Abstract
Human fetal exposure to valproic acid (VPA), a widely-used anti-epileptic and mood-stabilizing drug, leads to an increased incidence of behavioral and intellectual impairments including autism; VPA administration to pregnant rats and mice at gestational days 12.5 (E12.5) or E13.5 leads to autistic-like symptoms in the offspring and is widely used as an animal model for autism. We report here that this VPA administration protocol transiently increased both BDNF mRNA and BDNF protein levels 5–6-fold in the fetal mouse brain. VPA exposure in utero induced smaller increases in the expression of mRNA encoding the other neurotrophins, NT3 (2.5-fold) and NT4 (2-fold). Expression of the neurotrophin receptors, trkA, trkB and trkC were minimally affected, while levels of the low-affinity neurotrophin receptor, p75NTR, doubled. Of the nine 5′-untranslated exons of the mouse BDNF gene, only expression of exons I, IV and VI was stimulated by VPA in utero. In light of the well-established role of BDNF in regulating neurogenesis and the laminar fate of postmitotic neurons in the developing cortex, an aberrant increase in BDNF expression in the fetal brain may contribute to VPA-induced cognitive disorders by altering brain development.
Keywords: Autism spectrum disorder, Brain-derived neurotrophic factor, Brain development, Gene promoters, Neurotrophin, VPA
Introduction
Valproic acid (VPA) is a widely used anti-epileptic and mood-stabilizing drug; however, exposure to VPA in utero can adversely affect fetal brain development. Children of women taking VPA during pregnancy have an increased risk of congenital malformations and impaired cognitive function including reduced IQ and autism (Williams et al., 2001; Bromley et al., 2008; Meador et al., 2009; Ornoy et al., 2009). The critical time window for the induction of autistic symptoms occurs during the first trimester in humans (Surén et al. 2013) and gestational days 11 – 14 (E11 – 14) in rodents (Arndt et al., 2005; Ploeger et al., 2010), during which time the first neurons of the cerebral cortex are generated from proliferating neural progenitors and begin to differentiate into mature neurons. Consequently, a single administration of VPA to pregnant rats or mice during the second week of gestation has been extensively used as an animal model for autism (Rodier et al., al., 1997; Arndt et al., 2005; Murcia et al., 2005; Schneider and Przewlocki, 2005; Markram et al., 2007;Gogolla et al., 2009; Gandal et al., 2010; Roullet et al., 2010; Foley et al., 2012; Chomiak and Hu, 2013; and see Patterson, 2011), with the offspring exhibiting increased cortical excitation, learning and memory deficits as well as abnormal fear conditioning and social interactions (reviewed in Markram et al., 2007). Recently, mice exposed to VPA in utero were found to exhibit impaired communicative function, delayed auditory evoked potentials, and reduced γ-frequency phase locking factor, all of which are observed in autism (Gandal et al., 2010). The mechanism by which fetal VPA exposure leads to these endophenotypes is not known.
Several lines of evidence suggest that autism is associated with increased expression of the neurotrophin, brain-derived neurotrophic factor (BDNF) (Nelson et al., 2001; Miyazaki et al., 2004; Tsai, 2005; Connolly et al., 2006; Correia et al., 2010). While BDNF is required for normal brain function throughout the lifespan, enhanced BDNF signaling can sometimes be pathogenic. For example, adult transgenic mice overexpressing BDNF are prone to seizures (Binder et al., 2001) and elevation of BDNF enhances pain sensitivity (Merighi et al., 2008). Moreover, during early embryonic brain development, the cell cycle parameters of proliferating neuroblasts and the laminar fate of their progeny are highly sensitive to either increased or decreased BDNF signaling (Fukumitsu et al., 2006, Bartkowska et al., 2007). Thus, BDNF signaling must be tightly regulated to enable normal function in both the developing and adult nervous system.
Based on these considerations, we investigated the possibility that aberrant BDNF signaling mediates the effects of VPA on the developing brain by measuring not only BDNF mRNA and protein but also mRNAs encoding the BDNF receptor, trkB, the other neurotrophins, nerve growth factor (NGF), neurotrophin-3 (NT3) and neurotrophin-4 (NT4), as well as their receptors, using a VPA administration protocol that induces autistic behavior in mice and rats. Our results demonstrate that in utero VPA exposure increases expression of BDNF, and to a lesser extent, NT3 and NT4, in the fetal brain. Dysregulation of critical steps of prenatal cortical development occurring at the time of VPA exposure, which is predicted to result from increased BDNF signaling (Fukumitsu et al., 2006, Bartkowska et al., 2007), may contribute to postnatal behavioral and cognitive impairments.
Materials and methods
Animals
C57BL/6J mice were paired overnight; the day following the pairing was designated E0.5. Pregnant mice were injected at E12.5 with 400 mg/kg i.p. of VPA (sodium salt; Sigma-Aldrich, St. Louis, MO) in sterile phosphate-buffered saline (PBS) or PBS only. Administration of 600 mg/kg VPA resulted in an approximately 50% reduction in the number of viable fetuses 24 hr later (data not shown); consequently, the dose of VPA was limited to 400 mg/kg in this study. Fetal and maternal brains were removed 3, 6 or 24 hr after the injection.
All procedures using mice were carried out in compliance with the NIH Guide for the Care and Use of Laboratory Animals and the AVMA Guidelines on Euthanasia and were approved by the University of Maryland School of Medicine Institutional Animal Care and Use Committee.
