Abstract
The first reports of combined EEG and fMRI used for evaluation of epileptic spikes date back to the mid-90’s. At that time the technique was called EEG-triggered fMRI – the “triggered” corresponded to an epilepsy specialist reviewing live EEG while the patient was located in the scanner; after the spike was identified, scan was initiated to collect the data. Since then major progress has been made in combined EEG/fMRI data collection and analyses. These advances allow studying the electrophysiology of genetic generalized epilepsies (GGEs) in vivo in greater detail than ever. In addition to continuous data collection, we now have better methods for removing physiologic and fMRI-related artifacts, more advanced understanding of the hemodynamic response functions, and better computational methods to address the questions regarding the origins of the epileptiform discharge generators in patients with GGEs. These advances have allowed us to examine numerous cohorts of children and adults with GGEs while not only looking for spike and wave generators but also examining specific types of GGEs (e.g., juvenile myoclonic epilepsy or childhood absence epilepsy), drug-naïve patients, effects of medication resistance, or effects of epileptiform abnormalities and/or seizures on brain connectivity. While the discussion is ongoing, the prevailing thought is that the GGEs as a group are a network disorder with participation from multiple nodes including thalami and cortex with the clinical presentation depending on which node of the participating network is affected by the disease process. This review discusses the contributions of EEG/fMRI to our understanding of GGEs.
1. INTRODUCTION
The clinical characteristics of the genetic generalized epilepsies (GGEs) include various combinations of generalized seizures, myoclonic jerks and absence seizures; patients with GGEs have either normal EEG or exhibit bifrontally predominant generalized spike and wave discharges (GSWDs).(1) Substantial proportion of patients with GGEs also shows focal EEG abnormalities with some but not all studies associating these focal abnormalities with medication resistance. (2–5) The presence of these focal abnormalities may be consistent with focal cortical onset of these epilepsies and the “rapid bilateral synchrony” postulated by Gloor. (6) While in the past GGEs were thought to be of central (thalamic) onset with various thalamic nuclei implicated in the generation of the GSWDs, there is mounting evidence that this may not be true for all patients and that the location of the seizure onset may depend on which node of the thalamo-cortico-thalamic network for GSWD/seizure generation is affected by the disease process.
Several possible theories of GGEs and/or GSWD onset have been proposed. In general, these theories can be divided into “cortical onset” (cortical theory and cortical focus theory), “thalamic onset” (centrencephalic theory and thalamic clock theory), and the “cortico-reticular” theory which incorporates elements of the cortical and thalamic onset theories (for detailed review see e.g., (7)). Briefly, the cortical theory posits that GSWDs in GGEs originate from diffuse cortical areas rather than from the thalami (8, 9) while the cortical focus theory puts forth the somatosensory cortex as the originator of GSWDs. (10) In contrast, the “thalamic onset” theories suggest the onset of GSWDs and seizures to be thalamic. The difference between these theories is that the centrencephalic theory by Penfield and Jasper considers the thalamic structures and midbrain as the originators of the EEG abnormalities in GGEs (11) while the thalamic clock theory proposes that thalamic oscillations are the primary determinant and driver of the neocortical rhythmic events with the rhythmicity of the events maintained by cortex. (12, 13) Finally, the unifying corticoreticular theory maintains that excitable cortex is necessary for the production of GSWDs while the interplay between cortex and thalami is necessary for the maintenance of GSWDs in the excitable cortex via response to thalamic volley. Thus both, thalami and cortex, are necessary for the production and maintenance of GSWDs. (6) While the basic science experiments and human studies provide evidence in support of all these theories, the recent explosion of neuroimaging studies, in particular EEG/fMRI has further contributed to our understanding of GGEs. Thus, the questions posed by this targeted review are:
How has EEG/fMRI contributed to our understanding of the origins of the GSWDs and seizures in GGEs?
Can EEG/fMRI be used to assess the contribution of specific nuclei within the thalamus to generation and propagation of GSWDs?
What are the effects of GGEs and GSWDs on resting state and resting state connectivity?
Can fMRI be used to constrain source reconstruction of simultaneous EEG in order to further investigate the anatomical underpinnings of GSWDs generators?
