Abstract
Objective
Circulating branched-chain amino acids (BCAAs) are elevated in obesity and this has been linked to obesity comorbidities. However it is unclear how obesity affects alloisoleucine, a BCAA and pathognomonic marker of branched-chain keto acid dehydrogenase complex (BCKDC) disorders. It has been previously established that obese Zucker rats exhibit BCKDC impairments in fat and other tissues, whereas BCKDC impairments in adipose tissue of DIO rats are compensated by increased hepatic BCKDC activity. Therefore, alloisoleucine was investigated in these two obesity models.
Design and Methods
Amino acids were extracted from plasma and measured using ultra performance liquid chromatography mass spectrometry (UPLC-MS).
Results
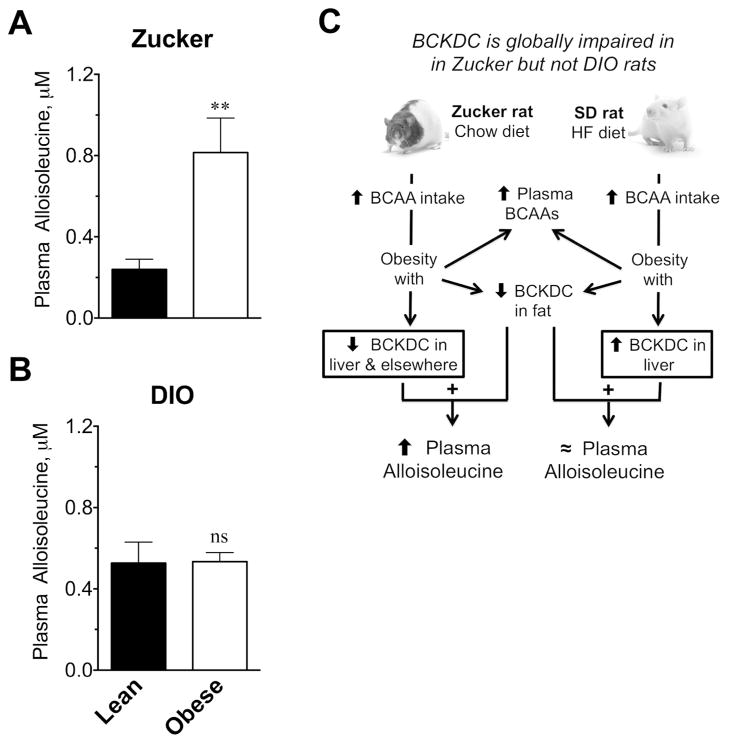
Plasma alloisoleucine was 238% higher in obese compared to lean Zucker rats. This elevation was greater than that of other BCAAs (107–124%). DIO rats had no significant change in alloisoleucine, despite elevations in other BCAAs (15–66%).
Conclusions
Alloisoleucine was elevated in obese Zucker but not DIO rats consistent with known global impairments of BCKDC in Zucker but not DIO rats. Cytotoxic branched-chain ketoacids (BCKAs) accumulate in genetic disorders affecting BCKDC. BCKAs increase reactive oxygen species, stress kinase activation and mitochondrial dysfunction. Inasmuch as these factors underlie obesity comorbidities, it may important to identify obese individuals with elevated alloisoleucine.
Keywords: branched-chain amino acids, isoleucine, branched-chain amino acid transaminase, maple syrup urine disease, alloisoleucine
Introduction
Branched-chain amino acid (BCAA) supplementation is frequently linked to endpoints associated with good health including satiety, diet-induced thermogenesis, improved glycemia and lean body mass 1, 2. Paradoxically, BCAAs are elevated in obesity where they have been described as a “metabolic signature” for insulin resistance, associated with glucose intolerance, and future diabetes 3, 4.
In different types of obesity, different processes affecting BCAA rates of appearance or disappearance (food intake, protein turnover, oxidation, protein synthesis and excretion) may be affected and their contribution or relative direction of change may differ between models 5–8 and perhaps individuals. On the other hand, a consistent observation across obesity models and humans is a loss of BCAA metabolism in adipose tissue 5. It has been hypothesized therefore that loss of BCAA metabolism in fat may contribute to the branched-chain aminoacidemia of obesity 5, 7–9.