Quantitative real-time polymerase chain reaction (qRT-PCR)
Whole fetal brains or 50 mg samples of maternal frontal cortex were agitated (600 Hz, 1 min) with 1 mm zirconia/silica beads (Biospec, Bartlesville, OK) in 1 ml of QIAzol lysis reagent (Qiagen, Germantown, MD). Total RNA was purified with RNeasy lipid tissue mini kit (Qiagen) including the on-column DNA digestion step. RNA (1 μg) was reverse-transcribed with Transcriptor High Fidelity (Roche, Indianapolis, IN). Primers were designed based on published genome sequences (NCBI, Gene database), except for the BDNF 5′-untranslated exons (I – IXa), which were obtained from Aid et al. (2007). Primers were synthesized by Integrated DNA Technologies (Coralville, IA, USA). Primer sequences and qRT-PCR parameters are provided in Appendix, Table A1. Gene expression for each target mRNA was normalized to GAPDH mRNA assayed in the same sample as described by Pfaffl (2001). mRNA levels in VPA-treated brains are reported relative to those in brains from mice that had received a PBS injection (1 = no effect of VPA).
BDNF ELISA
Each sample analyzed consisted of four pooled fetal brains from one pregnancy or 50 mg of the maternal frontal cortex; four different samples (4 pregnancies) were analyzed for BDNF protein expression at 3, 6, and 24 hrs after VPA administration. Tissue was placed in 400 μl or 1 ml of freshly prepared lysis buffer (Szapacs et al, 2004) containing 100 mM Pipes (pH 7.0), 500 mM NaCl, 0.2% Triton X-100, 0.1% NaN3, 2 mM EDTA-Na2•2H2O, 200 μM PMSF, 10 μM leupeptin, 0.3 μM aprotinin and 1 μM pepstatin (all reagents from Sigma-Aldrich), homogenized as described above and stored at −80°C. Total protein was assayed by the BCA method (Pierce, Rockford, IL). BDNF was assayed in triplicate by enzyme-linked immunosorbent assay (ELISA) following the manufacturer’s instructions (Promega, Madison, WI) omitting the optional acid pretreatment; bovine serum albumin (2%) was added to enhance sensitivity. Approximately 0.2 mg of fetal brain protein and 0.25 mg of maternal brain protein was loaded in each sample well.
Cell culture
Six fetal brains from one pregnancy obtained at E12.5 were placed in 1 ml of Liebowitz L15 medium without L-glutamine (Invitrogen, Carlsbad, CA), triturated with a narrow bore Pasteur pipette and suspended in Neurobasal medium supplemented with 2% B27, 1 mM l-glutamine and penicillin/streptomycin (Invitrogen). Cells were seeded at 3 x 105 cells/well in 24-well plates (0.5 ml/well) coated with poly-ornithine (Sigma-Aldrich) and laminin (Invitrogen). Two days after plating, cultures were treated for 7 hrs with VPA and lysed by addition of RNeasy lysis buffer (150 μl/well). Lysates from three wells in each plate were combined and processed for total RNA using an RNeasy mini kit (Qiagen). Four different cultures, each from a different pregnancy were analyzed for each VPA concentration.
Statistical analysis
In Figures 1, 2, 4, and 5B, each bar represents a different PCR reaction showing the VPA-induced change in mRNA expression relative to the PBS-injected controls ± sem (1 = no effect of VPA); asterisks indicate expression levels significantly different from control by t-test. In Figures 3 and 5A, BDNF mRNA or protein expression is shown relative to controls ± sem (1 = no effect of VPA); asterisks indicate expression levels significantly different from control by one-way ANOVA with pairwise multiple comparison by Tukey’s method. Unless otherwise stated, statistical analysis was performed using GraphPad InStat version 3.06 (GraphPad Software, San Diego, CA).
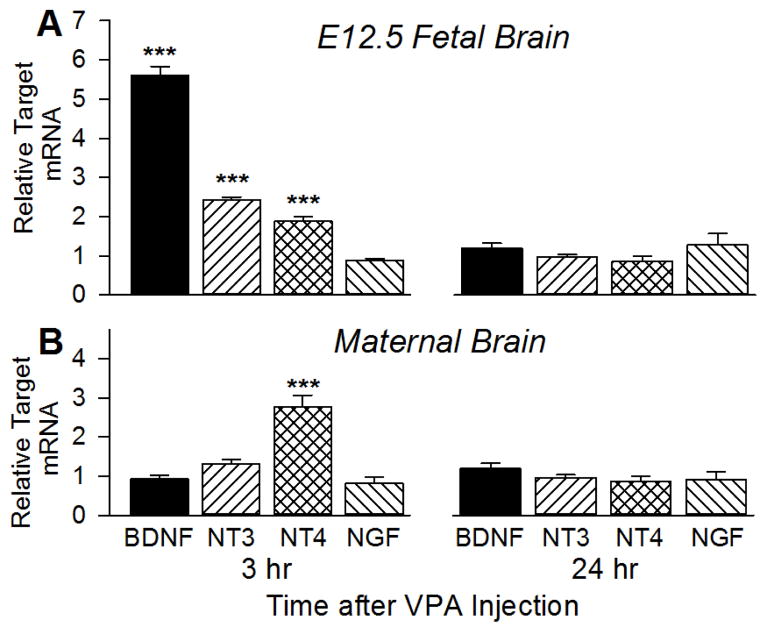
Fig. 1. VPA injection induced a transient increase in BDNF mRNA in the E12.5 fetal brain but not in the maternal brain.
