Abstract
Copper and zinc homeostasis systems in pathogenic bacteria are required to resist host efforts to manipulate the availability and toxicity of these metal ions. Central to this microbial adaptive response is the involvement of metal-trafficking and -sensing proteins that ultimately exercise control of metal speciation in the cell. Cu- and Zn-specific metalloregulatory proteins regulate the transcription of metal-responsive genes while metallochaperones and related proteins ensure that these metals are appropriately buffered by the intracellular milieu and delivered to correct intracellular targets. In this review, we summarize recent findings on how bacterial pathogens mount a metal-specific response to derail host efforts to win the “fight over metals.”
Metal Ions at the Host-Pathogen Interface
Strict control of the homeostasis of transition metal ions is essential to all forms of life. The cellular balance of metal ions is orchestrated by proteins and small molecules, and when cellular physiology is disrupted by aberrant metal metabolism, human disease can occur [1]. This need for cellular control of metal homeostasis is exploited by the innate immune system during a bacterial infection. Here, the host attempts to restrict the availability of essential nutrients in a process generally termed nutritional immunity [2, 3•, 4] while inundating the bacterial cell with a wide range of toxic insults, including low pH, reactive oxygen species (ROS), reactive nitrogen species (RNS), reactive chlorine species (RCS), and hydrolases [5,6]. A key aspect of this assault is an extensive perturbation of the availability of the four major transition metals required by the bacterium: iron (Fe), manganese (Mn), zinc (Zn), and copper (Cu). In contrast to host processes that attempt to limit a pathogen’s access to Fe and Mn, recent work reveals that high Cu concentrations are used to kill microbial invaders, particularly intracellular pathogens (Figure 1) [7,8]. For Zn, the work taken collectively supports a role for both host-mediated toxicity [4,7] and sequestration [3•,9] as a means to restrict pathogen viability upon host infection. Although these microbial defense mechanisms disrupt transition metal homeostasis of most bacteria [3•,4,9], successful pathogens have evolved mechanisms of adaptation to these perturbations [4,10,11].
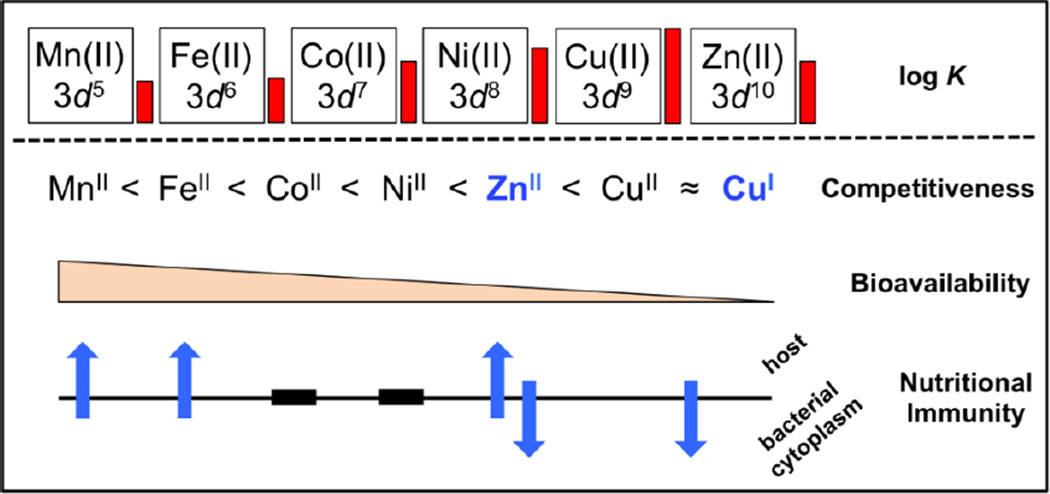
Figure 1.
Bioinorganic chemistry at the host-pathogen interface. Cu(II) has the highest affinity for a given ligand compared to other first row transition metals as exemplified by the height of the red bars which depict the NIST approved log K values relative to the Cu(II)-aspartic acid complex [Cu(II)-aspartic acid, log K = 8.9]. This empirical relationship is described as the Irving-Williams series and relates to the relative competitiveness of first-row transition metals in a cellular environment [18]. Cu(I) predominates in the cytoplasm and like Zn(II), forms high-affinity complexes with softer acids (histidine, cysteine, methionine), and is therefore also considered a highly competitive metal. Bioavailability is inversely proportional to competitiveness and has been roughly approximated on the basis of the relative binding affinities of metal-dependent transcriptional regulators [16]. Metal-centric nutritional immunity is defined as the host’s attempt to both sequester metal ions from cells (upward-facing blue arrows) and/or bombard the bacterial cytoplasm with metal-ion stress (downward-facing blue arrows). Roles of Co and Ni in nutritional immunity are not yet known (black bars).
Molecular Basis of Cu(I) and Zn(II) Toxicity
Cu and Zn speciation is defined by the types of ligands encountered in the cell [12,13], while metal specificity is collectively dictated by metal coordination number and geometry, the rates of exchange in and out of metal complexes, and redox state (valence) [14]. On the basis of the binding affinities of selected targeting, trafficking, and metal-sensing proteins [12,15,16], Cu(I) is thought to be buffered by a typical cell in the attomolar range, while Zn(II) is buffered in the nanomolar [17•] to picomolar [16] range; however, these values may vary for different bacteria. As a rule of thumb, chelate binding affinities for Cu(II) and Zn(II) are generally higher than for earlier first-row divalent transition metals for a given ligand, a trend known as the Irving-Williams series for divalent ions (Figure 1) [18]. The bioavailability of Cu and Zn is therefore generally low and inversely proportional to competitiveness relative to other first-row metals, which dictates that their availability in cells be tightly regulated. Further, the major redox state of copper is monovalent Cu(I) in the bacterial cytoplasm due to the low reduction potential maintained by low-molecular-weight thiols relative to the Cu(II)/Cu(I) redox couple (−0.22V and +0.15V, respectively, relative to the normal hydrogen electrode) [13]. The sulfur-containing amino acids cysteine and methionine play important roles as soft bases that readily coordinate the soft acid Cu(I). These properties make unregulated Cu(I) highly toxic as evidenced by the ability of Cu(I) to mediate disassembly of iron-sulfur (Fe-S) clusters leading to dysfunctional cellular metabolism [19, 20]. The vulnerability of Fe-S clusters to Cu(I) remains to be validated as a general mechanism of Cu toxicity in other bacteria, particularly those that lack significant Fe-S cluster-containing proteins in their metallomes [21]. Our molecular-level understanding of intracellular zinc toxicity is far less clear, although a model invoking mismetallation of metalloenzymes through competition is a reasonable, albeit largely untested one [22].
