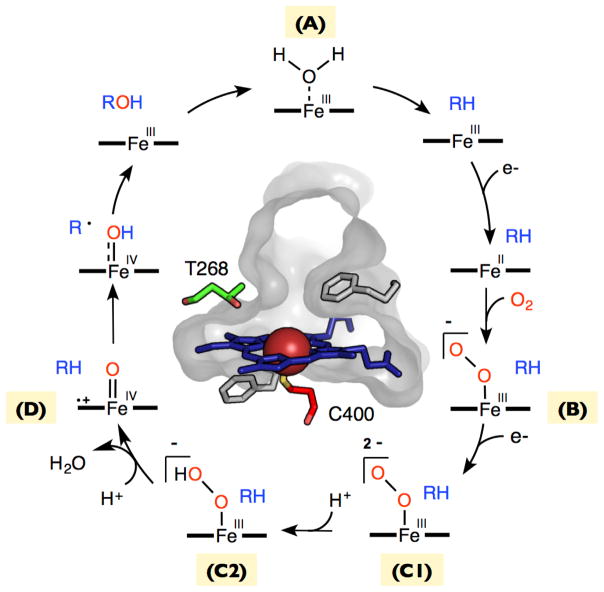
In the resting state of the enzyme, the catalytic iron is in the ferric (+3) oxidation state (intermediate A in Figure 1). P450s produce intermediates that are sufficiently reactive to attack even inert hydrocarbons. Consequently, P450 enzymes have evolved mechanisms that prevent initiation of the catalytic cycle in the absence of substrate. Substrate binding initiates the catalytic cycle by increasing the redox potential of the heme prosthetic group, which makes it possible for an associated reductase to reduce the heme to the ferrous (+2) state. The catalytic cycle continues with binding of molecular oxygen to the iron center to give a ferric superoxide complex (intermediate B in Figure 1). A second electron transfer generates an iron-peroxo intermediate (C1 in Figure 1), which is then protonated to give an iron-hydroperoxy intermediate (C2 in Figure 1). Subsequent protonation effects heterolytic cleavage of the O-O bond to form the high-valent iron-oxo intermediate known as compound I (intermediate D in Figure 1). In hydroxylation reactions, compound I abstracts a hydrogen atom from a substrate C-H bond (formally a proton-coupled 1-electron oxidation of the substrate) yielding compound II and a substrate radical. These two radical species then rapidly recombine to produce the hydroxylated product and the ferric resting state of the enzyme.
Figure 1.
The P450 catalytic cycle. The active site structure of P450BM3 is shown at center with conserved threonine (T268) and axial cysteine (C400) highlighted. Key intermediates include the ferric resting state (A), the ferric superoxide intermediate (B), the iron-peroxo or hydroperoxy intermediates (C1, C2), and compound I (D).
Although many interactions between the protein, its reductase partner, and heme prosthetic group contribute to the smooth operation of the catalytic cycle, a few key residues merit special mention. One is a conserved active-site threonine (T268 in Figure 1), which, through water, helps to protonate the iron-peroxo and iron-hydroperoxy intermediates, thus promoting O-O bond scission to generate compound I. Another key residue universally conserved among P450 enzymes is the axial cysteine that ligates the heme iron. Thiolate ligation is thought to serve several functions. For one, the electron-rich thiolate ligand makes the ferric heme a worse electron acceptor. This decrease in redox potential helps to prevent triggering of the catalytic cycle in the absence of substrate. Another key role of the axial cysteine is to promote heterolytic O-O bond scission of the iron-hydroperoxy intermediate. Finally, as Green has argued, thiolate ligation may bias compound I toward hydrogen abstraction chemistry [12]. In particular, the electron donating ability of the thiolate ligand makes compound I worse at performing 1-electron oxidations, but makes the 1-electron reduced form (known as compound II) much more basic, thus favoring proton-coupled 1-electron oxidations (i.e. hydrogen abstractions).