While fat appears to be a quantitatively important site of BCAA metabolism, it may be questioned whether a defect in BCAA metabolism in a single tissue is sufficient to elevate whole body BCAA concentrations. That is because (a) BCAAs are rapidly converted to branched-chain keto acids (BCKAs) in many peripheral tissues and can circulate in the blood to be metabolized elsewhere, (b) the BCKDC activity responsible for that metabolism is distributed across many tissues and (c) is substrate activated. Thus a systematic losses of BCAA metabolism in Maple Syrup Urine Disease (MSUD) or models thereof can be largely alleviated by transplantation of the liver or adipose tissue (reviewed in 10). Thus a fat specific impairment of BCKDC in obesity could theoretically be compensated for by increased activity in other tissues. This scenario is exemplified in DIO rats where BCKDC is impaired in fat, but in liver the activity is increased more than two-fold 6, 7. In contradistinction, in other models (e.g., ob/ob mice, ZDF rats and Otsuka Long Evans Tokushima Fatty rats), hepatic BCKDC is impaired by obesity 9, 11. In obese Zucker rats impairments of BCKDC in fat were matched by ~50% activity losses in liver and all other peripheral tissues tested 8, 9.
Whether BCKDC dysfunction extends to multiple tissues in obesity is an important question because systematic impairment of BCKDC could contribute to obesity comorbidities. Mutations in several BCKDC subunits (including BCKDHA) or BCKDC phosphatase (PPm1K, a.k.a. PP2Cm) can cause various forms of Maple Syrup Urine Disease (MSUD). Even in untreated milder forms of MSUD, potentially cytotoxic BCKAs circulate and accumulate in tissues. BCKA addition to MSUD cells activates stress kinases (JNK and p38), increases ROS generation and causes mitochondrial dysfunction 12, 13. These factors are linked to insulin resistance, diabetes and cardiovascular disease 12, 13. Consistently, both BCKDHA (encoding the subunit regulated by phosphorylation) and PPm1K (the BCKDHA phosphatase) are primary obesity/diabetes susceptibility genes 14–16, and PPm1K has also been implicated in cardiovascular disease17. Thus, subjects with type II diabetes had lower beta cell PPm1K, and partial silencing of PPm1K in beta cells impaired glucose stimulated insulin secretion 14. Furthermore, a specific allelic variation near PPm1K was associated with elevated BCAAs along with poorer glycemic and weight loss responses in the POUNDS LOST trial 16. Thus global BCKDC impairment in obesity could potentially contribute, along with other factors, to the development of obesity co-morbidities that higher concentrations of BCAAs appear to portend.
A practical means to identify obese types with partial global BCKDC impairments as opposed to those restricted to adipose tissue could be useful. Here we explored using alloisoleucine, the pathognomonic marker of MSUD, for that purpose. Due to its long half-life, alloisoleucine is not significantly impacted by acute factors such as nutritional status 18. Plasma alloisoleucine below the cut-off used for MSUD diagnosis, typically 2μM, are not usually measured with standards or reported by clinical laboratories 19, so it is unknown how alloisoleucine might vary due to obesity within the “normal range”. Given that impairments in BCKDC were observed in multiple tissues of obese Zucker rats, but were restricted to fat and compensated by increased hepatic activity in obese DIO rats 6, 8, 9, we tested the hypothesis that alloisoleucine might be elevated in Zucker but not DIO rats.
Methods and Procedures
Animals
All procedures were approved by the Penn State Hershey Institutional Animal Care and Use Committee (IACUC). Excess, banked (−80°C) heparinized plasma from two previous rat studies were used here. In both studies the plasma was collected about 3–4 h after the end of the dark cycle. In the Zucker rat study, male obese (fa/fa, 455 ± 5 gm body weight, n=10) and lean control (Fa/?, 280 ± 3 gm, n=10) rats from Charles River Laboratories (Cambridge, MA, USA) were maintained as previously described 8. The DIO samples were from ad libitum-fed Sprague-Dawley rats (Charles River Laboratories) maintained for more than 20 weeks on the same lean chow (396 ± 12 gm body weight, n=10) as the Zucker rats (Teklad 2018) or a 60% fat diet (Research Diets D12492) leading to DIO (867 ± 13 gm final body weight, n=10). The control DIO rats (396 ± 12 gm final body weight, n=10) as well as lean and obese Zucker rats were provided Teklad 2018 diet, a low fat diet.