A second potential impact of copper toxicity is the chemistry of Cu(I) with host-mediated hydrogen peroxide (H2O2) or superoxide (O2−•). Labile Fe(II) is accepted as a major source of intracellular oxidative damage in cells given its ability to heterolytically cleave H2O2 to form reactive hydroxyl radical OH• and oxidized Fe(III); this process becomes catalytic in the presence of cellular reductants [23,24]. Although uncomplexed Cu redox cycles faster than Fe in vitro [24–26], the degree to which Cu(I)-catalyzed Fenton chemistry is relevant in vivo remains uncertain due to the lack of a comprehensive understanding of Cu(I)-ligand speciation in the cell and how Cu(I)-complexes are modulated by myriad toxic insults at the host-pathogen interface.
Cu Sensing and Trafficking
Many pathogens accumulate micromolar levels of cell-associated Cu [12,22] despite possessing little or no clearly defined cytoplasmic need for the metal [27]. For Gram-negative bacteria, it is presumed that much of this Cu localizes to the periplasm and is bound to essential cuproproteins, although some pathogenic bacteria additionally harbor Cu(I) sequestration proteins in the cytoplasm [28,29]. Once inside the cell, bacterial copper chaperones generally represent a first line of defense against Cu(I) toxicity imparted by the host (Figure 2) [11]. The founding bacterial metallochaperone is Bacillus subtilis CopZ, which is structurally identical to Atx1 initially characterized in yeast (Figure 3a) [30]. CopZ adopts a ferredoxin-like fold where Cu(I) forms a bis-thiolato digonal coordination complex with a Cys-X-X-Cys loop sequence (where X is any amino acid) that is additionally capable of coordinating a small molecule from solvent. Metallochaperones buffer highly competitive Cu(I) to low levels in the cytoplasm [31] and shuttle Cu(I) to intracellular targets including cytoplasmic Cu(I) sensors and to Cu(I)-specific P-type ATPase effluxers with rapid exchange kinetics through an associative, ligand exchange mechanism that prevents release of Cu(I) into bulk solution (Figure 2) [10,32].
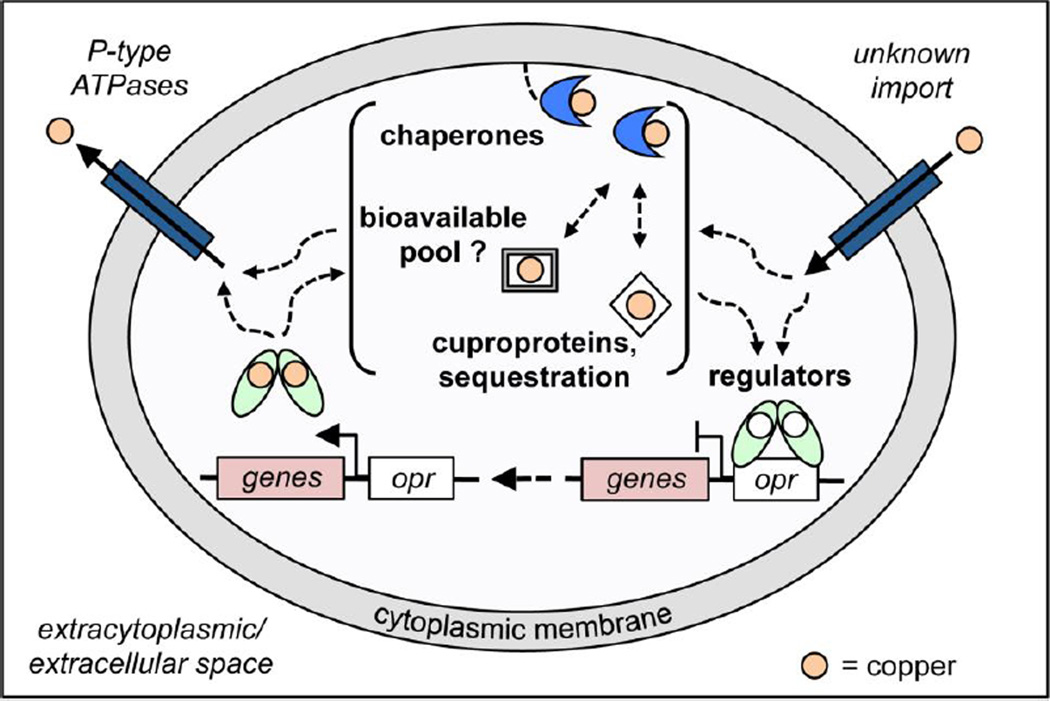
Figure 2.
Overview of copper sensing and trafficking within the bacterial cytoplasm. Copper enters the cytoplasm through largely unknown mechanisms. Copper speciation within the cell depends on the relative concentrations of Cu(I) bound to the bioavailable pool, e.g., copper bound to low-molecular-weight thiols, cytoplasmic binding proteins, e.g., MymT [28] and CutC [29]), chaperones, and Cu(I) sensors. The thermodynamics and kinetics of Cu(I) speciation remain incompletely understood and may be dictated by the concentrations at which copper homeostasis proteins become saturated. Importantly, Cu(I) overload must ultimately be sensed by Cu(I)-dependent metalloregulators (light green calipers) causing transcriptional derepression as a result of dissociation from the DNA operator-promoter region (white rectangle, opr) (or transcriptional activation) and expression of Cu(I) resistance genes (pink rectangle, labeled genes). It is these upregulated copper resistance proteins that ultimately function in Cu(I) resistance, either via sequestration or export through P-type ATPases.