2. KEY QUESTIONS
2.1. How has EEG/fMRI contributed to our understanding of the origins of the GSWDs and seizures in GGEs?
Generalized spike wave discharges observed on routine EEGs are pathognomonic for GGEs. (14) Although GSWDs may exhibit fronto-central predominance in some patients,(15) it is difficult to localize them more precisely than this using scalp EEG. Dense array EEGs and magnetoencephalography (MEG) studies have revealed less generalized patterns with medial frontal, orbitofrontal or prefrontal source localization. (16, 17) While intracerebral EEG recordings are the gold standard for seizure localization, little intracerebral data are available in patients with GGEs since they typically are not candidates for invasive EEG monitoring. (18–20) The combination of EEG with fMRI, as a noninvasive technique, offers high spatial resolution rendering it suitable for use in patients with GGEs. (21, 22)
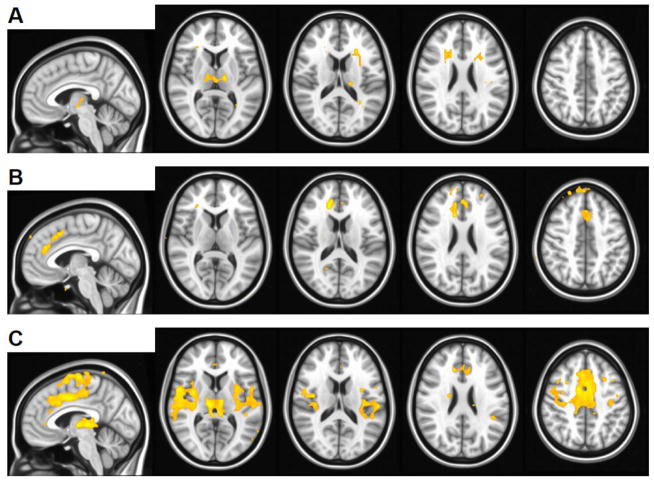
EEG/fMRI studies of GGEs utilizing standard fMRI data analysis methods typically show symmetric changes in thalamic and cortical blood oxygen level dependent (BOLD) signal related to GSWDs (Figure 1).(23–32) Observed cortical changes usually include both GSWD-related activation and deactivation in multiple regions. Thus, standard EEG/fMRI data analyses have not been able to identify any single region as an ictogenic focus. This may be a limitation of the EEG/fMRI data analysis methods, the EEG/fMRI methodology and the relatively poor temporal or spatial resolution of the fMRI (a single whole brain volume is imaged usually in 2–3 seconds with typical spatial resolution of 4 mm), various patient factors (e.g., controlled vs. not controlled GGE, GGE type), medication influence or other, up to date unidentified factors.
Figure 1.
Some GGE patients exhibit predominantly thalamic (A) or cortical (B) GSWD-related fMRI activation. A mixture of both thalamic and cortical activation (C) is the most commonly observed pattern. Activation (orange) for three representative subjects is overlaid on the MNI152 standard brain in radiological orientation with slice coordinates shown at bottom. Voxels with t > 3.5 (uncorrected p < 0.001) are shown. GSWD-related deactivation pattern is not shown. Pictures are in radiological convention (left on the picture is right in the brain).
Recently, instead of fitting a single canonical hemodynamic response function to the EEG/fMRI data attempts have been made to perform dynamic analyzes of the time courses of the BOLD signal changes related to either GSWDs or absence seizures (“sliding window” approach) and to assess the relationships between the activations using e.g., Granger causality methods. These studies were able to identify BOLD signal changes in cortical areas to precede thalamic activations (24, 30, 31, 33) and show effective connectivity directed from frontal cortex to thalami supporting cortical onset of GGEs in at least some patients. (30) Others have found that subcortical BOLD signal increases precede or coincide with cortical signal decreases in children with difficult to control GGEs. (34) But, this particular study included analyses of polyspike and wave discharges with a fast component of 10–12 Hz that is known, at least in the setting of Lennox-Gastaut syndrome, to produce positive global BOLD correlates in contrast to GSWDs that had the opposite effects on BOLD signal. (35) Thus, while it cannot be said that all EEG/fMRI studies support either the centrocephalic or cortical focus hypotheses exclusively, there is bias in the EEG/fMRI literature towards the cortical onset of GGEs. The observation of robust GSWD-related BOLD signal changes in both cortical and subcortical regions is most consistent with the corticoreticular hypothesis but differences between studies and the clinical characteristics of the included patients need to be taken into consideration especially the choice of new-onset or drug naïve patients vs. patients with multi-drug resistant GGEs and the possible effects of medication on the fMRI and EEG signals. (28, 34, 36)
Whereas the majority of patients with GGEs exhibit thalamic activation, cortical regions involved in GSWD-related activation are noted to vary (Figure 1C).(23, 25, 26) When medication responsive GGE patients are compared to patients who demonstrate pharmacoresistance, stronger activation is observed in cortical regions -- in medial frontal cortex and bilateral anterior insulae,(31) Further, increased GSWD-related changes in BOLD signal are associated with negative effects on cognitive performance. (25) Thus, EEG/fMRI is able to identify differences in GSWD-related brain activation associated with clinical features of GGEs.