Ultra pressure liquid chromatography mass spectrometry (UPLC-MS)
Amino acids and an internal standard were extracted from plasma using a Waters Oasis MCX 1cc solid phase vacuum extraction system according to the manufacturer’s instructions. Separation and analysis of alloisoleucine, Ile, Leu, and Val was then performed as previously described 10 on a Waters Synapt HDMS hybrid QTOF with Ion Mobility, housed in the Penn State College of Medicine Macromolecular Core Facility. Two injection volumes were used for each sample to maintain MS signals within linear range, 10μl for alloisoleucine and 0.25μl for the other amino acids. The standard curve included amino acid concentrations of 0.1μM and above.
Statistical Analysis
Data are expressed as mean ± SEM. Two-tailed unpaired t-tests and data correlation analyses was performed using Graphpad Prism 6.0 software (La Jolla, CA); p<0.05 was considered significant.
Results
BCAAs, Phe and alloisoleucine were measured in plasma by UPLC-MS. Compared to lean rats, obesity elevated BCAAs by 107–124% on average in plasma from obese Zucker rats (lean vs obese: Ile, 42 ± 3 vs 87 ± 3 μM; Leu, 85 ± 5 vs 190 ± 10 μM; Val, 125 ± 7 vs 266 ± 9 μM; n=9/group, p<0.001). Phenylalanine was elevated 24% (67 ± 1 vs 83 ± 2 μM, p<0.001). Alloisoleucine is the R-epimer of Ile, formed as a rare side reaction during the reversible transamination of Ile or S-ketomethlyvalerate (S-KMV) 20. The mechanism of its formation is schematically shown in Fig 1. In obese Zucker rats, alloisoleucine was elevated 238% (Fig 2A), with some individual values between 1.3 and 1.8 μM (not shown). No significant correlation was observed between plasma Ile and alloisoleucine concentrations in either lean or obese rats (data not shown).
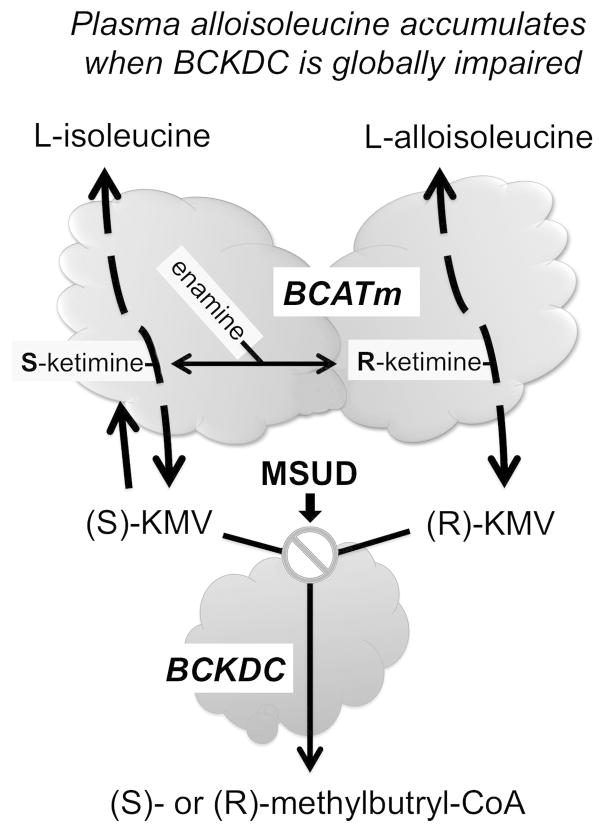
Figure 1.