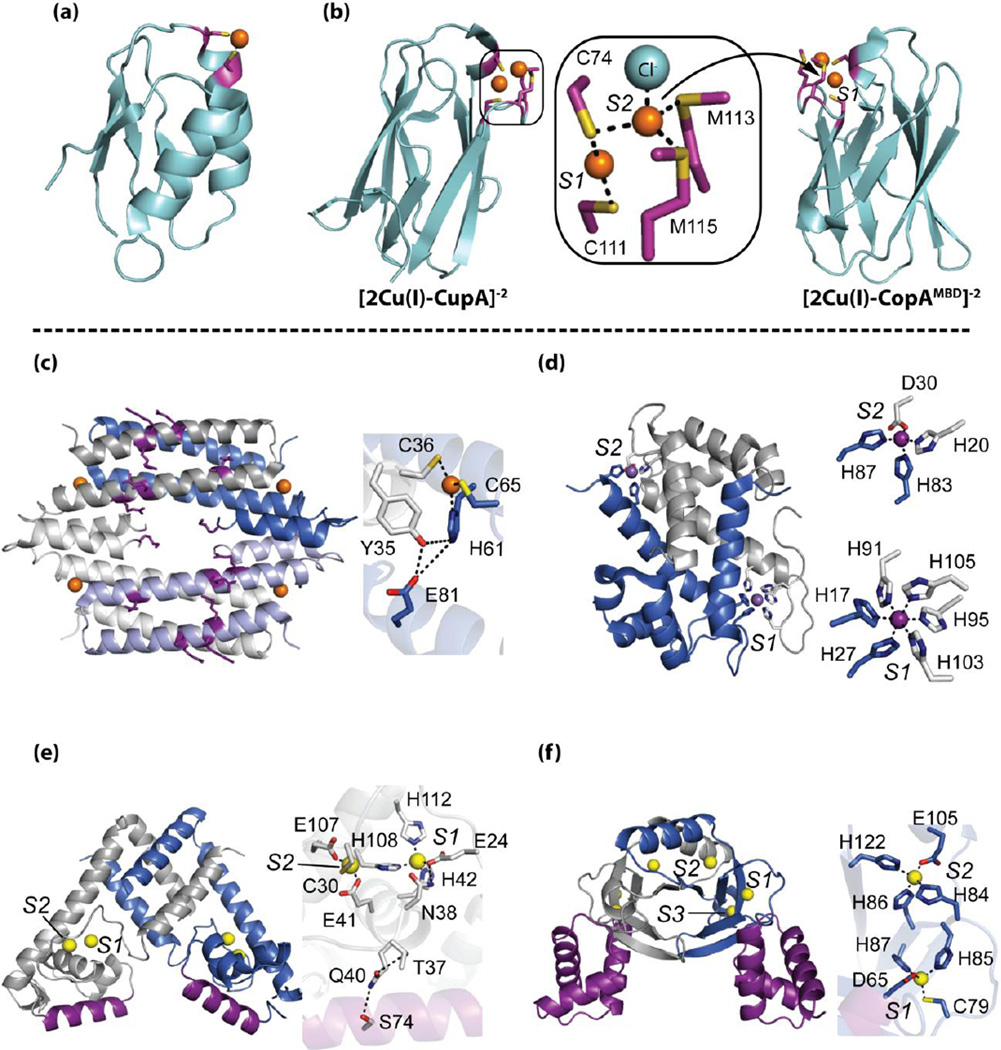
Figure 3.
Structural insights into the bioinorganic chemistry of proteins operating at the host-pathogen interface. Top panel, Cu(I)-trafficking proteins: (a) Cu(I)-bound CopZ [PDB 1K0V]; (b) binuclear Cu(I)-bound soluble CupA (sCupA) [PDB 4F2E] transfers Cu(I) from S1 Cu site to S2 Cu site of CopAMBD [PDB 4F2F]. Inset, Cu(I) binding sites of sCupA [33••]. Bottom panel, crystal structures of (c) Cu(I)-bound M. tuberculosis CsoR (PDB 2HH7) [36,39], (d) Mn(II)-bound calprotectin S100 A8/A9 heterodimer [51••] (Ca ions not shown for clarity) (PDB 4GGF), (e) Zn(II)-bound AdcR homodimer [PDB 3TGN] [60], and (f) Zn(II)-bound Streptomyces Zur homodimer [PDB 3MWM] [42•]. In each case, protomers are shaded blue and grey with known or probable DNA binding domains/residues shaded purple. Labels for metal-binding sites are in italics. Insets, metal-binding sites with dashed lines indicating either first-coordination sphere or second-sphere hydrogen-bonding interactions.
A new perspective on Cu(I) trafficking has been reported for the Gram-positive respiratory pathogen Streptococcus pneumoniae [33••]. This work reveals that the ancient cupredoxin fold [34] (Figure 3), known to play prominent roles in electron transfer and bacterial respiration, has been co-opted to function as a novel plasma membrane-anchored Cu(I) chaperone (CupA) that is capable of delivering Cu(I) to the N-terminal metal-binding domain (MBD) of the Cu(I)-effluxer CopA (CopAMBD) [33••]. This Cu(I) transfer is thermodynamically favorable and flows from the low affinity S2 site on CupA to the high affinity S1 site on CopA, presumably via transient docking of electrostatically complementary domains that are otherwise isostructural and harbor identical binuclear Cu(I) binding sites (Figure 3b). Unlike soluble Cu(I) chaperones in other bacteria, CupA is essential for cellular copper resistance. This suggests either an obligatory transfer of Cu(I) from CupA to CopA for efflux (both ΔcopA and ΔcupA strains accumulate excess copper), and/or CupA plays an as yet unknown function in Cu(I) delivery to other cellular targets, e.g., the copper sensor CopY or other unknown targets [10]. In other bacteria, such as Listeria monocytogenes, loss of the copper chaperone has little impact on copper resistance or metallation of downstream targets when copper is replete. In these cases where Cu(I) chaperones are nonessential, the bioavailable pool of Cu(I) remains adequately regulated, potentially by other metal-binding proteins or by small molecules, e.g., glutathione (GSH, see Figure 2) [31, 35•].
In some bacterial pathogens, a Cu(I) chaperone has not yet been identified, a noteworthy example of which is Mycobacterium tuberculosis. However, this pathogen encodes two Cu(I) metallosensors and a cytoplasmic Cu metallothionein (MymT) that provides resistance to cytoplasmic Cu toxicity (Figure 2) [28,36,37]. CsoR is the founding member of a large family of repressors that adopt an all α-helical dimer of dimers architecture in solution and represses transcription in the apo state [38]. In the presence of elevated copper, CsoR binds four equivalents of Cu(I) per tetramer in a trigonal planar coordination geometry, which in turn leads to derepression of the cso operon (Figure 3c). The specificity of the Cu(I) response appears to involve a second coordination shell of hydrogen bonding interactions and kinking of the long α2 helix, resulting in dissociation from the DNA via an as yet unknown structural mechanism [36,38,39]. A model of two CsoR tetramers bound to a single DNA operator has recently been reported for Streptomyces lividans CsoR and involves conserved residues that diagonally traverse an electropositive patch on one face of each tetramer (Figure 3c) [39,40]. S. lividans CsoR is most closely evolutionarily related to a second CsoR-like Cu(I) sensor in M. tuberculosis, regulated in copper repressor (RicR), which may play a more significant role in copper resistance than CsoR itself [37]. Similar sensor duality is also observed in the MerR family of transcriptional activators in Salmonella [41]. Why do two Cu(I) sensors of the same structure exist in the same cell? One hypothesis is that this arrangement, in tandem with Cu(I) chaperones, allows the evolution of multiple saturation “set points” of Cu(I) induction or finer combinatorial control of the copper stress response (Figure 2) [31,42•].
Copper Sensing without Cu(I) Binding?