Intermediates in the P450 catalytic cycle drive diverse natural chemical reactivity
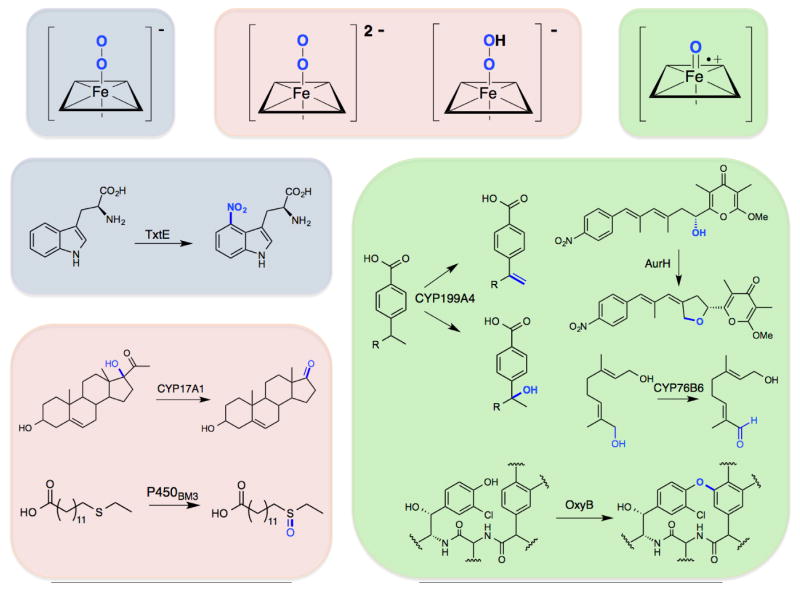
The expansive catalytic scope of P450 enzymes is obvious from even a partial listing of known P450-catalyzed reactions: aryl-aryl coupling, ring contractions and expansions, S- N-, and O-dealkylations, decarboxylation, oxidative cyclization, alcohol and aldehyde oxidation, desaturation, sulfoxidation, nitrogen oxidation, epoxidation, C-C bond scission, decarbonylation, and nitration. Many of these transformations (heteroatom demethylations, decarboxylation, alcohol and aldehyde oxidation, desaturation, and others) are mechanistically very similar to hydroxylation (Figure 1) and result from the ability of compound I to perform hydrogen atom abstractions; others involve compound I-mediated oxidations distinct from hydrogen atom abstraction. P450 enzymes, however, do not rely exclusively on compound I, as other intermediates in the catalytic cycle are responsible for some P450 transformations (Figure 2). For example, the iron-peroxo (or hydroperoxide) intermediate can mediate epoxidation and sulfoxidation under some circumstances [14,15]; in others this species carries out C-C bond cleavage, as described below. Likewise, the initial oxygen adduct with ferrous heme (the ferric-superoxide intermediate, Figure 2, blue) is proposed to play a key role in P450-catalyzed nitration [16••]. P450 catalytic diversification in nature is thus enabled by the generation of multiple potentially reactive species during the P450 catalytic cycle, as well as the potency of P450-derived oxidants, which can react with substrates in different ways. Though many potential oxidants occur during the cycle, natural P450s are often quite specific in the reactions that they catalyze. Specificity is directed by protein sequences molded by the force and filter of natural selection to favor certain intermediates while tuning their reactivity and selectivity.
Figure 2.
Key catalytic intermediates in the P450 cycle and examples of chemical reactions that rely on them. In blue are highlighted the ferric superoxide intermediate and the nitration reaction that is associated with this intermediate [16••]. In pink are highlighted the iron-peroxo and iron-hydroperoxy intermediates and C-C bond scission [30] and sulfoxidation [14] reactions. Highlighted in green are compound I and desaturation [23•], ring closure [17], sequential oxidation [22], and aryl coupling reactions [27].
Beginning with compound I-derived oxidations, one particularly interesting P450-mediated reaction occurs during biosynthesis of the natural product aureothin (Figure 2, green). The P450 enzyme AurH first catalyzes hydroxylation of the aureothin precursor, followed by intramolecular C-O bond formation to give a tetrahydrofuran ring, with both reactions presumably occurring with the intermediacy of compound I [17]. Hertweck and coworkers have exploited this unusual enzyme to accomplish a biomimetic total synthesis of aureothin, as well as the synthesis of several aureothin derivatives [18–20•]; one paper [20•] describes an active site mutation that converts AurH into a six-electron oxidase, leading to the conversion of a substrate methyl group all the way to a carboxylic acid.
Several natural examples of sequential hydroxylations to yield ketones or carboxylates from unactivated C-H bonds have been described recently [21,22]. For example, in xiamycin biosynthesis, the P450 enzyme XiaM was shown to catalyze sequential hydroxylation of a methyl group to a carboxylate [21]. Another example of multiple P450-catalyzed oxidations was published by Höfer et al. in their investigation of the first steps of the biosynthesis of bioactive alkaloids vinblastine and secologanin (Figure 2, green) [22].
Though more typical of di-iron monooxygenases and β-ketoglutarate-dependent dioxygenases, desaturation has been observed with a few P450 enzymes [8]. An interesting example of P450-catalyzed desaturation was recently reported by Bell et al. [23•]. CYP199A4 was previously found to catalyze demethylation of several aromatic compounds, including 4-methoxybenzoic acid and veratric acid, as well as hydroxylation (major product) and desaturation (minor product) of 4-ethylbenzoic acid. In their recent report, these authors found two active site mutations (F185V and F185I) that markedly increase desaturation of 4-ethylbenzoic acid to yield 4-vinylbenzoic acid, with the isoleucine variant giving exclusively the desaturation product (Figure 2, green).
Several examples of P450-catalyzed decarboxylation are associated with biosynthesis and drug metabolism. One biotechnologically interesting P450-catalyzed decarboxylation leads to the synthesis of terminal alkenes from fatty acids [24•]. The authors propose a mechanism in which compound I abstracts the β-hydrogen, followed by 1-electron oxidation of the resulting radical to yield a β-carbocation, which spontaneously decarboxylates to give the product.