Schematic showing how impairment of BCKDC increases alloisoleucine formation. The first step in BCAA metabolism is a reversible transamination catalyzed in most peripheral tissues except liver which lacks this activity by the mitochondrial isoform of branched-chain amino acid transaminase (BCATm, BCAT2). On the left, the interconversion of L-Ile and S-ketomethylvalerate [(S)-KMV] due to BCATm activity is shown (for simplicity, the transamination partners, usually Glu and α-ketoglutarate are not shown). BCATm’s catalytic mechanism involves a covalent linkage of intermediates to the pyridoxyl-5-phosphate cofactor which in turn undergo several transitions during the reaction 20. A Schiff base aldimine of Ile first forms with the cofactor (not shown) and that in turn rearranges to an S-ketamine (shown) which is finally released as S-KMV. S-KMV can then be metabolized by BCKDC which catalyzes the next step. If BCKDC is locally inhibited, S-KMV can either enter the circulation for metabolism in another tissue such as liver or undergo reverse transamination back to Ile. Occasionally, the S-ketamine of Ile may undergo a transition to an enamine. This enamine is susceptible to tautomerization leading to an R-ketamine intermediate of Ile (shown for simplicity on the right side). The likelihood of this secondary rearrangement and tautomerization increases when KMV accumulates due to global BCKDC impairment (⊘) such as in Maple Syrup Urine Disease (MSUD). The R-ketamine can be converted to R-KMV (also a BCKDC substrate) or in a stepwise fashion to L-alloisoleucine 20 which can also exit the mitochondria and enter the circulation. Since liver lacks BCAT activity, alloisoleucine is thought to be formed in other tissues.
Figure 2.
Plasma concentrations of alloisoleucine in Zucker and DIO rat models or obesity. Plasma alloisoleucine concentrations in (A) lean and obese Zucker rats fed a low fat (chow) diet or (B) lean and obese Sprague-Dawley DIO rats fed a HF diet as described in the Methods and Procedures. The results are mean ± SE, n=9–10, ** p=0.0051, ns indicates not significant. (C) Schematic summarizing previous findings in these two models related to branched chain amino acids. Zucker rats fed even a low fat diet and SD rats fed a high fat diet have increased caloric and BCAA intake and develop obesity associated with elevated BCAAs albeit BCAA elevations are greater in Zucker compared to DIO rats; both models exhibit impairments in adipose tissue BCAA metabolism that include BCKDC (see Results text statements and References 5, 7, 8). The combination of impaired BCKDC activity in fat 5,9 and along with ~50% decreased activity in multiple other peripheral tissues 8 of the obese Zucker rat is posited to explain the observed elevations in alloisoleucine (A). However in the DIO rat loss of BCKDC in fat 7, 9 is compensated for by >50% increase in BCKDC activity in liver as shown by Kadota et al.6. Thus no change in alloisoleucine was observed in DIO rats as S-KMV that is not metabolized in fat can circulate to the liver to be metabolized there.
Amino acids were also measured in DIO rats. BCAAs in the DIO rats were elevated 15–66% as follows for lean vs obese: Ile, 51 ± 3 vs 77 ± 3 μM (p<0.001); Leu, 136 ± 7 vs 157 ± 4 μM (p<0.05); Val, 124 ± 6 vs 206 ± 9 μM (p<0.001). Phenylalanine was unaltered (75 ± 3 vs 73 ± 2 μM, N.S.). Alloisoleucine was not changed by obesity in DIO rats (Fig 2B), in contrast to Zuckers (Fig 2A). A model reiterating why alloisoleucine may be elevated in obese Zucker but not DIO rats is shown in Fig 2C.
Discussion
In this paper, we have shown that the BCAA, alloisoleucine, is elevated in obese Zucker rats but not DIO rats. While all of the measured alloisoleucine concentrations in plasma from obese Zucker rats fell below the 2μM cut off used for MSUD diagnosis 19, some individual values approached that value. Consistent with dogma that elevations in Ile (pool size) without a systematic loss of metabolism do not elevate alloisoleucine 18, DIO rats had elevated Ile but no significant changes in plasma alloisoleucine.