It has recently been proposed that direct Cu(II) detection by the multiple antibiotic resistance regulator (MarR) in the E. coli cytoplasm underlies the microbial stress response to a range of antibiotics [43••]. In this work, Hao et al. propose that Cu(II) elicits transcriptional derepression of the MarR regulon via oxidative disulfide linkage of two MarR dimers into an inactive tetramer. Evidence was presented in this study that antibiotics lead to a change in Cu(I) speciation detectable by a fluorescent Cu(I) indicator, but intracellular Cu(II) was not directly detected. The mechanism of MarR oxidation by Cu(II) in the cell is not known, but could perhaps result from a thiol-disulfide redox imbalance where transient accumulation of copper-bound, oxidized glutathione (Cu(II)-GSSG) and/or Cu(II)-MarR2 occurs as a result of redox cycling facilitated by molecular oxygen (O2) [44]. Recent experiments suggest that antibiotic stress can occur in the absence of O2 indicating that more than one mechanism of toxicity could be operative in bacteria [45,46]. In any case, this study connects perturbation of cellular Cu speciation to antibiotic stress, which may impact the metallation state of cuproproteins, e.g., Cu,Zn-superoxide dismutase, in the extracytoplasmic compartment [47•].
Zn Homeostasis and the Host Response
Bacterial zinc homeostasis differs markedly from copper resistance in that bacteria have evolved both import and export mechanisms to strictly maintain accessible Zn(II) levels for zinc-requiring proteins and enzymes that function in diverse metabolic processes in the cell [48]. The need for sufficient bioavailable Zn(II) to supply a significant cellular demand while limiting Zn toxicity is maintained by the acquisition of zinc via ATP-binding cassette (ABC) transporters and cytoplasmic efflux through P-type ATPases or proton-coupled antiporters (Figure 4) [49,50••]. Therefore, host strategies to limit bacterial infection that exploit either zinc sequestration or zinc toxicity to override zinc homeostasis of microbial pathogens can be envisioned (Figure 1) [4]. The discovery of the involvement of neutrophil-derived calprotectin (CP) in metal homeostasis [9] and elucidation of its two metal-binding sites (S1 and S2) by structural and functional studies (Figure 3d) [51••,52••], as well as related calcium-activated S100 proteins [53], has brought into sharp focus the degree to which the host and bacteria participate in a “tug-of war” over Zn(II) and Mn(II). The ability of CP to chelate Mn(II) and Zn(II) represents a powerful antimicrobial weapon [9, 52]; however, two important Gram-negative respiratory pathogens, Acinetobacter baummanii [54•] and Neisseria meningitidus [55••] have been shown to overcome the CP defense response. These pathogens express an outer membrane receptor under Zn(II) deplete conditions, which in the case of Neisseria is proposed to bind Zn(II) [or Mn(II)]-bound CP directly in order to strip the metal from CP, analogous to what happens in bacterial iron “piracy” from transferrin [56]. Interestingly, Salmonella enterica also has the capability to out-compete CP for zinc in the gut via the high-affinity ZnuABC transport system, providing another illustration of how bacteria adapt to extracellular host defense strategies [57].
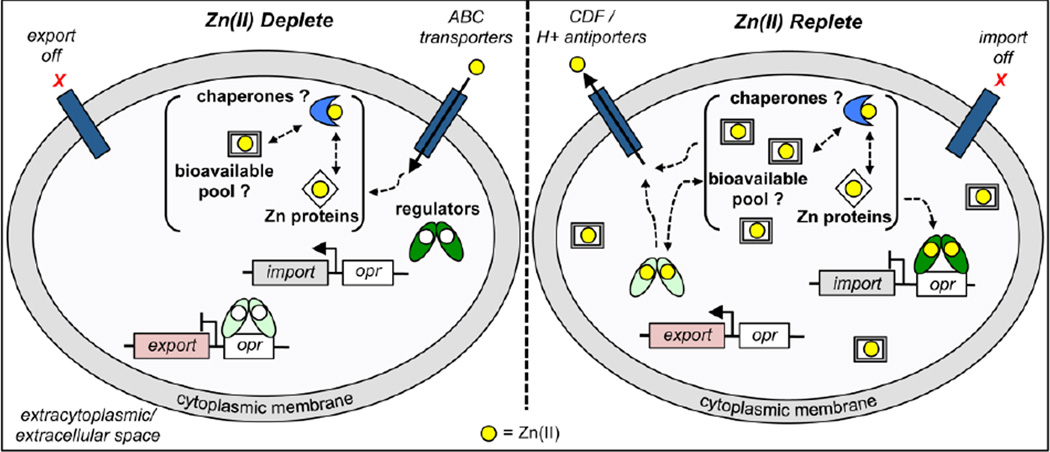
Figure 4.
Cellular response to either limited (left) or toxic (right) Zn(II) concentrations is mediated by the coordinate action of zinc uptake (dark green calipers) and efflux (light green calipers) transcriptional regulators that control the expression of import (grey boxes) and efflux (pink boxes) genes, respectively, as a result of a Zn(II)-regulated binding (activation or inhibition, respectively) to their DNA operators (white boxes, opr). Left panel, Zn(II) uptake regulators have low affinity for their DNA operator sequence in the apo state under zinc limiting conditions which allows for the expression of import genes. Zn(II) efflux regulators have high affinity for their DNA operator in the absence of Zn(II) and repress efflux. This response allows the cell to maintain a bioavailable concentration of Zn(II) that is sufficient for cellular needs. Trafficking of Zn(II) to selected proteins may involve the action of zinc chaperones, for which there is no definitive evidence. Right panel, under conditions of high extracellular zinc, the uptake regulators are metallated and bind with high affinity for their DNA operator, thereby repressing import. In the presence of Zn(II), efflux regulators dissociate from their operator sequence (shown), or become transcriptional activators, in the Zn(II)-bound state, resulting in the transcription of export genes. Zinc speciation in the cytoplasm is projected to involve small molecules, Zn-requiring metalloproteins, and possibly zinc chaperones, as indicated. There is some evidence that zinc-uptake and -efflux regulation occurs at distinct zinc concentrations added to cells, with repression of uptake genes occurring at lower total zinc relative to derepression/activation of export genes [22].
Zn Sensing and Trafficking
The extraordinary control of intracellular Zn(II) bioavailability is maintained by pairs of zinc sensors whose DNA operator-binding or activation functions are allosterically modulated by the direct binding of Zn(II) [12,16]. Uptake repressors facilitate repression of genes encoding uptake transporters upon zinc binding while efflux regulators control the expression of genes encoding zinc efflux systems upon zinc binding, either via transcriptional depression or activation [58] (Figure 4). The zinc specificity of these “allosteric switches” [58] is defined by the metal coordination environment and metal-induced structural changes that drive the appropriate allosteric response; in some single domain repressors a second-sphere hydrogen-bonding network appears to physically connect the metal- and DNA-binding sites [48,59••], analogous to CsoR discussed above (Figure 3c). For example, disruption or destabilization of this network in the zinc efflux repressor Staphylococcus aureus CzrA produces variable degrees of uncoupling of the allosteric response without effecting the binding free energies of either ligand (Zn(II) or DNA) [59••]. Another example involves the MarR-family zinc uptake repressor AdcR (adhesin competence regulator) from S. pneumoniae that features a second-coordination shell in the Zn(II)-bound state that is postulated to play an important role in allosteric coupling (Figure 3e) [60,61]. In addition to allostery, the roles of multiple metal-binding sites in multidomain repressors has been suggested to impart differential set points for expanding the dynamic range of sensing Zn(II) based on new crystallographic data of the zinc uptake repressor (Zur) that is proposed to be controlled by two functional sites (S1 and S2) (Figure 3f) [42•,62].