The above compound I-mediated transformations most likely proceed via hydrogen atom abstraction. Another mechanism by which compound I can mediate oxidation is through sequential 1-electron oxidations. Vancomycin and related antibiotics contain aryl C-C and C-O crosslinks catalyzed by P450-mediated 1-electron oxidations (Figure 2, green). Recent work on the biosynthesis of these antibiotics includes the solution of two crystal structures of P450s involved in aryl coupling reactions [25,26], as well as a study that examines the timing of P450-catalyzed crosslinking during vancomycin biosynthesis [27].
Biochemical evidence suggests that the iron-peroxo intermediate can behave as an alternative oxidant in epoxidation and sulfoxidation reactions [14,15], though until recently [28] theoretical studies cast doubt on its role in sulfoxidation [29]. It is generally accepted that the iron-peroxo species is the active oxidant in C-C cleavage reactions [30]. For example, recent work by Kincaid and coworkers supports the role of a substrate-influenced selectivity switch that promotes the stability of the iron-peroxo species, favoring C-C lyase chemistry for certain steroid derivatives [30] (Figure 2, pink).
One of the most interesting P450 reactions characterized recently is that of tryptophan nitration in thaxtomin biosynthesis (Figure 2, blue) [16••]. Here, neither compound I nor the iron-peroxo intermediate is thought to play the key role. Instead, the initial adduct between ferrous heme and dioxygen, the ferric superoxide intermediate (Figure 1, B), is proposed to react with in situ generated nitric oxide to form ferric peroxynitrite. The peroxynitrite species can then decompose via one of two pathways (neither of which has been directly supported so far). In pathway (1), peroxynitrite decomposes homolytically to yield NO2• and an iron-ferryl intermediate (compound II). Compound II then performs a 1-electron oxidation of tryptophan, giving a radical which recombines with NO2• to give the product. In pathway (2), heterolytic decomposition of the ferric peroxynitrite intermediate gives the ferric-hydroxide resting state and NO2+, which reacts with tryptophan by electrophilic aromatic substitution.
A recently characterized reaction of uncertain mechanism is P450-catalyzed synthesis of alkanes from fatty aldehydes to form insect protective coatings [31•]. In contrast to other known P450-catalyzed decarboxylation or decarbonylation reactions [24•], the product here is a fully saturated alkane. Although strong evidence that a P450 was responsible for this reaction was first presented in the 1990s [32], only recently has the specific P450 enzyme been identified [31•].
Manipulating conserved features of P450 catalysis allows access to reactions not observed in nature
The diverse set of naturally occurring P450 reactions has proven a rich source of inspiration for the field of biomimetic oxidation in synthetic chemistry. In an interesting reversal of roles, several classic papers as well as more recent works have shown that P450s can catalyze reactions first discovered by synthetic chemists. Unlike natural P450 reactions, which rely on various reactive oxygen intermediates, these new P450 reactions stem from alternative reactive species created through the use of activated reagents such as diazo compounds and azides. Some of the inspiration for non-natural P450 reactions came from the rich literature on P450 model complexes. Originally synthesized as functional or spectroscopic mimics of P450 enzymes, model P450 complexes (i.e. iron-porphyrins) were later found to catalyze several reactions distinct from oxygen transfers. Breslow and Gelman first demonstrated that iron-tetraphenyl porphyrin model complexes could catalyze intra- and intermolecular nitrene transfers to form benzosultams and substituted cyclohexanes when provided with iminoiodinane nitrene precursors [33]. Following up on this, Dawson and coworkers found that rabbit liver P450 enzymes could catalyze low levels (< 5 total turnovers, TTN) of the same reactions that had been described for the synthetic P450 model [34].
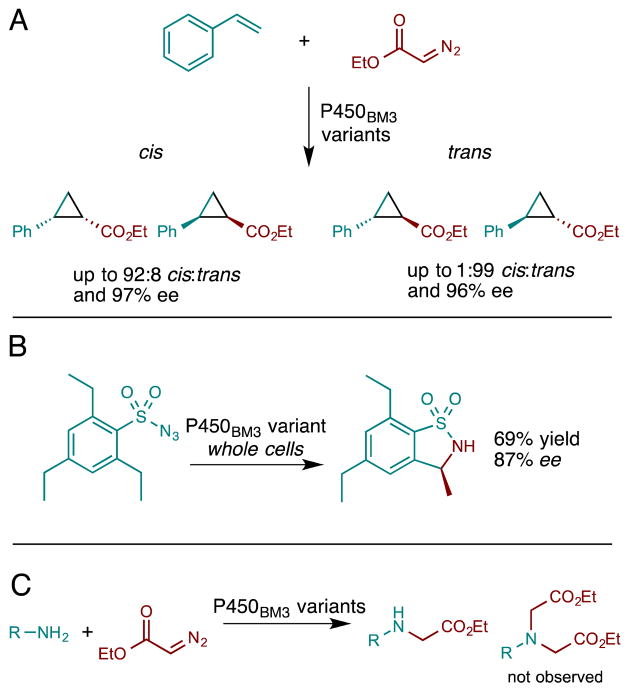
More recently, our group demonstrated several new P450 reactions that had previously been shown only for metalloporphyrins. It has been known since the 1990s that iron porphyrins catalyze the reaction of olefins with diazo carbene precursors to yield cyclopropanes [35]. Metalloporphyrin-catalyzed cyclopropanation is thought to proceed via a metal-carbenoid intermediate, analogous to P450 epoxidations that proceed through compound I. However, it was only recently shown by Coelho et al. [36•] that this reaction could be catalyzed at low levels by hemin in water as well as by several heme proteins, including P450BM3, although at lower levels even than free hemin. The selectivities of most of the heme proteins mirrored the trans-selectivity of hemin for the cyclopropanation of styrene with ethyl diazoacetate. P450BM3 showed very low activity, but in contrast to the other heme proteins produced