Abstract
Children with sickle cell disease (SCD) are repeatedly exposed to diagnostic radiation. We identified 938 children with SCD who had 9,246 radiographic tests. Mean number of tests/patient was 9.9 (95% CI: 8.9–10.9) over 8,817 patient-years. Mean rate was 1.5 tests/year (95% CI: 1.3–1.6). On average, a child with SCD will have 26.7 (95% CI: 24.1–29.3) radiographic tests by 18 years of age, and 5% will have ≥100 tests. Six percent have ≥3 CT scans, which may be associated with an increased risk of cancer. Strong consideration should be given to limiting the exposure of children with SCD to radiation.
Keywords: irradiation, radiation, radiograph, radiology, sickle cell disease
INTRODUCTION
Children with sickle cell disease (SCD) are repeatedly exposed to diagnostic radiation. Plain radiographs are frequently ordered for pain, and chest radiographs are often ordered for fever and respiratory symptoms [1–3]. Computed tomography (CT) and nuclear medicine scans are ordered for other suspected complications [1,2]. Each radiographic test exposes a child to ionizing or another form of radiation. Although evidence is limited and often extrapolated from survivors of atomic bombs and workers at nuclear power plants, exposure to 35 mSv, equivalent to three CT scans, increases the risk of cancer [4–6]. Recent studies provide epidemiological evidence for a direct link between diagnostic radiation and the development of cancer in children [7,8].
This risk engendered the ALARA (As Low As Reasonably Achievable) principles: there is no “safe” dose of radiation; the dose of diagnostic radiation should always be limited (decreasing dose/test and number of tests); and the risk-benefit ratio should always be optimized [9–11]. These principles are critical for growing children who are inherently more radiosensitive than adults [12]. Given the improving life expectancy and increasing use of hydroxyurea, a radio-sensitizing agent, in children with SCD [13–16], it is necessary to understand the magnitude of exposure to diagnostic radiation in this vulnerable population.
METHODS
We reviewed the medical records (1996–2009) of the Dallas Newborn Cohort [14,15] for the type, number and indication of all radiographic tests. Indications were considered inconsistent with our SCD center's guidelines if there was no specification of fever, erythema, edema, or effusions for plain radiographs of bony sites or respiratory signs/symptoms or thoracoabdominal pain for chest radiographs. Numbers of radiographs by age 18 years were projected from measured yearly rates. We identified patients who had ≥3 CT scans or >1 CT scan in the same calendar year. Fisher exact test was used to compare proportions. The IRB waived the requirement for informed consent.
RESULTS
We studied 938 SCD patients (52.8% male) with a mean follow-up of 9.4 years (range 0.1–20.6; 8,817 patient-years; Table I). Seven hundred eleven (76%) had at least one radiographic test. Patients with sickle cell anemia (HbSS) or sickle-β0-thalassemia (HbSβ0) were more likely to have had at least one radiographic test than sickle-hemoglobin C disease (HbSC) or sickle-β+-thalassemia (HbSβ+) patients (77% vs. 65%; P<0.001; Table I).
TABLE I.
Subjects and Radiographic Tests
| Diagnosis | Number of patients | Number of patients with ≥1 radiographic test (%) | |
|---|---|---|---|
| HbSS | 571 | 473 (83) |
 a
a
|
| HbSβ0 | 21 | 12 (57) | |
| HbSC | 283 | 187 (66) |
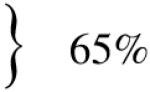 b
b
|
| HbSβ+ | 63 | 39 (62) | |
| Total | 938 | 711 (76) | |
| Type of study | Number of tests | ||
|
| |||
| Chest X-ray | 6,250 | ||
| X-ray of extremity | 1,287 | ||
| Abdominal plain film | 1,008 | ||
| Computerized tomography (CT) | 441 | ||
| CT head | 230 | ||
| CT abdomen/pelvis | 150 | ||
| CT face/orbits/sinuses | 26 | ||
| CT chest | 22 | ||
| CT neck/spine | 7 | ||
| CT extremity | 6 | ||
| Nuclear medicine studies | 108 | ||
| Fluoroscopy | 78 | ||
| Voiding cystourethrogram | 34 | ||
| Upper gastrointestinal series | 18 | ||
| Cerebral angiography | 13 | ||
| Barium enema | 5 | ||
| Panorex | 4 | ||
| Total | 9,246 | ||
HbSC, sickle-hemoglobin C disease; HbSβ0, sickle-β0-thalassemia; HbSβ+, sickle-β-thalassemia; HbSS, homozygous sickle cell anemia.