Although bona fide intracellular Zn(II)-specific chaperones have yet to be positively identified in bacteria, an unbiased mutant screen of A. baumannii stressed with CP-induced Zn(II) limitation identified components of the zur regulon, including a gene encoding a COG0523 family member [54•]. GOG0523 proteins are putative P-loop GTPases, one of which is a known Ni(II)-chaperone for urease in the pathogen Helicobactor pylori, [63] suggesting a role for this family of proteins in Zn(II) homeostasis [64,65•]. In addition to potential bona fide Zn(II) chaperones, copper chaperones may moonlight as Zn(II) binding proteins in vivo [66]. For example, the Zn(II)-mediated heterocomplex formed between Atx1 and the MBD of the PacS Cu(I)-transporting ATPase is significantly more stable than the corresponding Cu(I) complex [67]. Exchange of Cu(I) and Zn(II) on metalloregulators has also been proposed to occur under both unstressed and zinc-stressed growth conditions providing further support for the idea that cells must carefully control inappropriate access to these highly competitive metals [35•,66].
Conclusions and Perspectives
Transition metal ion homeostasis in pathogenic bacteria is characterized by the strict control of intracellular Cu(I) and Zn(II) speciation by metallosensors, metallochaperones, and metal transporters and is central to the adaptive response to host-mediated metal toxicity or restriction. The discovery and characterization of new bacterial copper- and zinc-trafficking proteins and host proteins that function in “metal-centric” nutritional immunity promises to provide further insights into this emerging aspect of the host-pathogen interface. The development of new methods to probe metal ion speciation in intact cells [68], particularly for spectroscopically challenged d10 metal ions Zn(II) and Cu(I) discussed here, remains a significant challenge, with low-throughput biochemical fractionation methods still state-of-the-art [69•]. The development of new synthetic and genetically encoded metal sensors, coupled with new biophysical/analytical methodologies that allow both temporal and spatial measurement of total metal and protein identification is certain to help unravel the complexities of transition metal homeostasis in liquid culture and in infected tissues [70•]. Obtaining such a sophisticated understanding of the dynamic in vivo metallome will further direct in vitro structural and functional studies, which in turn, may allow the rational design of new antibiotics that could provide a significant advantage to the host cell in the “fight over metals”.
Highlights.
The “fight over copper and zinc” at the host-pathogen interface is discussed.
Proteins involved in copper and zinc trafficking and sensing are discussed.
Cellular speciation of metals remains a significant challenge to address.
Characterizations of new host and bacterial players promises new biological insights.
Acknowledgments
Studies carried out in the Giedroc laboratory on bacterial metal homeostasis are supported by funding from the US National Institutes of Health (R01 GM042569 to D.P.G).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Note added in proof
Although we focus on mechanisms of intracellular Cu resistance in this review, a recent paper (Chaturvedi et al.) shows that the extracellular siderophore, yersiniabactin (Ybt), protects intracellular Escherichia coli from copper-mediated killing in phagocytes as a result of a catalytic superoxide dismutase-like activity of Cu(II)-Ybt complexes.
Chaturvedi KS, Hung CS, Giblin DE, Urushidani S, Austin AM, Dinauer MC, Henderson JP: Cupric yersiniabactin is a virulence-associated superoxide dismutase mimic. ACS Chem Biol 2014, in press (doi: 10.1021/cb400658k).
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as: • of special interest; •• of outstanding interest.
- 1.Kozlowski H, Janicka-Klos A, Brasun J, Gaggelli E, Valensin D, Valensin G. Copper, iron, and zinc ions homeostasis and their role in neurodegenerative disorders (metal uptake, transport, distribution and regulation) Coord Chem Rev. 2009;253:2665–2685. [Google Scholar]
- 2.Weinberg ED. Nutritional immunity: host's attempt to withhold iron from microbial invaders. J Am Med Assoc. 1975;231:39–41. doi: 10.1001/jama.231.1.39. [DOI] [PubMed] [Google Scholar]
- 3. Hood MI, Skaar EP. Nutritional immunity: transition metals at the pathogen-host interface. Nat Rev Microbiol. 2012;10:525–537. doi: 10.1038/nrmicro2836. A comprehensive review of how the host restricts the availability of some essential metals and induces toxicity with others in order to kill invading bacterial pathogens.