Most models of obesity, including Zucker and DIO rats exhibit changes in adipose tissue BCAA metabolism 5,7. In obese Zucker rats BCKDC impairments extend to other peripheral tissues 7–9, whereas in DIO rats, while BCKDC is impaired in fat, hepatic BCKDC activity is greatly increased 6. The mechanisms underlying these differences are unknown. It is tempting to speculate that alloisoleucine might be able to differentiate these situations in other models and obese individuals. It seems likely that there may be some obese individuals with global versus tissue selective effects on BCKDC because BCKDHA and PPm1K have been identified as type-2 diabetes and/or obesity susceptibility genes 14–16.
Proportionally, the elevation of alloisoleucine in obese Zucker rats was more than that observed for other BCAAs. Thus the signal to noise ratio of alloisoleucine elevations was greater than other BCAAs in Zucker rat obesity and the elevation appeared to be model selective since it did not change in obese DIO rats. Thus measuring alloisoleucine changes may provide a robust and selective marker of partial global impairments in BCKDC associated with certain types of obesity.
A potential limitation of our hypothesis is that the obese Zucker rats we studied tend to avoid diabetes. However on another genetic background, ZDF, the same loss of leptin signaling leads to diabetes. Obese diabetic ZDF rats also have been reported to exhibit BCKDC impairments affecting liver 11. A similar limitation is that in a small cohort of diabetes patients, alloisoleucine concentrations on average were not different from non-diabetic subjects, however the upper end of values for diabetic subjects were nevertheless very close to the diagnostic cut-off for MSUD used at the time for that older methodology 18.
Further studies are needed to understand the potential pathological significance of global BCKDC impairments versus those restricted to fat and compensated by liver activity changes in different models of obesity. It also remains to be determined whether alloisoleucine is more robust or has a different pattern or selectivity in longitudinal studies compared to the major BCAAs that correlate with or predict obesity co-morbidities.
What is already known about this subject?
Genetic disruption of BCKDC or its phosphatase are associated with elevated plasma alloisoleucine along with potentially cytotoxic branched-chain keto acids (BCKAs)
Impairments in adipose tissue branched-chain keto acid dehydrogenase complex (BCKDC) occur in all known forms of obesity.
Obesity associated BCKDC dysfunction in fat may or may not extend to other tissues; obese Zucker and DIO rats are different in this regard.
What does this study add?
While both Zucker and DIO rats had elevated plasma BCAAs, plasma alloisoleucine was only elevated in obese Zucker rats.
Alloisoleucine differentiated the obesity branched-chain aminoacidemia of Zucker rats (where BCKDC has been found to be impaired in fat and other peripheral tissues) from DIO rats (BCKDC is impaired in fat but activity is increased in liver).
Acknowledgments
We thank Dongxiao Sun and Penn State Macromolecular Core Facility for their UPLC-MS expertise. The research was supported by NIH grant DK091784.
Footnotes
Competing interests: the authors have no competing interests.
References
- 1.Layman DK, Walker DA. Potential importance of leucine in treatment of obesity and the metabolic syndrome. J Nutr. 2006;136:319S–23S. doi: 10.1093/jn/136.1.319S. [DOI] [PubMed] [Google Scholar]
- 2.Blouet C, Jo YH, Li X, Schwartz GJ. Mediobasal hypothalamic leucine sensing regulates food intake through activation of a hypothalamus-brainstem circuit. J Neurosci. 2009;29:8302–11. doi: 10.1523/JNEUROSCI.1668-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Newgard CB, An J, Bain JR, et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009;9:311–26. doi: 10.1016/j.cmet.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang TJ, Larson MG, Vasan RS, et al. Metabolite profiles and the risk of developing diabetes. Nat Med. 2011;17:448–53. doi: 10.1038/nm.