Pregnant mice received VPA (400 mg/kg, i.p.) at E12.5. 3 or 24 hrs later, RNA was isolated from both whole fetal brain and maternal frontal cortex and reverse-transcribed for analysis of neurotrophin (and neurotrophin receptor) mRNA levels by qRT-PCR. mRNA levels in VPA-treated brains are reported relative to those in brains from mice that had received a PBS injection (1 = no effect of VPA). A. Relative BDNF levels in fetal brain increased 5.6-fold at 3 hrs and returned to control levels by 24 hr. NT3 and NT4 mRNA levels were also transiently increased 2.5- and 2-fold. For BDNF, 7 control (3 pregnancies) and 14 VPA-treated (3) fetal brains were analyzed at 3 hr; 7 control (3) and 10 VPA-treated (3) fetal brains were analyzed at 24 hr. For NT3, NT4 and NGF, 18 control (4) and 17 VPA-treated (4) fetal brains were analyzed at 3 hr; 13 control (3) and 14 VPA-treated (3) fetal brains were analyzed at 24 hr. ***, significantly different from PBS-injected control, p<.001. B. VPA exposure at E12.5 had no effect on neurotrophin mRNA levels in the maternal brain at either time except for NT4, which was increased about 2.5-fold at 3 hr. For BDNF, 3 control and 3 VPA-treated maternal brains were analyzed at 3 hr and at 24 hr. For NT3, NT4 and NGF, 4 control and 4 VPA-treated maternal brains were analyzed at 3 hr and 3 control and 3 VPA-treated maternal brains were analyzed at 24 hr. ***, significantly different from control, p<.001.
Figure 2. Effects of VPA on expression of neurotrophin receptors in E12.5 brain.

Data are expressed relative to the mRNA level in control (PBS treated) brains (1 = no effect of VPA). Same fetal brain samples that were analyzed for NGF/NT3/NT4 in Fig. 1. A. 3-hr treatment with 400 mg VPA/kg increased the expression of mRNAs encoding trkA (≈50%), trkB.FL (≈25%) and p75NTR (≈100%), while an approximately 20% decrease was observed for trkB.shc and trkC mRNAs; trkB.T1 mRNA expression was unchanged. Significantly different from control: *** =p<.001 and * = p<.05. B. Neurotrophin receptor mRNA expression in fetal brain 24 hrs after a single in utero VPA exposure (400 mg/kg) at E12.5. Levels of trkA, trkB.FL, and trkB.T1 mRNA were unaffected 24 hrs after VPA exposure, while trkB.shc, trkC and p75NTR mRNA levels remained suppressed by 20 – 25%. Significantly different from control: ** = p<.01 and * = p<.05.
Fig. 4. VPA injection stimulated expression of BDNF 5′-untranslated exons I, IV and VI in fetal brain.

Pregnant mice received VPA (400 mg/kg, i.p.) at E12.5. 3 hrs later, RNA was isolated from fetal brains and reverse transcribed for analysis of BDNF 5′-untranslated exons I – IXa mRNA expression by qRT-PCR. Only exon I-, IV- and VI-specific mRNAs were detected in fetal brain following VPA administration and expression of those 5′-untranslated exons was stimulated about 4-, 13- and 15-fold, respectively. Same fetal brain samples analyzed for NT3, NT4 and NGF in Fig. 1. ***, significantly different from PBS-injected control, p<.001, n = 14.
Fig. 5. VPA stimulated expression of BDNF mRNA (coding region, exon IX) (A) and 5′-untranslated exons I, II, IV and VI (B) in cultures of E12.5 fetal mouse brain.
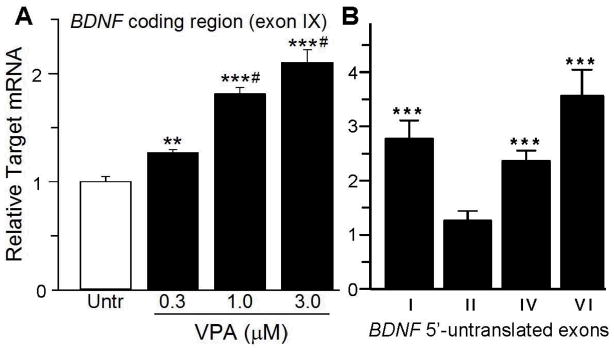
Brain cell cultures were prepared from fetal brain at E12.5 as described in Materials and Methods. Two days after plating, fetal mouse brain cultures were treated for 6 hr with 0.3, 1.0 or 3.0 mM (A) or 3 mM VPA (B) and then harvested for determination of BDNF coding region (exon IX) mRNA (A) or 5′-untranslated exon I, II, IV and VI mRNA (B) by qRT-PCR. A. Four independent cultures were analyzed for each VPA concentration; all three concentrations of VPA significantly stimulated exon IX mRNA expression. Data were analyzed by ANOVA: F(3,12) = 50 (p<.001). Statistical analysis was performed using SigmaPlot v.12.3 (Systat Software, Chicago, IL). ***, significantly different from untreated cultures (p<.001); **, significantly different from untreated cultures (p<.02); #, significantly different from 0.3 μM VPA (p<.01). B. Only exon I, II, IV and VI mRNAs were detected and of these only exons I, IV and VI mRNAs were stimulated by VPA. Four control and 4 VPA stimulated cultures were analyzed for each BDNF 5′ untranslated exon. ***, significantly different from untreated cultures.
Fig. 3. VPA injection induced a transient increase in BDNF protein in the E12.5 fetal brain but not in the maternal brain.
Pregnant mice received PBS (control) or VPA (400 mg/kg, i.p.) at E12.5. 3, 6 or 24 hrs later, fetal and maternal brains were removed and BDNF protein expression was quantified by ELISA. Each sample analyzed consisted of 4 pooled fetal brains from one pregnancy. Four separate samples (4 pregnancies) plus a sample of each maternal frontal cortex were analyzed ± VPA at each time point; i.e., a total of 16 fetuses and 4 dams were analyzed ± VPA at each time point. BDNF protein levels in VPA-treated brains are reported relative to those in brains from mice that had received a PBS injection (1 = no effect of VPA). Left. BDNF levels had increased 6-fold in fetal brains by 6 hr after VPA treatment following a delay of at least 3 hr and returned to slightly below unstimulated levels by 24 hr. Note that the level of BDNF mRNA was elevated 3 hr following VPA administration (Fig. 1). Right. Consistent with BDNF mRNA levels, there was no increase in BDNF protein expression in maternal brains following VPA injection (right). BDNF protein levels in fetal and maternal brains exposed to PBS were 11.7 ± 3.3 and 24.1 ± 2.8 pg/mg total protein, respectively. Data were analyzed by ANOVA; F(2,9) = 200 (p<.001) for fetal brain and F(2,9) = 22 (p<.001) for maternal brain. ***, significantly different from PBS-injected control, p<.001. Statistical analysis was performed with SigmaPlot v.12.3 (Systat Software, Chicago, IL).