- 4.Botella H, Stadthagen G, Lugo-Villarino G, de Chastellier C, Neyrolles O. Metallobiology of host-pathogen interactions: an intoxicating new insight. Trends Microbiol. 2012;20:106–112. doi: 10.1016/j.tim.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 5.Plüddemann A, Mukhopadhyay S, Gordon S. Innate immunity to intracellular pathogens: macrophage receptors and responses to microbial entry. Immunol Rev. 2011;240:11–24. doi: 10.1111/j.1600-065X.2010.00989.x. [DOI] [PubMed] [Google Scholar]
- 6.Gray MJ, Wholey W-Y, Jakob U. Bacterial responses to reactive chlorine species. Annu Rev Microbiol. 2013;67:141–160. doi: 10.1146/annurev-micro-102912-142520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wagner D, Maser J, Lai B, Cai Z, Barry CE, 3rd, Höner zu Bentrup K, Russell DG, Bermudez LE. Elemental analysis of Mycobacterium avium-, Mycobacterium tuberculosis-, and Mycobacterium smegmatis-containing phagosomes indicates pathogen-induced microenvironments within the host cell’s endosomal system. J Immunol. 2005;174:1491–1500. doi: 10.4049/jimmunol.174.3.1491. [DOI] [PubMed] [Google Scholar]
- 8.White C, Lee J, Kambe T, Fritsche K, Petris MJ. A role for the ATP7A copper-transporting ATPase in macrophage bactericidal activity. J Biol Chem. 2009;284:33949–33956. doi: 10.1074/jbc.M109.070201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Corbin BD, Seeley EH, Raab A, Feldmann J, Miller MR, Torres VJ, Anderson KL, Dattilo BM, Dunman PM, Gerads R, et al. Metal chelation and inhibition of bacterial growth in tissue abscesses. Science. 2008;319:962–965. doi: 10.1126/science.1152449. [DOI] [PubMed] [Google Scholar]
- 10.Samanovic MI, Ding C, Thiele DJ, Darwin KH. Copper in microbial pathogenesis: meddling with the metal. Cell Host Microbe. 2012;11:106–115. doi: 10.1016/j.chom.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hodgkinson V, Petris MJ. Copper homeostasis at the host-pathogen interface. J Biol Chem. 2012;287:13549–13555. doi: 10.1074/jbc.R111.316406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Finney LA, O'Halloran TV. Transition metal speciation in the cell: insights from the chemistry of metal ion receptors. Science. 2003;300:931–936. doi: 10.1126/science.1085049. [DOI] [PubMed] [Google Scholar]
- 13.Davis AV, O'Halloran TV. A place for thioether chemistry in cellular copper ion recognition and trafficking. Nat Chem Biol. 2008;4:148–151. doi: 10.1038/nchembio0308-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rubino JT, Franz KJ. Coordination chemistry of copper proteins: how nature handles a toxic cargo for essential function. J Inorg Biochem. 2012;107:129–143. doi: 10.1016/j.jinorgbio.2011.11.024. [DOI] [PubMed] [Google Scholar]
- 15.Changela A, Chen K, Xue Y, Holschen J, Outten CE, O'Halloran TV, Mondragon A. Molecular basis of metal-ion selectivity and zeptomolar sensitivity by CueR. Science. 2003;301:1383–1387. doi: 10.1126/science.1085950. [DOI] [PubMed] [Google Scholar]
- 16.Reyes-Caballero H, Campanello GC, Giedroc DP. Metalloregulatory proteins: metal selectivity and allosteric switching. Biophys Chem. 2011;156:103–114. doi: 10.1016/j.bpc.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang D, Hosteen O, Fierke CA. ZntR-mediated transcription of zntA responds to nanomolar intracellular free zinc. J Inorg Biochem. 2012;111:173–181. doi: 10.1016/j.jinorgbio.2012.02.008. Cell-based evidence that intracellular bioavailable zinc is considerably higher than proposed from known zinc affinities of zinc metalloregulatory proteins using a genetically encoded zinc sensor.
- 18.Irving H, Williams RJP. Order of stability of metal complexes. Nature. 1948;162:746–747. [Google Scholar]
- 19.Macomber L, Imlay JA. The iron-sulfur clusters of dehydratases are primary intracellular targets of copper toxicity. Proc Natl Acad Sci U S A. 2009;106:8344–8349. doi: 10.1073/pnas.0812808106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Djoko KY, McEwan AG. Antimicrobial action of copper is amplified via inhibition of heme biosynthesis. ACS Chem Biol. 2013;8:2217–2223. doi: 10.1021/cb4002443. [DOI] [PubMed] [Google Scholar]
- 21.Pericone CD, Park S, Imlay JA, Weiser JN. Factors contributing to hydrogen peroxide resistance in Streptococcus pneumoniae include pyruvate oxidase (SpxB) and avoidance of the toxic effects of the Fenton reaction. J Bacteriol. 2003;185:6815–6825. doi: 10.1128/JB.185.23.6815-6825.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jacobsen FE, Kazmierczak KM, Lisher JP, Winkler ME, Giedroc DP. Interplay between manganese and zinc homeostasis in the human pathogen Streptococcus pneumoniae. Metallomics. 2011;3:38–41. doi: 10.1039/c0mt00050g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Imlay JA, Chin SM, Linn S. Toxic DNA damage by hydrogen peroxide through the Fenton reaction in vivo and in vitro. Science. 1988;240:640–642. doi: 10.1126/science.2834821. [DOI] [PubMed] [Google Scholar]
- 24.Lemire JA, Harrison JJ, Turner RJ. Antimicrobial activity of metals: mechanisms, molecular targets and applications. Nat Rev Microbiol. 2013;11:371–384. doi: 10.1038/nrmicro3028. [DOI] [PubMed] [Google Scholar]
- 25.Letelier ME, Sánchez-Jofré S, Peredo-Silva L, Cortés-Troncoso J, Aracena-Parks P. Mechanisms underlying iron and copper ions toxicity in biological systems: pro-oxidant activity and protein-binding effects. Chem-Biol Interact. 2010;188:220–227. doi: 10.1016/j.cbi.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 26.Halliwell B, Gutteridge JMC. [1] Role of free radicals and catalytic metal ions in human disease: an overview. Methods Enzymol. 1990;186:1–85. doi: 10.1016/0076-6879(90)86093-b. [DOI] [PubMed] [Google Scholar]
- 27.Waldron KJ, Robinson NJ. How do bacterial cells ensure that metalloproteins get the correct metal? Nat Rev Microbiol. 2009;7:25–35. doi: 10.1038/nrmicro2057. [DOI] [PubMed] [Google Scholar]
- 28.Gold B, Deng H, Bryk R, Vargas D, Eliezer D, Roberts J, Jiang X, Nathan C. Identification of a copper-binding metallothionein in pathogenic mycobacteria. Nat Chem Biol. 2008;4:609–616. doi: 10.1038/nchembio.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li Y, Du J, Zhang P, Ding J. Crystal structure of human copper homeostasis protein CutC reveals a potential copper-binding site. J Struct Biol. 2010;169:399–405. doi: 10.1016/j.jsb.2009.10.012. [DOI] [PubMed] [Google Scholar]
- 30.Boal AK, Rosenzweig AC. Structural biology of copper trafficking. Chem Rev. 2009;109:4760–4779. doi: 10.1021/cr900104z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Corbett D, Schuler S, Glenn S, Andrew PW, Cavet JS, Roberts IS. The combined actions of the copper-responsive repressor CsoR and copper-metallochaperone CopZ modulate CopAmediated copper efflux in the intracellular pathogen Listeria monocytogenes. Mol Microbiol. 2011;81:457–472. doi: 10.1111/j.1365-2958.2011.07705.x. [DOI] [PubMed] [Google Scholar]
- 32.Banci L, Bertini I, Ciofi-Baffoni S, Kandias NG, Robinson NJ, Spyroulias GA, Su XC, Tottey S, Vanarotti M. The delivery of copper for thylakoid import observed by NMR. Proc Natl Acad Sci U S A. 2006;103:8320–8325. doi: 10.1073/pnas.0600142103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fu Y, Tsui HC, Bruce KE, Sham LT, Higgins KA, Lisher JP, Kazmierczak KM, Maroney MJ, Dann CE, 3rd, Winkler ME, et al. A new structural paradigm in copper resistance in Streptococcus pneumoniae. Nat Chem Biol. 2013;9:177–183. doi: 10.1038/nchembio.1168. Identification and structural characterization of a new cellular strategy to effect copper resistance through use of an unprecedented membrane-tethered Cu(I) chaperone (CupA) that is essential to cell viability under copper stress.