Cortical deactivations observed in patients with GGEs are usually symmetric and widespread (Figure 2); they are consistently described as occupying a set of brain regions known as the default mode network (DMN).(26, 28, 37, 38) The DMN is a well-studied resting state network thought to support consciousness. (39–42) Its deactivation by GSWDs is thought to contribute to absence seizure semiology (27, 37) via the network inhibition hypothesis. (43, 44)
Figure 2.
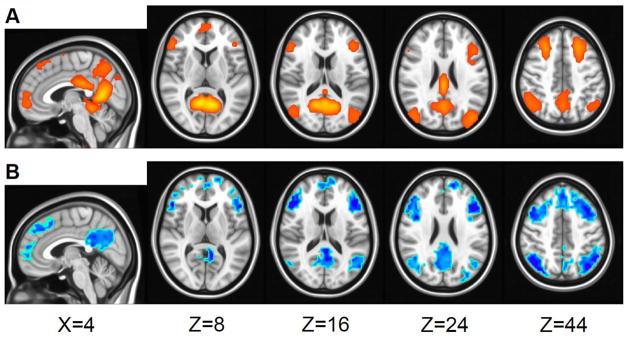
Brain regions in the default mode network (A) undergo GSWD-related deactivation (B). A: Default mode network independent component, modified from Figure 1 in Kay et al. 2013.(90) B: Deactivation (activation not shown) 3 seconds after GSWD onset, modified from Figure 1 in Szaflarski et al. 2013.(31) Pictures are in radiological convention (left on the picture is right in the brain).
2.2. Can EEG/fMRI be used to assess the contribution of specific nuclei within the thalamus to generation and propagation of GSWDs?
The thalamus is thought to be an important part of the circuit that generates GSWDs in GGEs. (45) However, the thalamus is not a monolithic structure. It is somatotopically organized with different nuclei connecting to different regions in cortex. (46) The morphology (47) of these different nuclei and their white-matter connections with cortex (48) are affected unequally by GGE. Furthermore, the activation patterns, as detected by EEG/fMRI, vary between studies and between patients (Figure 1) thus making generalized inferences regarding specific thalamic nuclei is difficult. In this context it is important to consider whether or not EEG/fMRI can assess the contribution of specific nuclei within the thalamus toward producing GSWDs.
Imaging the thalamus is difficult because it is a relatively compact structure. Each lobe is approximately 20 mm wide (46) compared to a 4 mm fMRI voxel size. Statistical techniques that assume large,(49) smooth,(50) clusters of significant voxels are thus ill-suited to detection of individual thalamic nuclei at low spatial resolution. The use of other,(51) non-parametric (52) statistical techniques may be more appropriate when studying the thalamus in the context of GGEs. High-field fMRI using the early BOLD response increases spatial resolution (53–55) and allows for the detection of individual thalamocortical networks. (56) However, artifacts associated with simultaneous EEG/fMRI are increased at higher field strengths, and their removal at 7 Tesla remains a challenge with respect to testing the roles of specific thalamic nuclei in GGEs. (57)
At least one EEG/fMRI study has found that the timing of GSWD-related activations is different for different thalamic nuclei, implying that they may play different roles in initiation vs. maintenance of seizures. (32) When the thalamus is considered as a single unit, EEG/fMRI does not consistently reveal changes in thalamic functional connectivity associated with GGE. (58) However, an EEG/fMRI study that subdivided the thalamus into regions based on morphology has found changes in functional connectivity in the medial dorsal nucleus (MDN) but not the pulvinar. (59) Even when significant changes in thalamic activity are not observed, subthreshold thalamic connectivity is found to be organized into discrete clusters. (60) Therefore, results of EEG/fMRI studies support the hypothesis that different thalamic nuclei may be affected differently by GGEs or that that they may have different roles in the maintenance of epileptiform discharges and/or seizures. But, we are far from reaching conclusive evidence because of the inherent limitation of the technique – the large size of the fMRI voxels. Thus, future EEG/fMRI studies of epileptiform discharges in patients with GGEs should focus on collecting fMRI data using smaller voxel size (e.g., <2mm) in order to better differentiate the thalamic structures.