Results
In utero VPA exposure stimulates BDNF expression in fetal brain
We investigated whether in utero exposure to VPA can modulate neurotrophin expression in the fetal brain. A single i.p. injection of VPA (400 mg/kg) to pregnant females induced a 5.6-fold increase in BDNF exon IX mRNA expression in the fetal brain as determined by qRT-PCR (Fig. 1A). Exon IX encodes the proBDNF protein, which is enzymatically processed to the mature, biologically active form subsequent to secretion. The BDNF primer pair used in Figure 1 (Table A1) amplified a sequence entirely within the mature BDNF coding sequence. Virtually identical results were obtained using a primer pair that amplified the N-terminal sequence, which is cleaved from proBDNF in the process of generating mature BDNF (data not shown). BDNF exon IX mRNA fell to baseline levels by 24 hr. Smaller (2–2.5-fold) transient increases in NT3 and NT4 expression were also observed in the fetal brain 3 hr after VPA administration, while NGF expression was unaffected at either time. Rapid metabolism of VPA in the mother (serum half-life in adult mouse ≈ 1 hr) (Nau and Löscher, 1982) may account for the transient nature of the increase in fetal neurotrophin mRNAs.
We also measured the expression of BDNF mRNA in the frontal cortex of the VPA-injected pregnant females. Surprisingly, BDNF mRNA expression in the maternal brain was not stimulated by VPA at either 3 or 24 hr (Fig 1B); however, expression of NT4, which, like BDNF, activates trkB, was increased 2.8-fold in the maternal brain at 3 hr after VPA administration. Thus, the profile of neurotrophin mRNA responses to administration of VPA at E12.5 is different in fetal brain and maternal frontal cortex (Fig. 1). We did not conduct a regional study of BDNF mRNA expression in either the fetal or the maternal brain and it remains possible that VPA may induce BDNF expression in areas of the maternal brain other than the frontal cortex.
We also determined the effects of in utero VPA exposure on levels of mRNAs encoding the full-length, catalytically-active neurotrophin receptors, trkA, trkB, and trkC, the dominant-negative, truncated trkB isoforms, trkB.T1 and trkB.shc, as well as the low-affinity, non-specific neurotrophin receptor, p75NTR, in the fetal brain (Fig. 2). At 3 hr, VPA exposure had induced only modest increases (<50%; trkA, trkB.FL) or decreases (<25%; trkB.shc; trkC) in the trk receptors; interestingly, p75NTR expression had doubled. As p75NTR mediates neurotrophin signaling independently of the trk receptors (Schor, 2005), enhanced p75NTR signaling may also contribute to the effects of VPA on the fetal brain; however, the role of p75NTR signaling in brain development has not been studied. By 24 hr, increases in neurotrophin receptor mRNA levels had returned to levels at or slightly below baseline (Fig. 2B).
VPA increases expression of BDNF protein in fetal brain
To determine if the up-regulation of BDNF mRNA by in utero VPA exposure (Fig. 1A) is accompanied by an increase in BDNF protein expression, we assayed total BDNF protein by ELISA in the fetal and maternal brains. There was no increase in BDNF levels in the fetal brains 3 hr after VPA administration but BDNF protein levels had increased 6-fold at 6 hr after VPA administration and returned to control levels by 24 hr (Fig. 3). Thus, the transient VPA-induced increase in BDNF mRNA precedes the corresponding rise in BDNF protein by about 3 hr in the fetal brain. As expected from the results of BDNF mRNA determination (Fig. 1B), VPA injection did not stimulate expression of BDNF protein in the maternal brain (Fig. 3).
5′-untranslated BDNF exons mediating the effects of VPA
BDNF expression is regulated by alternative splicing of one or more 5′-untranslated exons, each of which has its own unique promoter. In the mouse and rat, there are nine 5′-untranslated exons (I – IXa) plus a single common exon (IX) which encodes proBDNF protein (Aid et al., 2007). To investigate the mechanism of VPA-induced BDNF mRNA expression, we used qRT-PCR to measure expression of the nine 5′-untranslated exons of the mouse BDNF gene in the same fetal brain samples used to analyze NGF, NT3 and NT4 expression in Fig. 1A. Whereas all nine exons were detected in adult mouse brain by qRT-PCR (not shown), only BDNF exon I, IV and VI expression was detected in the fetal brains. VPA stimulated BDNF exons I, IV and VI expression about 4-, 12.5- and 15-fold, respectively (Fig. 4). These data suggest that the VPA-induced increase in BDNF expression probably results from activation of BDNF exons I, IV and/or VI.
VPA stimulates BDNF mRNA expression in brain cell cultures
Because VPA is rapidly metabolized in vivo and some of its metabolites might be biologically active (Nau and Löscher, 1982), we investigated whether VPA induces BDNF mRNA expression in a cell culture system where there is no drug metabolism by the maternal liver. Cultures were prepared from E12.5 mouse brain; after 2 days in vitro (DIV) cultures were stimulated with VPA (0.3 – 3 mM) for 7 hours. This concentration range was examined because it spans the circulating VPA levels of patients undergoing VPA treatment (Löscher, 1981). RNA was reverse-transcribed and analyzed by qRT-PCR. VPA stimulated expression of mRNA encoding the BDNF protein (exon IX) with a 2-fold increase observed at 3 mM, the highest concentration tested (Fig. 5A). We also assayed the expression of BDNF 5′-untranslated exons I – IXA in the cultures. Only exons I, II, IV and VI were detectable. VPA (3 mM) induced a 2 – 4-fold stimulation of BDNF exons I, IV and VI, while exon II was not affected (Fig. 5B). Thus, as was observed in the fetal brain in vivo (Fig. 4), VPA stimulates BDNF gene expression via exons I, IV and VI in fetal brain cultures.