- 34.Dupont CL, Butcher A, Valas RE, Bourne PE, Caetano-Anollés G. History of biological metal utilization inferred through phylogenomic analysis of protein structures. Proc Natl Acad Sci U S A. 2010;107:10567–10572. doi: 10.1073/pnas.0912491107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tottey S, Patterson CJ, Banci L, Bertini I, Felli IC, Pavelkova A, Dainty SJ, Pernil R, Waldron KJ, Foster AW, et al. Cyanobacterial metallochaperone inhibits deleterious side reactions of copper. Proc Natl Acad Sci U S A. 2012;109:95–100. doi: 10.1073/pnas.1117515109. In vivo evidence that both Cu(I) chaperones and glutathione play important roles in metal trafficking within cyanobacteria and function to minimize misallocation of Cu(I) to adventitious sites in the cell.
- 36.Liu T, Ramesh A, Ma Z, Ward SK, Zhang L, George GN, Talaat AM, Sacchettini JC, Giedroc DP. CsoR is a novel Mycobacterium tuberculosis copper-sensing transcriptional regulator. Nat Chem Biol. 2007;3:60–68. doi: 10.1038/nchembio844. [DOI] [PubMed] [Google Scholar]
- 37.Festa RA, Jones MB, Butler-Wu S, Sinsimer D, Gerads R, Bishai WR, Peterson SN, Darwin KH. A novel copper-responsive regulon in Mycobacterium tuberculosis. Mol Microbiol. 2011;79:133–148. doi: 10.1111/j.1365-2958.2010.07431.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Higgins KA, Giedroc DP. Insights into protein allostery in the CsoR/RcnR family of transcriptional repressors. Chem Lett. 2014 doi: 10.1246/cl.130965. in press (doi:10.1246/cl.2014.130965) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tan BG, Vijgenboom E, Worrall JAR. Conformational and thermodynamic hallmarks of DNA operator site specificity in the copper sensitive operon repressor from Streptomyces lividans. Nucleic Acids Res. 2014 doi: 10.1093/nar/gkt902. in press (doi:10.1093/nar/gkt902) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chang FM, Lauber MA, Running WE, Reilly JP, Giedroc DP. Ratiometric pulse-chase amidination mass spectrometry as a probe of biomolecular complex formation. Anal Chem. 2011;83:9092–9099. doi: 10.1021/ac202154r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Osman D, Waldron KJ, Denton H, Taylor CM, Grant AJ, Mastroeni P, Robinson NJ, Cavet JS. Copper homeostasis in Salmonella is atypical and copper-CueP is a major periplasmic metal complex. J Biol Chem. 2010;285:25259–25268. doi: 10.1074/jbc.M110.145953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Shin J-H, Jung HJ, An YJ, Cho Y-B, Cha S-S, Roe J-H. Graded expression of zinc-responsive genes through two regulatory zinc-binding sites in Zur. Proc Natl Acad Sci U S A. 2011;108:5045–5050. doi: 10.1073/pnas.1017744108. Crystallographic and functional analysis of two distinct regulatory zinc sites in Streptomyces coelicolor Zur that may expand the range of Zn(II)-induced repression dictated by metallation state of the repressor.
- 43. Hao Z, Lou H, Zhu R, Zhu J, Zhang D, Zhao BS, Zeng S, Chen X, Chan J, He C, et al. The multiple antibiotic resistance regulator MarR is a copper sensor in Escherichia coli. Nat Chem Biol. 2014;10:21–28. doi: 10.1038/nchembio.1380. In vitro and in vivo evidence is provided that supports the hypothesis that Cu(II) is directly sensed by MarR as a general response to antibiotic stress.
- 44.Aliaga ME, López-Alarcón C, García-Río L, Martín-Pastor M, Speisky H. Redox-changes associated with the glutathione-dependent ability of the Cu(II)-GSSG complex to generate superoxide. Bioorg Med Chem. 2012;20:2869–2876. doi: 10.1016/j.bmc.2012.03.027. [DOI] [PubMed] [Google Scholar]
- 45.Liu Y, Imlay JA. Cell death from antibiotics without the involvement of reactive oxygen species. Science. 2013;339:1210–1213. doi: 10.1126/science.1232751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Keren I, Wu Y, Inocencio J, Mulcahy LR, Lewis K. Killing by bactericidal antibiotics does not depend on reactive oxygen species. Science. 2013;339:1213–1216. doi: 10.1126/science.1232688. [DOI] [PubMed] [Google Scholar]
- 47. Osman D, Patterson CJ, Bailey K, Fisher K, Robinson NJ, Rigby SEJ, Cavet JS. The copper supply pathway to a Salmonella Cu,Zn-superoxide dismutase (SodCII) involves P1B-type ATPase copper efflux and periplasmic CueP. Mol Microbiol. 2013;87:466–477. doi: 10.1111/mmi.12107. This study shows that a periplasmic copper binding protein plays a pivotal role in metallation of a periplasmic superoxide dismutase using intracellularly derived Cu(I) that is supplied by a copper effluxer.
- 48.Guerra AJ, Giedroc DP. Metal site occupancy and allosteric switching in bacterial metal sensor proteins. Arch Biochem Biophys. 2012;519:210–222. doi: 10.1016/j.abb.2011.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Padilla-Benavides T, McCann CJ, Argüello JM. The mechanism of Cu+ transport ATPases: interaction with Cu+ chaperones and the role of transient metal-binding sites. J Biol Chem. 2013;288:69–78. doi: 10.1074/jbc.M112.420810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Coudray N, Valvo S, Hu M, Lasala R, Kim C, Vink M, Zhou M, Provasi D, Filizola M, Tao J, et al. Inward-facing conformation of the zinc transporter YiiP revealed by cryoelectron microscopy. Proc Natl Acad Sci U S A. 2013;110:2140–2145. doi: 10.1073/pnas.1215455110. The combined use of cyroelectron microscopy, x-ray crystallography, and molecular dynamics simulations provides the most up-to-date and detailed mechanistic understanding of Zn(II) efflux by a cation diffusion facilitator (CDF) transporter.
- 51. Damo SM, Kehl-Fie TE, Sugitani N, Holt ME, Rathi S, Murphy WJ, Zhang Y, Betz C, Hench L, Fritz G, et al. Molecular basis for manganese sequestration by calprotectin and roles in the innate immune response to invading bacterial pathogens. Proc Natl Acad Sci U S A. 2013;110:3841–3846. doi: 10.1073/pnas.1220341110. Crystallographic evidence of an unprecedented hexa-histidine-coordinated manganese binding site in calprotectin and elucidation of a general role that Mn(II) sequestration by calprotectin likely plays at the host-pathogen interface.