the cyclopropane product with low but measurable enantioselectivity. Mutations in P450BM3, including at highly conserved residues such T268, dramatically improved the productivity as well as the diastereo- and enantioselectivity of this reaction (Figure 3A). Although in natural P450s, this conserved active site threonine acts as a proton shuttle, for non-natural chemistry, mutation of T268 to less bulky alanine presumably relieves steric impediments to reactivity, as evidenced by strong alterations in the stereoselectivity of cyclopropanation; future studies may shed light on the effect of this mutation by assaying alternative substitutions at this position. Enzyme engineering could even overcome the natural selectivity of the prosthetic group to achieve >90% cis selectivity. And, while free hemin gives a racemic mixture of products, the P450BM3 cyclopropanation catalysts exhibited enantioselectivites of up to 97%. In a second publication, Coelho and coworkers demonstrated that mutation of the axial coordinating cysteine, universally conserved among P450s, to serine was highly activating, particularly in vivo (vide infra) [37••]. The strong effect of axial ligand substitution was attributed in part to the significant increase in reduction potential (>100 mV increase) for the serine-ligated enzyme, facilitating reduction to the active ferrous state. Axial thiolate ligation, absolutely essential for monooxygenation, is unnecessary for this non-natural reaction. Whereas thiolate ligation is important for O-O bond scission, no such strong electron donation is required to decompose the less-stable diazo substrates employed by Coelho et al.
Figure 3.
Several recent examples of non-natural P450 reactions: (A) cyclopropanation of styrene catalyzed by P450BM3 variants [36•], (B) intramolecular C-H amination catalyzed by P450BM3 variants expressed in whole cells [38•], and (C) intermolecular carbene insertion into N-H bonds yielding secondary amines [40•]
Another metalloporphyrin reaction shown to be catalyzed by P450s is C-H amination from azides as nitrene sources (Figure 3B) [38•]. The iminoiodinane precursors that Dawson and coworkers had used to obtain low levels of C-H amination with P450s [34] are problematic due to their insolubility in protein-compatible solvents. Thus we tested the more atom-efficient and convenient azide-based nitrene precursors. In spite of the fact that azide-based C-H amination reactions have been found to be markedly less efficient with iron-porphyrins (as compared to cobalt or ruthenium complexes) and require high temperatures and anhydrous conditions [39], we found that wild-type P450BM3 could catalyze low levels of intramolecular C-H amination to yield benzosultams [38•]. As found for cyclopropanation, enzyme engineering could improve the enantioselectivity and activity of the new C-H amination enzymes. Indeed several of the mutations that increased cyclopropanation activity (at the active site threonine and axial cysteine) were found to strongly modulate C-H amination activity, leading to catalysts that were capable of catalyzing several hundred turnovers in vitro and roughly double that amount in vivo. Here again, mutation to the conserved axial cysteine was highly activating: its positive effect on C-H amination in vitro was even greater than observed for cyclopropanation.
In another approach to P450-catalyzed C-N bond formation, Wang et al. have shown that engineered P450 enzymes can also catalyze carbene N-H insertions [40•]. The reaction of ethyl diazoacetate with a diverse set of amine acceptors was found to proceed with high turnover numbers. Although many other C-N bond forming methodologies lead to product mixtures via multiple nucleophilic additions, the enzyme-catalyzed N-H insertions gave only the desired secondary amines (Figure 3C). Of note is that free hemin produces a mixture of secondary and tertiary amines, which emphasizes the important role of the enzyme in regulating substrate access to the reactive center.
An interesting aspect of these new reactions is that both cyclopropanation and C-H amination proceed well in whole cells. P450BM3-derived cyclopropanation catalysts, in particular, were more than six-fold faster when used in whole cells (on a per enzyme basis) and catalyzed more than 60,000 total turnovers under saturating substrate concentrations [37••]. Thus the enzyme is as good as any transition metal catalyst reported to date. Although NADPH-driven heme reduction in vitro requires P450BM3’s reductase domain, in whole cells the reductase was not strictly necessary: even the isolated heme domain could catalyze over 1,000 total turnovers of styrene cyclopropanation. In the reducing environment of anaerobic whole cells, other electron donors apparently can facilitate reduction to the active ferrous state. For C-H amination the effect of carrying out reactions in whole cells was less profound (roughly two-fold higher activity), perhaps due to the higher levels of azide reduction (which competes with C-H amination) in whole cells than in vitro.