Discussion
Regulation of fetal brain development by BDNF
The development of the fetal cortex is tightly regulated by BDNF signaling. For example, BDNF injected into mouse cerebral ventricles in utero led to precocious neurogenesis with a shorter S-phase, premature exit from the cell cycle of the proliferating neuroblasts and altered laminar fate of the postmitotic cortical neurons (Fukumitsu et al., 2006). Abnormal precursor proliferation and cortical development was also observed by Bartkowska et al. (2007), who used an in utero electroporation approach to either increase or decrease BDNF signaling in the embryonic mouse brain. It should be noted that BDNF expression levels are normally low in the fetal brain and gradually increase to a maximum in the adult (Maisonpierre et al., 1990), however, a rapid, transient increase in BDNF in the fetal brain is clearly abnormal. Thus, a transient VPA-induced increase in BDNF expression in the fetal brain of the magnitude we observed (Figs. 1a and 3) would be expected to both alter the proliferation and differentiation of neural progenitors and modify the laminar fate of their progeny, regardless of the levels achieved in the normal postnatal and adult brain. Such errors in neurogenesis may leave a “signature” that is not corrected during subsequent cortical development (Ben-Ari, 2008), thereby leading to permanent connectivity defects that could impair cognition and behavior in the postnatal animal.
Other modulators of brain development may be regulated by VPA
In this study, we focused on expression of the neurotrophins and their receptors and found that BDNF is the principal neurotrophin target of VPA in the fetal brain. However, BDNF is not the only potential modulator of brain development affected by VPA. For example, as revealed in Fig. 1A, NT3 and NT4 mRNA levels are also elevated, albeit to lesser extents than BDNF. NT3 acts at a different neurotrophin receptor (trkC) than do BDNF and NT4 (trkB), but little is known about its role in regulating fetal brain development. Thus, effects of VPA on the developing fetal brain could be due to the combined actions of elevated BDNF, NT3 and NT4. Further conclusions regarding NT3 and NT4 must await protein measurements as was done for BDNF (Fig. 3).
Factors other than neurotrophins may also mediate the effects of VPA on brain development. For example, mRNA encoding the homeotic gene, Hoxa1, is elevated in rat embryos after in utero VPA treatment (Stodgell et al., 2006); increased HoxA1 expression has the potential to alter multiple developmental processes in the fetal brain, particularly during critical windows of vulnerability. Prenatal VPA exposure in mice has also been reported to reduce expression of the postsynaptic cell-adhesion molecule, neuroligin-3 (NLGN3), mutations in which have been reported in patients with autism (Kolozsi et al., 2009). Thus, exposure to VPA alters the expression of multiple genes encoding critical regulators of brain development.
Although the findings reported here and by others (Stodgell et al., 2006; Kolozsi et al., 2009) suggest plausible mechanisms for the effects of in utero exposure to VPA on brain development, they do not, by themselves, demonstrate that altered expression of a single gene product causes the developmental defects. The degree to which altered expression of any one gene affects cortical neurogenesis and, consequently, cognition and behavior, could be studied using in utero viral-mediated siRNA gene silencing strategies (c.f., Bartkowska et al., 2007) or, for BDNF, using transgenic mice with genetically modified trkB, which is sensitive to inhibition by systemically-administered pharmacological blockers (Chen et al., 2005).
Possible mechanism of VPA-induced stimulation of BDNF expression
Because VPA is a histone deacetylase (HDAC) inhibitor, our results raise the possibility that stimulation of BDNF transcription may be due to increased acetylation of histones associated with the promoters of 5′-untranslated exons I, IV and/or VI (Fig. 4). Indeed, histone modifications at one or more of these BDNF promoters have been reported in adult animal models of epilepsy and depression (Huang et al., 2002; Tsankova et al., 2006) and stimulation of BDNF exon IV expression by VPA in neuron cultures has been attributed to HDAC inhibition (Yasuda et al., 2009). Our finding that expression of exons I, IV and VI is stimulated in fetal brain by administration of VPA to the pregnant dam (Fig. 4) and by direct application of VPA to fetal brain cultures (Fig. 5B), raises the possibility that epigenetic modulation of these exons is also important for normal and pathological brain development. Use of fetal brain cultures (Fig. 5), in which responses to VPA were observed to be similar to those in fetal brain in vivo (Fig. 4), may facilitate determination of the extent to which the promoters of exons I, IV and VI are modulated histone acetylation.
Genes versus environment
Recent research has linked genetic variants of a number of genes to autism and several of those genes are known to play a role in either brain development or synaptic function (Abrahams and Geschwind, 2008; State and Levitt, 2011; Berg and Geschwind, 2012). Those findings are not incompatible with studies demonstrating that environmental influences such as in utero VPA exposure can also lead to autistic behavior. Indeed, as has been previously suggested (Arndt et al., 2005), a combination of genetic and environmental influences may act synergistically to produce autism as has recently been discussed for schizophrenia (Lipina et al. 2013; Kannan et al. 2013), affective disorders (Renoir et al. 2013) and pre-term births (Cha et al. 2013). The results of the present study and others (Stodgell et al., 2006; Kolozsi et al., 2009) demonstrate that environmental stressors such as VPA can alter the expression of genes that are critical for normal brain development. Such stressors may be more likely to induce autistic symptoms on a background of inherited genetic abnormalities.