Percent of HbSS and HbSβ0 patients as a group who had at least one radiographic test;
percent of HbSC and HbSβ+ patients as a group who had at least one radiographic test.
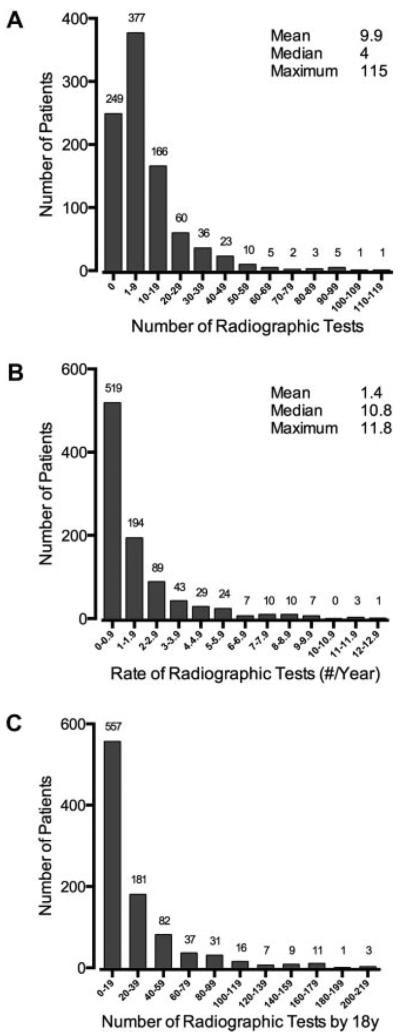
We identified 9,246 radiographic tests (Table I). The mean was 9.9 tests/patient (95% CI: 8.9–10.9; range 0–115; Fig. 1). Twenty-seven patients (2.9%) had ≥50 tests, and 2 (0.2%) had ≥100 tests during 9.4 years of mean follow-up. The mean rate of radiographic tests was 1.4/year (95% CI: 1.3–1.6; range 0–27.3). We estimate that a patient with SCD will be exposed to radiation from 26.7 (95% CI: 24.1–29.3; range 0–492.1) tests, on average, by 18 years of age. Approximately 5% of patients will be exposed to 100 or more radiographic tests during childhood. During the study period, the number and rate of radiographic tests increased by year (Supplemental Figure).
Fig. 1.
Number and rate of radiographic tests in patients with sickle cell disease. A: Histogram of number of radiographic tests/patient. B: Histogram of yearly rate of radiographic tests/patient calculated from the 13-year study interval (mean of 9.4 years of follow-up/patient). C: Histogram of the projected number of radiographic tests patients will receive by 18 years of age. For all panels, the number of patients in each bin is shown above the bar (e.g., 166 patients each had 10–19 radiographic tests), and population summary statistics are shown in the top right-hand corner of the graphs.
One hundred eighty-two patients (19.4%) had at least one CT scan (range 1–17) of a body region, and 56 (6.0%) had ≥3 CT scans each. Forty-five (4.8%) had >1 CT scans in the same calendar year (range 2–7/year). HbSS/HbSβ0 patients were more likely to have ≥1 CT scan than HbSC/HbSβ+ patients (23.8% vs. 11.8%; P<0.0001) and more likely to have ≥3 CT scans (8.1% vs. 2.3%; P<0.001).
The indication for 273 of 1,287 (21%) plain radiographs of a bony site was pain only. The indication was fever only for 581 of 6,250 chest radiographs (9%). Excluding the chest radiographs whose indications provided insufficient clinical details (e.g., “rule-out chest syndrome”; N=1,407), the indication was fever only for 581 of 4,843 (12%).
DISCUSSION
A typical child with SCD in our practice will have >25 radiographic tests by 18 years of age. Five percent will have >100 tests. These 18-year extrapolations may be low because the rate of tests increased during the study. Despite our institutional guidelines designed to limit diagnostic radiation, including only imaging sites of bony pain with atypical features [1] and not obtaining chest radiographs of febrile children without respiratory signs/symptoms or chest pain, 10–20% of radiographs had potentially inappropriate indications. Institutional practices regarding indications for radiographs in children with SCD vary [3], so children with SCD at other institutions might have higher exposures to diagnostic radiation.