2.3. What are the effects of GGEs and GSWDs on resting state and resting state connectivity?
Epilepsy is also a disorder of brain connectivity. (61) Structural brain connectivity in GGE has been studied using diffusion tensor imaging (DTI), which measures anatomical connectivity, and resting-state fMRI, which measures functional connectivity. Functional connectivity reflects anatomical connectivity (62) and is sensitive to changes associated with GGE. (61) In the case of resting-state fMRI, the availability of simultaneous EEG data allows for the exclusion of data points, scans, or subjects that are “contaminated” by GSWDs. However, resting-state fMRI studies that do or do not use EEG to exclude GSWDs have yielded similar findings (63, 64) and we are not aware of any studies that specifically examine whether GSWDs have a confounding effect on functional connectivity. The practice of excluding GSWDs from resting-state analysis provides emerging evidence that GSWDs have an adverse effect on cognitive performance. (25)
Deactivation of the default mode network (DMN) by GSWDs is thought to contribute to the semiology of absence seizures (Figure 2).(37) Connectivity in the DMN has been well-studied using fMRI and EEG/fMRI and was found to be reduced in patients with GGEs when compared to healthy controls. Moreover, the degree of reduction in DMN connectivity in patients with GGEs is correlated with the length of time since diagnosis. (59, 61, 64–66) Reduction in DMN connectivity is greater, and its rate of decline steeper, in patients with GGEs demonstrating features of pharmacoresistance. (65)
Patients with GGEs may have various cognitive deficits as part of their syndrome e.g., frontal lobe dysfunction which may be genetically determined. (67, 68) Thus, it is not surprising that the EEG/fMRI studies have revealed changes in the functional connectivity patterns of frontal regions (33, 48, 59, 60) that correlate with cognitive performance. (69, 70) GGE-associated changes are also observed in the functional connectivity of subcortical regions including thalamus,(48, 59) basal ganglia, and cerebellum,(60, 66) although these are not consistently reproduced. (58)
Simultaneous EEG data also allow for direct examination of the effects of GSWDs on functional connectivity. One approach is to compare data from patients with GGEs who do and do not manifest GSWDs during the fMRI scan. Changes in subcortical connectivity associated with GGEs vs. healthy controls were greater in patients with GGEs who exhibited GSWDs during scanning. (66) Another strategy is to use GSWD frequency as a regressor while excluding timepoints “contaminated” with GSWDs. Patients with GGEs with higher GSWD frequency were found to have increased connectivity between a paracingulate seed region and executive, DMN, and subcortical brain regions. (60)
2.4. Can fMRI be used to constrain source reconstruction of simultaneous EEG in order to further investigate the anatomical underpinnings of GSWD generators?
Simultaneous EEG/fMRI is used to identify brain regions exhibiting fMRI BOLD activation correlated with epileptiform discharges observed on the EEG. (22) However the temporal resolution of the fMRI BOLD signal, on the order of seconds,(71) limits its usefulness for investigating the order in which these brain regions activate. The high temporal resolution of EEG is a potentially useful adjunct to the fMRI data in determining the order of fMRI cluster activation. (72)
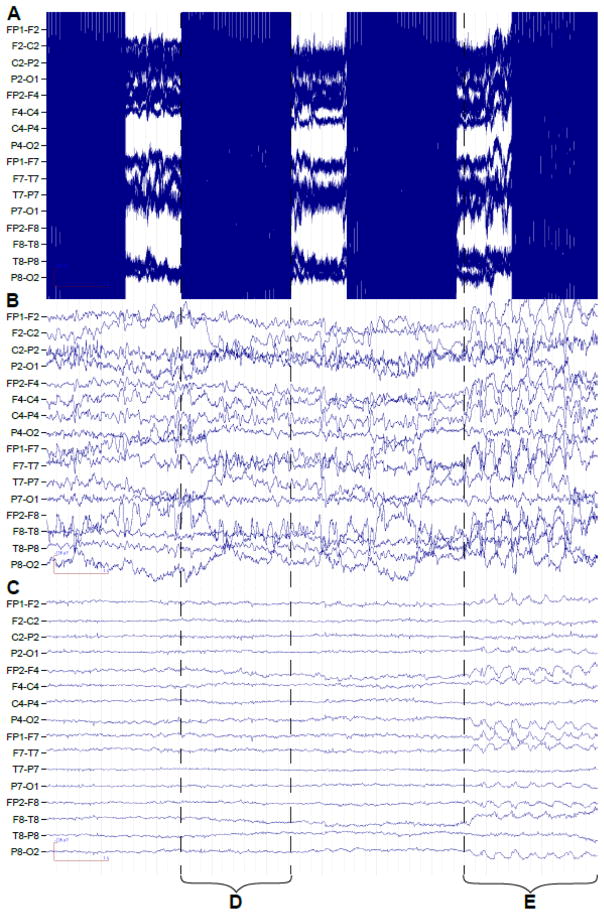
In the setting of focal epilepsy, EEG source reconstruction is useful in the localization of epileptiform discharges and can correctly identify the seizure focus up to 90% of the time. (73) EEG/fMRI yields better spatial resolution than EEG alone and is useful in cases of multiple or ambiguous foci. (74, 75) By comparing the results of source estimation from both EEG and EEG/fMRI modalities, the order in which brain regions activate may be inferred. (72) For example, it is possible to distinguish between regions associated with seizure onset vs. seizure propagation. (76) Although EEG source reconstruction has been attempted in GGE,(16) to our knowledge this has never been combined with EEG/fMRI analyses in the same study. Combining EEG and EEG/fMRI source estimation is thus a promising future direction in the study of GGEs, however, the lack of available intracranial EEG (icEEG) data from patients with GGEs will pose a problem for validation and the need for multi-level processing of the EEG data prior to visualization (Figure 3) may affect the results of further EEG source localization analyses.