Acknowledgments
This work was supported by Ruth L. Kirschstein National Research Service Award (NRSA) 5T32DE007309-12 (LEFA) and NIH grants R01NS048095 (BKK) and R01HD067135 (BKK and E.M. Powell). The authors would like to thank Drs. M. Bond, S. G. Dorsey, A. Keller, T. J. Kingsbury and E.M. Powell (University of Maryland Baltimore) for their critiques of the manuscript.
Abbreviations
- BDNF
brain-derived neurotrophic factor
- ELISA
enzyme-linked immunosorbent assay
- NGF
nerve growth factor
- NT3
neurotrophin-3
- NT4
neurotrophin-4
- trkB
tropomyosin-related kinase B
- VPA
valproic acid
Appendix
Table A.1.
List of primers used in qRT-PCR experiments. All primers were designed using the NCBI web site, except for primers amplifying BDNF exons, which were obtained from Aid et al., (2007). Primers were synthesized by Integrated DNA Technologies (Coralville, IA, USA). The efficiency (Eff) for each primer pair is also indicated.
| BDNF Exon IX (coding region, mature protein) (Eff = 2.0) | |
| Forward | AAAGTCCCGGTATCCAAAGGCCAA |
| Reverse | TAGTTCGGCATTGCGAGTTCCAGT |
| BDNF All 5′ non-coding exons | |
| Reverse | GAAGTGTACAAGTCCGCGTCCTTA |
| BDNF Exon I (Eff = 2.1) | |
| Forward | GTGTGACCTGAGCAGTGGGCAAAGGA |
| BDNF Exon II (Eff = 2.0) | |
| Forward | GGAAGTGGAAGAAACCGTCTAGAGCA |
| BDNF Exon III (Eff = 2.0) | |
| Forward | GCTTTCTATCATCCCTCCCCGAGAGT |
| BDNF Exon IV (Eff = 2.0) | |
| Forward | CTCTGCCTAGATCAAATGGAGCTTC |
| BDNF Exon V (Eff = 1.9) | |
| Forward | CTCTGTGTAGTTTCATTGTGTGTTC |
| BDNF Exon VI (Eff = 1.8) | |
| Forward | GCTGGCTGTCGCACGGTTCCCATT |
| BDNF Exon VII (Eff = 2.1) | |
| Forward | CCTGAAAGGGTCTGCGGAACTCCA |
| BDNF Exon VIII (Eff = 1.9) | |
| Forward | GTGTGTGTCTCTGCGCCTCAGTGGA |
| BDNF Exon IXa (Eff = 2.0) | |
| Forward | CCCAAAGCTGCTAAAGCGGGAGGAAG |
| NGF (Eff = 2.0) | |
| Forward: | CCAAGGACGCAGCTTTCTATAC |
| Reverse: | CTGCCTGTACGCCGATCAAAA |
| NT3 (Eff = 2.0) | |
| Forward: | GGAGTTTGCCGGAAGACTCTC |
| Reverse: | GGGTGCTCTGGTAATTTTCCTTA |
| NT4 (Eff = 1.9) | |
| Forward: | TGAGCTGGCAGTATGCGAC |
| Reverse: | CAGCGCGTCTCGAAGAAGT |
| trkA (Eff = 2.2) | |
| Forward | CCTTCCGTTTCACCCCTCGGC |
| Reverse | GGTCAGGTCCTGTAGGGAGAGGC |
| trkB.FL (Eff = 2.0) | |
| Forward | TTTCCTTGCCGAGTGCTACAACCT |
| Reverse | TGAAAGTCCTTGCGTGCATTGTCG |
| trkB.T1 (Eff = 1.9) | |
| Forward | ATAAGATCCCACTGGATGGG |
| Reverse | CGTATAGTCAAACAGCTCGC |
| trkB.shc (Eff = 2.0) | |
| Forward | GGAATGACCAAGATTCCTGTTATTGAA |
| Reverse | GAACCTCTGGGCCATGTGTCT |
| trkC (Eff = 2.0) | |
| Forward | CTGAGTGCTACAATCTAAGCCC |
| Reverse | CACACCCCATAGAACTTGACAAT |
| p75NTR (Eff = 1.9) | |
| Forward | CTAGGGGTGTCCTTTGGAGGT |
| Reverse | CAGGGTTCACACACGGTCT |
| GAPDH (EFF = 1.9) | |
| Forward | TGATGACATCAAGAAGGTGGTGAAG |
| Reverse | TCCTTGGAGGCCATGTAGGCCAT |
Footnotes
The authors declare no conflicts of interest.