A simplifying feature of enzyme-catalyzed carbene and nitrene transfers is the enzyme’s decreased dependence on the reductase domain for activity. For C-H amination and carbene transfers, although initial reduction to ferrous heme is necessary, after bond formation the heme is returned to the active ferrous state, thus eliminating the need for stoichiometric NADPH. Decreased dependence on the reductase may also prove to be problematic as it may lead to the generation of reactive carbon or nitrogen species in the absence of substrate, which for stronger electrophiles may lead to heme or protein destruction.
An interesting lesson from these efforts to generate enzymes for whole new reactions is that conserved residues whose function was highly specific to the chemistry catalyzed by the natural enzyme became particularly important for tuning the new activities. The active site threonine, which normally helps to catalyze O-O bond scission via protonation, and the axial coordinating cysteine, whose importance in oxygenation reactions is profound [12], can both be substituted to greatly increase activity for C-H amination and cyclopropanation (and abolish monooxygenase activity). Many other protein residues contribute to oxygen activation in P450s, and it is likely that at least some can be mutated to further enhance non-natural reactivity. As observed with natural P450 enzymes, enhancing the reactivity of enzyme-carbenoid and nitrenoid intermediates may facilitate an expanded catalytic scope for these new chemistries.
Conclusions
What information can be gleaned from the diverse natural and non-natural chemistry catalyzed by P450 enzymes that might inform other efforts to genetically encode new reactions? As noted above, much of the natural diversity of P450 chemistry is driven by the reactive nature of oxygen activation intermediates. In this vein, it is worth noting that many other natural enzymes are capable of generating highly reactive species, such as other oxygenase enzymes (di-iron monooxygenases, Rieske monooxygenases, etc.), radical SAM enzymes, and adenosylcolbalamin-dependent enzymes, among others [41]. Although they may prove more difficult to engineer than P450s, these enzymes should not be overlooked in the search for new biocatalytic transformations.
For recent non-natural P450 chemistry, the reactive intermediates derive from the reaction of enzyme with synthetic reagents. That these reactions do not require the sophisticated P450 catalytic cycle with its well-timed reductions and bond cleavages can be attributed to the activated nature of the reagents, which undergo relatively facile decomposition to yield reactive carbon and nitrogen species. Exploring the reactions of synthetic reagents with natural enzymes has proven fruitful for finding new genetically encoded catalysts in other contexts [42–44] and is likely to bring more synthetic chemistry into biology. While the reactivity of a free prosthetic group is not necessarily predictive of activity within an enzyme, for each reaction type we explored thus far [36•,38•,40•], free heme was found to give at least some basal activity with most (though not all) substrates under the assay conditions. Thus investigations of metal/cofactor-reagent pairs may yield useful starting points for identifying possible new enzyme reactivities. Of course, what is different from past efforts [34] is the availability of enzyme engineering tools such as directed evolution, which can reliably improve even very low activities, especially when the activities are exhibited by an (evolvable) enzyme rather than some other protein framework.
Although non-natural chemistries that rely on synthetic reagents may be challenging to employ within cellular biosynthetic pathways, a great deal of useful biocatalysis is conducted in vitro [45] where access to the synthetic reagent is not a problem. Current efforts to develop biosynthesis in cell-free extracts [46] could allow for relatively straightforward integration of non-natural prosthetic groups and reagents in biosynthetic pathways. Thus working within the constraints of biology may ultimately prove unnecessary—even for enzyme engineers.
Highlights.
Discovering enzymes for new reactions is important but challenging
Cytochrome P450 enzymes catalyze many different chemical reactions in nature
P450s can be engineered to catalyze reactions first discovered by synthetic chemists
Engineering enhances the activity and selectivity of P450s in non-natural reactions
Acknowledgments
The authors acknowledge the support of the Jacobs Institute of Molecular Medicine at Caltech and the Office of Naval Research (grant N00014-11-1-0205). JAM is supported by an NIH Ruth L. Kirschstein National Research Service Award (F32GM101792) and CCF is supported by an NSF Graduate Research Fellowship. The content is solely the responsibility of the authors and does not represent the official views of any of the funding agencies. We thank Devin Trudeau, Jackson Cahn, Jane Wang, Ryan Lauchli, Sabine Brinkmann-Chen, Sheel Dodani, Tillman Heinisch, Todd Hyster, and Martin Enqvist for helpful comments on several versions of this review.