Most tests were plain radiographs, which provide exposure to relatively low-dose radiation. However, there is no safe dose of radiation according to ALARA. A chest radiograph provides a relevant organ dose of 0.01–0.02 mSv depending on the age of the patient, whereas one CTof the chest at 10 mSv is equivalent to 500–1,000 chest radiographs. Six percent of our study population had three or more CT scans, a number associated with an increased risk of cancer in several studies [4–6]. About 5% had multiple CT scans in a calendar year.
Other chronically ill children are exposed to diagnostic radiation and, consequently, have an increased risk of cancer. Children with congenital heart disease had a lifetime cumulative radiation effective dose (RED) of 7.7 mSv and a lifetime attributable risk of cancer of 1 in 331 for females and 1 in 859 for males [17]. Children with cystic fibrosis had an average cumulative RED of 6.2 mSv during an 8.5-year period and an increased risk of radiation-induced cancer (0.02% for males, 0.07% for females) [18,19]. Children with Crohn's disease or ulcerative colitis received a mean RED of 26.6 and 10.5 mSv during an 8-year period [20]. Although these studies rely upon extrapolation from survivors of atomic bombs, recent studies provide evidence for a direct link between diagnostic radiation exposure and the development of cancer in children [7,8]. Given our findings, we propose that children with SCD are another high-risk population for excessive exposure to diagnostic radiation that could potentially increase the risk of cancer. Hydroxyurea therapy, a radio-sensitizing agent [21,22], could increase the risk of exposure to diagnostic radiation, but hydroxyurea could also decrease overall radiation exposure by reducing the frequency of SCD-related complications for which radiographs may be obtained.
Our study has several limitations. First, we likely underestimated the number of radiographic tests because this was a retrospective analysis that did not include tests performed at other facilities. Second, we extrapolated the cumulative, 18-year numbers of tests from annual rates because we did not have 18 years of follow-up for each patient, although a mean follow-up of 9.4 years/patient and a total of 8,817 patient-years of observation are substantial. Third, it was impractical to determine the RED, an index of radiation exposure to the body that considers both the tissues exposed and the type of irradiation, for 9,246 tests. This prevents us from quantifying the potential for any increased risk of cancer, directly comparing our findings to other populations, and studying the effects of diagnostic radiation on different organs (especially the eyes, breasts, thyroid and gonads, which are most radiosensitive).
In summary, children with SCD are frequently exposed to diagnostic radiation. There is no safe dose of radiation, so a justifiable indication is necessary for all radiographic tests. The frequency of radiographic tests should be minimized and the lowest reasonably achievable dose of radiation used for each test. Research is needed to understand the consequences of exposure to diagnostic radiation in this potentially vulnerable population, and practice guidelines are needed to limit judiciously the exposure of children with SCD to diagnostic radiation.
Supplementary Material
ACKNOWLEDGMENTS
We thank Drs. Timothy Blackburn and Jon Anderson from the Department of Radiology at the University of Texas Southwestern Medical Center for their assistance with the conduct of this study.
Grant sponsor: National Institutes of Health to GRB and CTQ; Grant number: U54-HL0705088
Footnotes
Additional Supporting Information may be found in the online version of this article.
Conflict of interest: Nothing to declare.
Author contribution: CLV collected and analyzed the data and wrote the manuscript. GRB analyzed the data and wrote the manuscript. CTQ designed the study, supervised the data collection, analyzed the data, and wrote the manuscript.