References
- Abrahams BS, Geschwind DH. Advances in autism genetics: on the threshold of a new neurobiology. Nat Rev Genet. 2008;9:341–355. doi: 10.1038/nrg2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aid T, Kazantseva A, Piirsoo M, Palm K, Timmusk T. Mouse and rat BDNF gene structure and expression revisited. J Neurosci Res. 2007;85:525–535. doi: 10.1002/jnr.21139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arndt TL, Stodgell CJ, Rodier PM. The teratology of autism. Int J Devl Neurosci. 2005;23:189–199. doi: 10.1016/j.ijdevneu.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Bartkowska K, Paquin A, Gauthier AS, Kaplan DR, Miller FD. Trk signaling regulates neural precursor cell proliferation and differentiation during cortical development. Development. 2007;134:4369–4380. doi: 10.1242/dev.008227. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y. Neuro-archaeology: pre-symptomatic architecture and signature of neurological disorders. Trends Neurosci. 2008;31:626–636. doi: 10.1016/j.tins.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Berg JM, Geschwind DH. Autism genetics: searching for specificity and convergence. Genome Biology. 2012;13:247–263. doi: 10.1186/gb-2012-13-7-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder DK, Croll SD, Gall CM, Scharfman HE. BDNF and epilepsy: too much of a good thing? Trends Neurosci. 2001;24:47–53. doi: 10.1016/s0166-2236(00)01682-9. [DOI] [PubMed] [Google Scholar]
- Bromley RL, Mawer G, Clayton-Smith J, Baker GA. Autism spectrum disorders following in utero exposure to antiepileptic drugs. Neurology. 2008;71:1923–1924. doi: 10.1212/01.wnl.0000339399.64213.1a. [DOI] [PubMed] [Google Scholar]
- Cha J, Bartos A, Egasira M, Hafraguchi H, Saito-Fugita T, Leishman E, Bradshaw G, Dey SK, Hirota Y. Combinatory approaches prevent preterm birth profoundly exacerbated by gene-environment interactions. J Clin Invest. 2013;123:4063–4075. doi: 10.1172/JCI70098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Ye H, Kuruvilla R, Ramanan N, Scangos KW, Zhang C, Johnson NM, England PM, Shokat KM, Ginty DD. A chemical-genetic approach to studying neurotrophin signaling. Neuron. 2005;46:13–21. doi: 10.1016/j.neuron.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Chomiak T, Hu B. Alterations of neocortical development and maturation in autism: Insight from valproic acid exposure and animal models of autism 2013. Neurotoxicol Teratol. 2013;36:57–66. doi: 10.1016/j.ntt.2012.08.005. [DOI] [PubMed] [Google Scholar]
- Connolly AM, Chez M, Streif EM, Keeling RM, Golumbek PT, Kwon JM, Riviello JJ, Robinson RG, Neuman RJ, Deuel RM. Brain-derived neurotrophic factor and autoantibodies to neural antigens in sera of children with autistic spectrum disorders, Landau-Kleffner syndrome, and epilepsy. Biol Psychiat. 2006;59:354–363. doi: 10.1016/j.biopsych.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Correia CT, Coutinho AM, Sequeira AF, Sousa IG, Lourenco Venda L, Almeida JP, Abreu RL, Lobo C, Miguel TS, Conroy J, Cochrane L, Gallagher L, Gill M, Ennis S, Oliveira GG, Vicente AM. Increased BDNF levels and NTRK2 gene association suggest a disruption of BDNF/TrkB signaling in autism. Genes Brain Behav. 2010;9:841–848. doi: 10.1111/j.1601-183X.2010.00627.x. [DOI] [PubMed] [Google Scholar]
- Foley AG, Gannon S, Rombach-Mullan N, Prendergast A, Barry C, Cassidy AW, Regan CM. Class I histone deacetylase inhibition ameliorates social cognition and cell adhesion molecule plasticity deficits in a rodent model of autism spectrum disorder. Neuropharmacol. 2012;63:750–760. doi: 10.1016/j.neuropharm.2012.05.042. [DOI] [PubMed] [Google Scholar]
- Fukumitsu H, Ohtsuka M, Murai R, Nakamura H, Itoh K, Furukawa S. Brain-derived neurotrophic factor participates in determination of neuronal laminar fate in the developing mouse cerebral cortex. J Neurosci. 2006;26:13218–13230. doi: 10.1523/JNEUROSCI.4251-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandal MJ, Edgar JE, Ehrlichman RS, Mehta M, Roberts TPL, Siegel SJ. Validating γ oscillations and delayed auditory responses as translational biomarkers of autism. Biol Psychiat. 2010;68:1100–1106. doi: 10.1016/j.biopsych.2010.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Doherty JJ, Dingledine R. Altered histone acetylation at glutamate receptor 2 and brain-derived neurotrophic factor genes is an early event triggered by status epilepticus. J Neurosci. 2002;22:8422–8428. doi: 10.1523/JNEUROSCI.22-19-08422.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannan G, Sawa A, Pletnikov Mouse models of gene-environment interactions in schizophrenia. Neurobiol Dis. 2013;57:5–11. doi: 10.1016/j.nbd.2013.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolozsi E, Mackenzie RN, Roullet FI, Decatanzaro D, Foster JA. Prenatal exposure to valproic acid leads to reduced expression of synaptic adhesion molecule neuroligin 3 in mice. Neurosci. 2009;163:1201–1210. doi: 10.1016/j.neuroscience.2009.07.021. [DOI] [PubMed] [Google Scholar]
- Lipina TV, Zai C, Hlousek D, Roder JC, Wong AHC. Maternal immune activation during gestation interacts with Disc1 point mutation to exacerbate schizophrenia-related behaviors in mice. J Neurosci. 2013;3:7654–7666. doi: 10.1523/JNEUROSCI.0091-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löscher W. Concentration of metabolites of valproic acid in plasma of epileptic patients. Epilepsia. 1981;22:169–78. doi: 10.1111/j.1528-1157.1981.tb04098.x. [DOI] [PubMed] [Google Scholar]
- Maisonpierre PC, Belluscio L, Friedman B, Alderson RF, Wiegand SJ, Furth ME, Lindsay RM, Yancopoulos GD. NT-3, BDNF, and NGF in the developing rat nervous system: Parallel as well as reciprocal patterns of expression. Neuron. 1990;5:501–509. doi: 10.1016/0896-6273(90)90089-x. [DOI] [PubMed] [Google Scholar]