Footnotes
Conflicts of interest
The authors are aware of no conflicts of interest regarding the preparation and submission of this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
Papers of particular interest, published within the annual period of the review, have been highlighted as:
• Of special interest
•• Of outstanding interest
- 1.Keasling JD, Mendoza A, Baran PS. Synthesis: A constructive debate. Nature. 2012;492(7428):188–189. doi: 10.1038/492188a. [DOI] [PubMed] [Google Scholar]
- 2.Blomberg R, Kries H, Pinkas DM, Mittl PR, Grütter MG, Privett HK, Mayo SL, Hilvert D. Precision is essential for efficient catalysis in an evolved Kemp eliminase. Nature. 2013;503(7476):418–421. doi: 10.1038/nature12623. [DOI] [PubMed] [Google Scholar]
- 3.Siegel JB, Zanghellini A, Lovick HM, Kiss G, Lambert AR, St Clair JL, Gallaher JL, Hilvert D, Gelb MH, Stoddard BL, Houk KN, et al. Computational design of an enzyme catalyst for a stereoselective bimolecular Diels-Alder reaction. Science. 2010;329(5989):309–313. doi: 10.1126/science.1190239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gerlt JA, Babbitt PC. Enzyme (re)design: Lessons from natural evolution and computation. Curr Opin Chem Biol. 2009;13(1):10–18. doi: 10.1016/j.cbpa.2009.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Glasner ME, Gerlt JA, Babbitt PC. Evolution of enzyme superfamilies. Curr Opin Chem Biol. 2006;10(5):492–497. doi: 10.1016/j.cbpa.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 6.Dellus-Gur E, Toth-Petroczy A, Elias M, Tawfik DS. What makes a protein fold amenable to functional innovation? Fold polarity and stability tradeoffs. J Mol Biol. 2013;425(14):2609–2621. doi: 10.1016/j.jmb.2013.03.033. [DOI] [PubMed] [Google Scholar]
- 7.Guengerich FP, Munro AW. Unusual cytochrome P450 enzymes and reactions. J Biol Chem. 2013;288(24):17065–17073. doi: 10.1074/jbc.R113.462275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guengerich FP. Common and uncommon cytochrome P450 reactions related to metabolism and chemical toxicity. Chem Res Toxicol. 2001;14(6):611–650. doi: 10.1021/tx0002583. [DOI] [PubMed] [Google Scholar]
- 9•.Podust LM, Sherman DH. Diversity of P450 enzymes in the biosynthesis of natural products. Nat Prod Rep. 2012;29(10):1251–1266. doi: 10.1039/c2np20020a. This review discusses several interesting P450-catalyzed transformations that contribute to the biosynthesis of natural products. Several of the important mechanistic features of P450 catalysts including the role of the active-site threonine are explained. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10•.Whitehouse CJ, Bell SG, Wong LL. P450BM3 (CYP102A1). Connecting the dots. Chem Soc Rev. 2012;41(3):1218–1260. doi: 10.1039/c1cs15192d. An excellent comprehensive look at the enzymology and applications of the well-studied P450BM3. [DOI] [PubMed] [Google Scholar]
- 11.Krest CM, Onderko EL, Yosca TH, Calixto JC, Karp RF, Livada J, Rittle J, Green MT. Reactive intermediates in cytochrome P450 catalysis. J Biol Chem. 2013;288(24):17074–17081. doi: 10.1074/jbc.R113.473108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Green MT. C-H bond activation in heme proteins: The role of thiolate ligation in cytochrome P450. Curr Opin Chem Biol. 2009;13(1):84–88. doi: 10.1016/j.cbpa.2009.02.028. [DOI] [PubMed] [Google Scholar]
- 13.Fasan R. Tuning P450 enzymes as oxidation catalysts. ACS Catal. 2012;2(4):647–666. [Google Scholar]
- 14.De Voss JJ, Cryle MJ. Is the ferric hydroperoxy species responsible for sulfur oxidation in cytochrome P450s? Angew Chem Int Ed Engl. 2006;45(48):8221–8223. doi: 10.1002/anie.200603411. [DOI] [PubMed] [Google Scholar]
- 15.Jin S, Markris TM, Bryson TA, Sligar SG, Dawson JH. Epoxidation of olefins by hydroperoxo-ferric cytochrome P450. J Am Chem Soc. 2003;125(12):3406–3407. doi: 10.1021/ja029272n. [DOI] [PubMed] [Google Scholar]
- 16••.Barry SM, Kers JA, Johnson EG, Song L, Aston PR, Patel B, Krasnoff SB, Crane BR, Gibson DM, Loria R, Challis GL. Cytochrome P450-catalyzed l-tryptophan nitration in thaxtomin phytotoxin biosynthesis. Nat Chem Biol. 2012;8(10):814–816. doi: 10.1038/nchembio.1048. Although several nitro-containing natural products are known, none were believed to result from direct nitration. Here a P450 enzyme, TxtE, is shown to catalyze the regioselective nitration of tryptophan in vitro when supplied with nitric oxide donors. Gene disruption studies implicate a nitric oxide synthase in biosynthesis, leading the authors to propose a NO-dependent mechanism. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.He J, Muller M, Hertweck C. Formation of the aureothin tetrahydrofuran ring by a bifunctional cytochrome P450 monooxygenase. J Am Chem Soc. 2004;126(51):16742–16743. doi: 10.1021/ja046104h. [DOI] [PubMed] [Google Scholar]