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adams SH. Emerging perspectives on essential amino acid metabolism in obesity and the insulin-resistant state. Adv Nutr. 2011;2:445–56. doi: 10.3945/an.111.000737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kadota Y, Toyoda T, Kitaura Y, Adams SH, Shimomura Y. Regulation of hepatic branched-chain α-ketoacid dehydrogenase complex in rats fed a high-fat diet. Obesity Res & Clin Prac. 2013 doi: 10.1016/j.orcp.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lackey DE, Lynch CJ, Olson KC, et al. Regulation of adipose branched-chain amino acid catabolism enzyme expression and cross-adipose amino acid flux in human obesity. Am J Physiol Endocrinol Metab. 2013;304:E1175–87. doi: 10.1152/ajpendo.00630.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.She P, Olson KC, Kadota Y, et al. Leucine and protein metabolism in obese Zucker rats. PLoS One. 2013;8:e59443. doi: 10.1371/journal.pone.0059443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.She P, Van Horn C, Reid T, Hutson SM, Cooney RN, Lynch CJ. Obesity-related elevations in plasma leucine are associated with alterations in enzymes involved in branched-chain amino acid metabolism. Am J Physiol Endocrinol Metab. 2007;293:E1552–63. doi: 10.1152/ajpendo.00134.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zimmerman HA, Olson KC, Chen G, Lynch CJ. Adipose transplant for inborn errors of branched chain amino acid metabolism in mice. Molecular Genetics and Metabolism. 2013 doi: 10.1016/j.ymgme.2013.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuzuya T, Katano Y, Nakano I, et al. Regulation of branched-chain amino acid catabolism in rat models for spontaneous type 2 diabetes mellitus. Biochem Biophys Res Commun. 2008;373:94–8. doi: 10.1016/j.bbrc.2008.05.167. [DOI] [PubMed] [Google Scholar]
- 12.Oyarzabal A, Martinez-Pardo M, Merinero B, et al. A novel regulatory defect in the branched-chain alpha-keto acid dehydrogenase complex due to a mutation in the PPM1K gene causes a mild variant phenotype of maple syrup urine disease. Hum Mutat. 2013;34:355–62. doi: 10.1002/humu.22242. [DOI] [PubMed] [Google Scholar]
- 13.Bridi R, Braun CA, Zorzi GK, et al. alpha-keto acids accumulating in maple syrup urine disease stimulate lipid peroxidation and reduce antioxidant defences in cerebral cortex from young rats. Metab Brain Dis. 2005;20:155–67. doi: 10.1007/s11011-005-4152-8. [DOI] [PubMed] [Google Scholar]
- 14.Taneera J, Lang S, Sharma A, et al. A systems genetics approach identifies genes and pathways for type 2 diabetes in human islets. Cell Metabolism. 2012;16:122–34. doi: 10.1016/j.cmet.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 15.Tiffin N, Adie E, Turner F, et al. Computational disease gene identification: a concert of methods prioritizes type 2 diabetes and obesity candidate genes. Nucleic acids research. 2006;34:3067–81. doi: 10.1093/nar/gkl381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu M, Qi Q, Liang J, et al. Genetic determinant for amino acid metabolites and changes in body weight and insulin resistance in response to weight-loss diets: the preventing overweight using novel dietary strategies (POUNDS LOST) trial. Circulation. 2013;127:1283–9. doi: 10.1161/CIRCULATIONAHA.112.000586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun H, Lu G, Ren S, Chen J, Wang Y. Catabolism of branched-chain amino acids in heart failure: insights from genetic models. Pediatr Cardiol. 2011;32:305–10. doi: 10.1007/s00246-010-9856-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schadewaldt P, Bodner-Leidecker A, Hammen HW, Wendel U. Significance of L-alloisoleucine in plasma for diagnosis of maple syrup urine disease. Clin Chem. 1999;45:1734–40. [PubMed] [Google Scholar]
- 19.Oglesbee D, Sanders KA, Lacey JM, et al. Second-tier test for quantification of alloisoleucine and branched-chain amino acids in dried blood spots to improve newborn screening for maple syrup urine disease (MSUD) Clin Chem. 2008;54:542–9. doi: 10.1373/clinchem.2007.098434. [DOI] [PubMed] [Google Scholar]
- 20.Mamer OA, Reimer ML. On the mechanisms of the formation of L-alloisoleucine and the 2-hydroxy-3-methylvaleric acid stereoisomers from L-isoleucine in maple syrup urine disease patients and in normal humans. J Biol Chem. 1992;267:22141–7. [PubMed] [Google Scholar]