- Markram H, Rinaldi T, Markram K. The Intense World Syndrome: an alternative hypothesis for autism. Frontiers Neurosci. 2007;1:77–96. doi: 10.3389/neuro.01.1.1.006.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meador KJ, Baker GA, Browning N, Clayton-smith J, Combs-Cantrell DT, Cohen M, Kalayjian LA, Kanner A, Liporace JD, Pennell PB, Privitera M, Loring DW. Cognitive Function at 3 Years of Age after Fetal Exposure to Antiepileptic Drugs. N Eng J Med. 2009;360:1597–1605. doi: 10.1056/NEJMoa0803531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merighi A, Salio C, Ghirri A, Lossi L, Ferrini F, Betelli C, Bardoni R. BDNF as a pain modulator. Prog Neurobiol. 2008;85:297–317. doi: 10.1016/j.pneurobio.2008.04.004. [DOI] [PubMed] [Google Scholar]
- Miyazaki K, Narita N, Sakuta R, Miyahara T, Naruse H, Okado N, Narita M. Serum neurotrophin concentrations in autism and mental retardation: a pilot study. Brain Dev. 2004;26:292–295. doi: 10.1016/S0387-7604(03)00168-2. [DOI] [PubMed] [Google Scholar]
- Murcia CL, Gulden F, Herrup K. A question of balance: a proposal for new mouse models of autism. Int J Devl Neurosci. 2005;23:265–275. doi: 10.1016/j.ijdevneu.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Nau H, Löscher W. Valproic acid: Brain and plasma levels of the drug and its metabolites, anticonvulsant effects and γ-aminobutiric acid (GABA) metabolism in the mouse. J Pharmacol Exp Therapeu. 1982;220:654–659. [PubMed] [Google Scholar]
- Nelson KB, Grether JK, Croen LA, Dambrosia JM, Dickens BF, Jelliffe LL, Hansen RL, Phillips TM. Neuropeptides and neurotrophins in neonatal blood of children with autism or mental retardation. Ann Neurol. 2001;49:597–606. [PubMed] [Google Scholar]
- Ornoy A. Valproic acid in pregnancy: how much are we endangering the embryo and fetus? Reprod Toxicol. 2009;28:1–10. doi: 10.1016/j.reprotox.2009.02.014. [DOI] [PubMed] [Google Scholar]
- Patterson PH. Modeling autistic features in animals. Ped Res. 2011;69:34R–40R. doi: 10.1203/PDR.0b013e318212b80f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nuc Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ploeger A, Rajimakers ME, van der Maas HL, Galis F. The association between autism and errors in early embryogenesis: what is the causal mechanism? Biol Psychiat. 2010;67:602–607. doi: 10.1016/j.biopsych.2009.10.010. [DOI] [PubMed] [Google Scholar]
- Renoir T, Pang TY, Hannan AJ. Effects of environmental manipulations in genetically targeted animal models of affective disorders. Neurobiol Dis. 2013;57:12–27. doi: 10.1016/j.nbd.2012.04.003. [DOI] [PubMed] [Google Scholar]
- Rodier PM, Ingram JL, Tisdale B, Croog VJ. Linking etiologies in humans and animal models: studies of autism. Reprod Toxicol. 1997;11:417–422. doi: 10.1016/s0890-6238(97)80001-u. [DOI] [PubMed] [Google Scholar]
- Roullet FI, Wollaston L, deCatanzaro D, Foster JA. Behavioral and molecular changes in the mouse in response to prenatal exposure to the anti-epileptic drug valproic acid. Neurosci. 2010;170:514–522. doi: 10.1016/j.neuroscience.2010.06.069. [DOI] [PubMed] [Google Scholar]
- Schneider T, Przewlocki R. Behavioral alterations in rats prenatally exposed to valproic acid: animal model of autism. Neuropsychopharmacol. 2005;30:80–89. doi: 10.1038/sj.npp.1300518. [DOI] [PubMed] [Google Scholar]
- Schor NF. The p75 neurotrophin receptor in human development and disease. Prog Neurobiol. 2005;77:201–214. doi: 10.1016/j.pneurobio.2005.10.006. [DOI] [PubMed] [Google Scholar]
- State MW, Levitt P. The conundrums of understanding genetic risks for autism spectrum disorders. Nature Neurosci. 2011;14:1499–1506. doi: 10.1038/nn.2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stodgell CJ, Ingram JL, O’Bara M, Tisdale BK, Nau H, Rodier PM. Induction of homeotic gene Hoxa1 through valproic acid’s teratogenic mechanism of action. Neurotoxicol Teratol. 2006;28:617–624. doi: 10.1016/j.ntt.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Surén P, Roth C, Bresnahan M, Haugen M, Hornig M, Hirtz D, Lie KK, Lipkn WI, Magnus P, Reichborn-Kjennerud TR, Schjølberg S, Smith GD, Øyen AS, Susser E, Stolterberg C. Associate between maternal use of folic acid supplements and risk of autism spectrum disorders in children. J Am Med Assoc. 2013;309:570–577. doi: 10.1001/jama.2012.155925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szapacs ME, Mathews TA, Tessarollo L, ErnestLyons W, Mamounas LA, Andrews AM. Exploring the relationship between serotonin and brain-derived neurotrophic factor: analysis of BDNF protein and extraneuronal 5-HT in mice with reduced serotonin transporter or BDNF expression. J Neurosci Meth. 2004;140:81–92. doi: 10.1016/j.jneumeth.2004.03.026. [DOI] [PubMed] [Google Scholar]
- Tsai SJ. Is autism caused by early hyperactivity of brain-derived neurotrophic factor? Med Hypoth. 2005;65:79–82. doi: 10.1016/j.mehy.2005.01.034. [DOI] [PubMed] [Google Scholar]
- Tsankova NM, Berton O, Renthal W, Kumar A, Neve RL, Nestler EJ. Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nature Neurosci. 2006;9:519–525. doi: 10.1038/nn1659. [DOI] [PubMed] [Google Scholar]
- Williams G, King J, Cunningham M, Stephan M, Kerr B, Hersh JH. Fetal valproate syndrome and autism: additional evidence of an association. Dev Med Child Neurol. 2001;43:202–206. [PubMed] [Google Scholar]
- Yasuda S, Liang MH, Marinova Z, Yahyavi A, Chuang DM. The mood stabilizers lithium and valproate selectively activate the promoter IV of brain-derived neurotrophic factor in neurons. Molec Psychiat. 2009;14:51–59. doi: 10.1038/sj.mp.4002099. [DOI] [PubMed] [Google Scholar]