- 18.Henrot M, Richter ME, Maddaluno J, Hertweck C, De Paolis M. Convergent asymmetric synthesis of (+)-aureothin employing an oxygenase-mediated resolution step. Angew Chem Int Ed Engl. 2012;51(38):9587–9591. doi: 10.1002/anie.201204259. [DOI] [PubMed] [Google Scholar]
- 19.Richter M, Busch B, Ishida K, Moore BS, Hertweck C. Pyran formation by an atypical CYP-mediated four-electron oxygenation-cyclization cascade in an engineered aureothin pathway. Chem Bio Chem. 2012;13(15):2196–2199. doi: 10.1002/cbic.201200406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20•.Zocher G, Richter ME, Mueller U, Hertweck C. Structural fine-tuning of a multifunctional cytochrome P450 monooxygenase. J Am Chem Soc. 2011;133(7):2292–2302. doi: 10.1021/ja110146z. The crystal structure of tetrahydrofuran ring-forming P450 AurH is solved in substrate-free and inhibitor-bound forms. Structure guided mutagenesis is used to uncover a new activity of AurH in catalyzing the successive oxidation of the methyl group normally involved in ring formation. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Q, Li H, Li S, Zhu Y, Zhang G, Zhang H, Zhang W, Shi R, Zhang C. Carboxyl formation from methyl via triple hydroxylations by XiaM in xiamycin A biosynthesis. Org Lett. 2012;14(24):6142–6145. doi: 10.1021/ol302782u. [DOI] [PubMed] [Google Scholar]
- 22.Höfer R, Dong L, Andre F, Ginglinger JF, Lugan R, Gavira C, Grec S, Lang G, Memelink J, Van Der Krol S, Bouwmeester H, et al. Geraniol hydroxylase and hydroxygeraniol oxidase activities of the CYP76 family of cytochrome P450 enzymes and potential for engineering the early steps of the (seco)iridoid pathway. Metab Eng. 2013 doi: 10.1016/j.ymben.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 23•.Bell SG, Zhou R, Yang W, Tan AB, Gentleman AS, Wong LL, Zhou W. Investigation of the substrate range of CYP199A4: Modification of the partition between hydroxylation and desaturation activities by substrate and protein engineering. Chemistry. 2012;18(52):16677–16688. doi: 10.1002/chem.201202776. Desaturation is a relatively rare P450 reaction. Mutagenesis is used to generate an enzyme that is completely selective for desaturation over C-H bond hydroxylation of 4-ethylbenzoic acid. [DOI] [PubMed] [Google Scholar]
- 24•.Rude MA, Baron TS, Brubaker S, Alibhai M, Del Cardayre SB, Schirmer A. Terminal olefin (1-alkene) biosynthesis by a novel P450 fatty acid decarboxylase from jeotgalicoccus species. Appl Environ Microbiol. 2011;77 (5):1718–1727. doi: 10.1128/AEM.02580-10. The authors identify several microbes that carry out the synthesis of terminal olefins. One strain was further analyzed, and a P450 enzyme, OleT, purified from cell extracts was found to generate terminal olfeins via fatty acid decarboxylation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Z, Rupasinghe SG, Schuler MA, Nair SK. Crystal structure of a phenol-coupling P450 monooxygenase involved in teicoplanin biosynthesis. Proteins. 2011;79(6):1728–1738. doi: 10.1002/prot.22996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cryle MJ, Staaden J, Schlichting I. Structural characterization of CYP165D3, a cytochrome P450 involved in phenolic coupling in teicoplanin biosynthesis. Arch Biochem Biophys. 2011;507(1):163–173. doi: 10.1016/j.abb.2010.10.017. [DOI] [PubMed] [Google Scholar]
- 27.Schmartz PC, Wolfel K, Zerbe K, Gad E, El Tamany el S, Ibrahim HK, Abou-Hadeed K, Robinson JA. Substituent effects on the phenol coupling reaction catalyzed by the vancomycin biosynthetic P450 enzyme OxyB. Angew Chem Int Ed Engl. 2012;51(46):11468–11472. doi: 10.1002/anie.201204458. [DOI] [PubMed] [Google Scholar]
- 28.Wang B, Chunsen L, Cho KB, Nam W, Shaik S. The FeIII(H2O2) complex as a highly efficient oxidant in sulfoxidation reactions: Revival of an underrated oxidant in cytochrome P450. J Chem Theory Comput. 2013;9(6):2519–2525. doi: 10.1021/ct400190f. [DOI] [PubMed] [Google Scholar]
- 29.Li C, Zhang L, Zhang C, Hirao H, Wu W, Shaik S. Which oxidant is really responsible for sulfur oxidation by cytochrome P450? Angew Chem Int Ed Engl. 2007;46(43):8168–8170. doi: 10.1002/anie.200702867. [DOI] [PubMed] [Google Scholar]