REFERENCES
- 1.Berger E, Saunders N, Wang L, et al. Sickle cell disease in children: Differentiating osteomyelitis from vaso-occlusive crisis. Arch Pediatr Adolesc Med. 2009;163:251–255. doi: 10.1001/archpediatrics.2008.545. [DOI] [PubMed] [Google Scholar]
- 2.Almeida A, Roberts I. Bone involvement in sickle cell disease. Br J Haematol. 2005;129:482–490. doi: 10.1111/j.1365-2141.2005.05476.x. [DOI] [PubMed] [Google Scholar]
- 3.Morris C, Vichinsky E, Styles L. Clinician assessment for acute chest syndrome in febrile patients with sickle cell disease: Is it accurate enough? Ann Emerg Med. 1999;34:64–69. doi: 10.1016/s0196-0644(99)70273-8. [DOI] [PubMed] [Google Scholar]
- 4.Hall EJ, Brenner DJ. Cancer risks from diagnostic radiology. Br J Radiol. 2008;81:362–378. doi: 10.1259/bjr/01948454. [DOI] [PubMed] [Google Scholar]
- 5.Brenner DJ, Doll R, Goodhead DT, et al. Cancer risks attributable to low doses of ionizing radiation: Assessing what we really know. Proc Natl Acad Sci U S A. 2003;100:13761–13766. doi: 10.1073/pnas.2235592100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brenner DJ. Extrapolating radiation-induced cancer risks from low doses to very low doses. Health Phys. 2009;97:505–509. doi: 10.1097/HP.0b013e3181ad7f04. [DOI] [PubMed] [Google Scholar]
- 7.Bartley K, Metayer C, Selvin S, et al. Diagnostic X-rays and risk of childhood leukaemia. Int J Epidemiol. 2010;39:1628–1637. doi: 10.1093/ije/dyq162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pearce MS, Salotti JA, Little MP, et al. Radiation exposure from CT scans in childhood and subsequent risk of leukaemia and brain tumours: A retrospective cohort study. Lancet. 2012;380:499–505. doi: 10.1016/S0140-6736(12)60815-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.The ALARA (as low as reasonably achievable) concept in pediatric CT intelligent dose reduction. Multidisciplinary conference organized by the Society of Pediatric Radiology. August 18–19, 2001. Pediatr Radiol. 2002;32:217–313. doi: 10.1007/s00247-002-0665-z.
- 10.Slovis TL. The ALARA concept in pediatric CT: Myth or reality? Radiology. 2002;223:5–6. doi: 10.1148/radiol.2231012100. [DOI] [PubMed] [Google Scholar]
- 11.Slovis TL. Children, computed tomography radiation dose, and the as low as reasonably achievable (ALARA) concept. Pediatrics. 2003;112:971–972. doi: 10.1542/peds.112.4.971. [DOI] [PubMed] [Google Scholar]
- 12.Brenner DJ, Hall EJ. Computed tomography: An increasing source of radiation exposure. N Engl J Med. 2007;357:2277–2284. doi: 10.1056/NEJMra072149. [DOI] [PubMed] [Google Scholar]
- 13.Ware RE. How I use hydroxyurea to treat young patients with sickle cell anemia. Blood. 2010;115:5300–5311. doi: 10.1182/blood-2009-04-146852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quinn CT, Rogers ZR, McCavit TL, et al. Improved survival of children and adolescents with sickle cell disease. Blood. 2010;115:3447–3452. doi: 10.1182/blood-2009-07-233700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Quinn CT, Rogers ZR, Buchanan GR. Survival of children with sickle cell disease. Blood. 2004;103:4023–4027. doi: 10.1182/blood-2003-11-3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heeney MM, Ware RE. Hydroxyurea for children with sickle cell disease. Hematol Oncol Clin North Am. 2010;24:199–214. doi: 10.1016/j.hoc.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ait-Ali L, Andreassi MG, Foffa I, et al. Cumulative patient effective dose and acute radiation-induced chromosomal DNA damage in children with congenital heart disease. Heart. 2010;96:269–274. doi: 10.1136/hrt.2008.160309. [DOI] [PubMed] [Google Scholar]
- 18.O'Reilly R, Ryan S, Donoghue V, et al. Cumulative radiation exposure in children with cystic fibrosis. Ir Med J. 2010;103:43–46. [PubMed] [Google Scholar]
- 19.de Gonzalez AB, Kim KP, Samet JM. Radiation-induced cancer risk from annual computed tomography for patients with cystic fibrosis. Am J Respir Crit Care Med. 2007;176:970–973. doi: 10.1164/rccm.200704-591OC. [DOI] [PubMed] [Google Scholar]
- 20.Peloquin JM, Pardi DS, Sandborn WJ, et al. Diagnostic ionizing radiation exposure in a population-based cohort of patients with inflammatory bowel disease. Am J Gastroenterol. 2008;103:2015–2022. doi: 10.1111/j.1572-0241.2008.01920.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Piver MS, Barlow JJ, Vongtama V, et al. Hydroxyurea: A radiation potentiator in carcinoma of the uterine cervix. A randomized double-blind study. Am J Obstet Gynecol. 1983;147:803–808. doi: 10.1016/0002-9378(83)90043-1. [DOI] [PubMed] [Google Scholar]
- 22.Piver MS, Barlow JJ, Vongtama V, et al. Hydroxyurea as a radiation sensitizer in women with carcinoma of the uterine cervix. Am J Obstet Gynecol. 1977;129:379–383. doi: 10.1016/0002-9378(77)90580-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.