- 52. Hayden JA, Brophy MB, Cunden LS, Nolan EM. High-affinity manganese coordination by human calprotectin is calcium-dependent and requires the histidine-rich site formed at the dimer interface. J Am Chem Soc. 2013;135:775–787. doi: 10.1021/ja3096416. A comprehensive quantitative analysis of the affinities and stoichiometries of the binding of Mn(II) to calprotectin and thermodynamic linkage of transition metal binding to calcium binding.
- 53.Moroz O, Wilson K, Bronstein I. The role of zinc in the S100 proteins: insights from the Xray structures. Amino Acids. 2011;41:761–772. doi: 10.1007/s00726-010-0540-4. [DOI] [PubMed] [Google Scholar]
- 54. Hood MI, Mortensen BL, Moore JL, Zhang Y, Kehl-Fie TE, Sugitani N, Chazin WJ, Caprioli RM, Skaar EP. Identification of an Acinetobacter baumannii zinc acquisition system that facilitates resistance to calprotectin-mediated zinc sequestration. PLoS Pathog. 2012;8:e1003068. doi: 10.1371/journal.ppat.1003068. An unbaised mutant screen carried out with the bacterial pathogen A. baumannii employing calprotectin-mediated zinc depletion leads to the discovery of a panel of Zn(II)-Zur regulated genes which functions beyond zinc uptake.
- 55. Stork M, Grijpstra J, Bos MP, Manas Torres C, Devos N, Poolman JT, Chazin WJ, Tommassen J. Zinc piracy as a mechanism of Neisseria meningitidis for evasion of nutritional immunity. PLoS Pathog. 2013;9:e1003733. doi: 10.1371/journal.ppat.1003733. Discovery of a new extracytoplasmic outer membrane-localized calprotectin-binding protein that is thought to allow bacteria to acquire zinc from bound CP-Zn(II) complexes.
- 56.Noinaj N, Easley NC, Oke M, Mizuno N, Gumbart J, Boura E, Steere AN, Zak O, Aisen P, Tajkhorshid E, et al. Structural basis for iron piracy by pathogenic Neisseria. Nature. 2012;483:53–58. doi: 10.1038/nature10823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu Janet Z, Jellbauer S, Poe AJ, Ton V, Pesciaroli M, Kehl-Fie TE, Restrepo Nicole A, Hosking MP, Edwards Robert A, Battistoni A, et al. Zinc sequestration by the neutrophil protein calprotectin enhances Salmonella growth in the inflamed gut. Cell Host Microbe. 2012;11:227–239. doi: 10.1016/j.chom.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Giedroc DP, Arunkumar AI. Metal sensor proteins: nature's metalloregulated allosteric switches. Dalton Trans. 2007;29:3107–3120. doi: 10.1039/b706769k. [DOI] [PubMed] [Google Scholar]
- 59. Campanello GC, Ma Z, Grossoehme NE, Guerra AJ, Ward BP, Dimarchi RD, Ye Y, Dann CE, 3rd, Giedroc DP. Allosteric inhibition of a zinc-sensing transcriptional repressor: insights into the arsenic repressor (ArsR) family. J Mol Biol. 2013;425:1143–1157. doi: 10.1016/j.jmb.2013.01.018. The first in-depth analysis of the structural versus dynamic contributions of allosteric coupling in a model zinc-sensing arsenic repressor (ArsR) protein placed in the context of new insights into the evolution of distinct metal-binding sites in this ubiquitous family of repressors.
- 60.Guerra AJ, Dann CE, 3rd, Giedroc DP. Crystal structure of the zinc-dependent MarR family transcriptional regulator AdcR in the Zn(II)-bound state. J Am Chem Soc. 2011;133:19614–19617. doi: 10.1021/ja2080532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chakravorty DK, Parker TM, Guerra AJ, Sherrill CD, Giedroc DP, Merz KM., Jr Energetics of zinc-mediated interactions in the allosteric pathways of metal sensor proteins. J Am Chem Soc. 2013;135:30–33. doi: 10.1021/ja309170g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ma Z, Gabriel SE, Helmann JD. Sequential binding and sensing of Zn(II) by Bacillus subtilis Zur. Nucleic Acids Res. 2011;39:9130–9138. doi: 10.1093/nar/gkr625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sydor AM, Liu J, Zamble DB. Effects of metal on the biochemical properties of Helicobacter pylori HypB, a maturation factor of [NiFe]-hydrogenase and urease. J Bacteriol. 2011;193:1359–1368. doi: 10.1128/JB.01333-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Haas C, Rodionov D, Kropat J, Malasarn D, Merchant S, de Crecy-Lagard V. A subset of the diverse COG0523 family of putative metal chaperones is linked to zinc homeostasis in all kingdoms of life. BMC Genomics. 2009;10:470. doi: 10.1186/1471-2164-10-470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Sydor AM, Jost M, Ryan KS, Turo KE, Douglas CD, Drennan CL, Zamble DB. Metal binding properties of Escherichia coli YjiA, a member of the metal homeostasis-associated COG0523 family of GTPases. Biochemistry. 2013;52:1788–1801. doi: 10.1021/bi301600z. Detailed bioinorganic analysis of Zn(II) binding properties of a putative COG0523 zinc chaperone.
- 66.Dainty S, Patterson C, Waldron K, Robinson N. Interaction between cyanobacterial copper chaperone Atx1 and zinc homeostasis. J Biol Inorg Chem. 2010;15:77–85. doi: 10.1007/s00775-009-0555-z. [DOI] [PubMed] [Google Scholar]
- 67.Badarau A, Basle A, Firbank SJ, Dennison C. Crosstalk between Cu(I) and Zn(II) homeostasis via Atx1 and cognate domains. Chem Commun. 2013;49:8000–8002. doi: 10.1039/c3cc42709a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sharma A, Gaidamakova EK, Matrosova VY, Bennett B, Daly MJ, Hoffman BM. Responses of Mn2+ speciation in Deinococcus radiodurans and Escherichia coli to gamma-radiation by advanced paramagnetic resonance methods. Proc Natl Acad Sci U S A. 2013;110:5945–5950. doi: 10.1073/pnas.1303376110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Tottey S, Waldron KJ, Firbank SJ, Reale B, Bessant C, Sato K, Cheek TR, Gray J, Banfield MJ, Dennison C, et al. Protein-folding location can regulate manganese-binding versus copper- or zinc-binding. Nature. 2008;455:1138–1142. doi: 10.1038/nature07340. A report establishing how the cellular compartment where a metalloprotein folds is able to dictate the binding of cognate metal over other, highly competitive metals. Detailed biochemical fractionation methodologies to discover metal-containing proteins are outlined in this report.
- 70. New EJ. Tools to study distinct metal pools in biology. Dalton Trans. 2013;42:3210–3219. doi: 10.1039/c2dt31933k. A review of available methodologies including small molecule and genetic metal sensors along with biophyscial and analytical approaches for examining different pools of metals in cells.