- 30.Gregory M, Mak PJ, Sligar SG, Kincaid JR. Differential hydrogen bonding in human CYP17 dictates hydroxylation versus lyase chemistry. Angew Chem Int Ed Engl. 2013;52(20):5342–5345. doi: 10.1002/anie.201300760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31•.Qiu Y, Tittiger C, Wicker-Thomas C, Le Goff G, Young S, Wajnberg E, Fricaux T, Taquet N, Blomquist GJ, Feyereisen R. An insect-specific P450 oxidative decarbonylase for cuticular hydrocarbon biosynthesis. Proc Natl Acad Sci USA. 2012;109(37):14858–14863. doi: 10.1073/pnas.1208650109. An insect P450 enzyme from the CYP4G family is shown through gene knock-down and heterologous expression to catalyze hydrocarbon formation from fatty aldehydes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reed JR, Vanderwel D, Choi S, Pomonis JG, Reitz RC, Blomquist GJ. Unusual mechanism of hydrocarbon formation in the housefly: Cytochrome P450 converts aldehyde to the sex pheromone component (Z)-9-tricosene and CO2. Proc Natl Acad Sci U S A. 1994;91(21):10000–10004. doi: 10.1073/pnas.91.21.10000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Breslow R, Gellman SH. Tosylamidation of cyclohexane by a cytochrome P-450 model. J Chem Soc Chem Commun. 1982:1400–1401. [Google Scholar]
- 34.Svastits EW, Dawson JH, Breslow R, Gellman SH. Functionalized nitrogen atom transfer catalyzed by cytochrome P450. J Am Chem Soc. 1985;107(22):6427–6428. [Google Scholar]
- 35.Wolf JR, Hamaker CG, Djukic JP, Kodadek T, Woo LK. Shape and stereoselective cyclopropanation of alkenes catalyzed by iron porphyrins. J Am Chem Soc. 1995;117(36):9194–9199. [Google Scholar]
- 36•.Coelho PS, Brustad EM, Kannan A, Arnold FH. Olefin cyclopropanation via carbene transfer catalyzed by engineered cytochrome P450 enzymes. Science. 2013;339(6117):307–310. doi: 10.1126/science.1231434. P450 enzymes and other hemoproteins are shown for the first time to catalyze olefin cyclopropanation. Protein engineering tuned the activity as well as the enantio- and diasteroselectivity of the reaction. Although free hemin shows strong trans selectivity, several mutant enzymes were found to be cis-selective, with up to 90% diastereoselectivity. [DOI] [PubMed] [Google Scholar]
- 37••.Coelho PS, Wang ZJ, Ener ME, Baril SA, Kannan A, Arnold FH, Brustad EM. A serine-substituted P450 catalyzes highly efficient carbene transfer to olefins in vivo. Nat Chem Biol. 2013;9(8):485–487. doi: 10.1038/nchembio.1278. P450BM3 variants are shown to catalyze cyclopropanation efficiently in vivo. Mutation of the axial coordinating cysteine to serine strongly enhances this activity, leading to some of the most active and selective cyclopropanation catalysts yet reported. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38•.McIntosh JA, Coelho PS, Farwell CC, Wang ZJ, Lewis JC, Brown TR, Arnold FH. Enantioselective intramolecular C-H amination catalyzed by engineered cytochrome P450 enzymes in vitro and in vivo. Angew Chem Int Ed Engl. 2013;52(35):9309–9312. doi: 10.1002/anie.201304401. C-H amination is a challenging synthetic transformation with wide applicability in chemical synthesis. Here engineered P450 enzymes are shown to be highly active and enantioselective in the intramolecular formation of C-N bonds using unnatural sulfonyl azides as substrates. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ruppel JV, Kamble RM, Zhang XP. Cobalt-catalyzed intramolecular C-H amination with arylsulfonyl azides. Org Lett. 2007;9(23):4889–4892. doi: 10.1021/ol702265h. [DOI] [PubMed] [Google Scholar]
- 40•.Wang ZJ, Peck NE, Renata H, Arnold FH. Cytochrome P450 catalysed insertion of carbenoids into N-H bonds. Chem Sci. 2014 doi: 10.1039/C3SC52535J. Epub ahead of print Here the scope of P450-catalyzed carbene transfer is expanded to include insertions into N-H bonds. The enzyme is selective for the single-insertion secondary amine product, whereas hemin produces a mixture of single- and double-insertion products. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lewis JC, Coelho PS, Arnold FH. Enzymatic functionalization of carbon-hydrogen bonds. Chem Soc Rev. 2011;40(4):2003–2021. doi: 10.1039/c0cs00067a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Muller A, Sturmer R, Hauer B, Rosche B. Stereospecific alkyne reduction: Novel activity of old yellow enzymes. Angew Chem Int Ed Engl. 2007;46 (18):3316–3318. doi: 10.1002/anie.200605179. [DOI] [PubMed] [Google Scholar]
- 43.Hasnaoui-Dijoux G, Majeric Elenkov M, Lutje Spelberg JH, Hauer B, Janssen DB. Catalytic promiscuity of halohydrin dehalogenase and its application in enantioselective epoxide ring opening. Chem Bio Chem. 2008;9(7):1048–1051. doi: 10.1002/cbic.200700734. [DOI] [PubMed] [Google Scholar]
- 44.Savile CK, Magloire VP, Kazlauskas RJ. Subtilisin-catalyzed resolution of N-acyl arylsulfinamides. J Am Chem Soc. 2005;127(7):2104–2113. doi: 10.1021/ja045397b. [DOI] [PubMed] [Google Scholar]
- 45.Huisman GW, Collier SJ. On the development of new biocatalytic processes for practical pharmaceutical synthesis. Curr Opin Chem Biol. 2013;17(2):284–292. doi: 10.1016/j.cbpa.2013.01.017. [DOI] [PubMed] [Google Scholar]
- 46.Harris DC, Jewett MC. Cell-free biology: Exploiting the interface between synthetic biology and synthetic chemistry. Curr Opin Biotechnol. 2012;23 (5):672–678. doi: 10.1016/j.copbio.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]