Figure 3.
Processing of scanner artifact in EEG data acquired simultaneous with fMRI is a precursor to GSWD identification and source reconstruction. Ten seconds of EEG data are shown raw (A), after gradient artifact removal with average artifact subtraction (B), and after attenuation of the ballistocardiographic artifact with a linear spatial filter (C). Examples of gradient artifact (D) and GSWDs (E) are marked. Standard double banana montage is presented with EEG leads indicated on left.
Any attempt to interpret EEG source reconstruction with EEG/fMRI must consider that the EEG and fMRI BOLD signal measure different physiologic phenomena arising from the same underlying neuronal activation (77) such that positive EEG/fMRI activation corresponds to low frequency energy in the EEG recording, i.e. slow waves. (78) Indeed, a comparison of EEG and EEG/fMRI with the gold standard for neuronal recording, intracranial EEG (icEEG), reveals that in focal epilepsies EEG and EEG/fMRI are more concordant with icEEG than they are with each other. (78, 79) There are multiple techniques for quantifying the concordance of EEG and EEG/fMRI sources. (80) They are, in general, concordant,(72, 74, 81) with the distance between estimated sources ranging from 16 mm for evoked response potentials (ERPs) (82) to on the order of 30 mm for detection of discharges in focal epilepsies. (76, 83)
Among experimental factors contributing to concordance, a greater number of EEG electrodes has been shown to improve concordance between electric source imaging (ESI) results using the scalp EEG and the ictal onset zone; ESI was superior to other non-EEG imaging techniques. (73) This effect is especially notable when using beamforming algorithms for source reconstruction in place of dipoles, in which case doubling the number of EEG electrodes improves the signal-to-noise ratio (SNR) by a factor of 1.6.(84) Interestingly, the beamformer technique appears to yield more concordant sources in the absence of filtering to remove fMRI-related artifact from the EEG recording. If epileptiform discharges are treated as reproducible events, then EEG data from outside the MRI scanner may be used for source reconstruction and has been found to explain a greater proportion of discharge related variation in fMRI BOLD signal than simultaneous EEG data from inside the MRI scanner, possibly because of reduced contamination by fMRI-related artifact. (85) Magnetic field strength is thus another important consideration, as fMRI-related artifacts in the EEG recording are greater at higher fields. (57) EEG source reconstruction from simultaneous EEG/fMRI data has been performed at up to 7 Tesla with concordance on the order of 10 mm for a phantom and as low as 24 mm for healthy human subjects undergoing median nerve stimulation. (84)
Using the location of EEG/fMRI to constrain EEG source reconstruction provides a quantitative model for combining spatiotemporal information from EEG and fMRI sources. (86, 87) However, such models should be used with caution as the underlying assumption that each EEG source corresponds to an EEG/fMRI source is not always valid. In fact, discordance on the order of up to 60 mm is not uncommon. (88) An alternative symmetric model that estimates EEG and EEG/fMRI sources jointly has been shown to produce good spatial agreement with icEEG in focal epilepsies. (89) Interestingly, this model suggests that the time courses of EEG and EEG/fMRI sources may be discordant, calling into question the validity of EEG/fMRI studies that make inferences about timing on the basis of EEG/fMRI sources alone. (24, 30, 34, 89)
3. SUMMARY
EEG/fMRI, as an investigational technique, has undergone tremendous development over the past two decades. Despite this progress, its use for in vivo investigation of patients with GGEs is limited by insufficient temporal and spatial resolution of the fMRI signal. The fMRI-related artifacts make it difficult to apply EEG source reconstruction to EEG/fMRI data as a means of improving overall temporal resolution. Higher field strengths would improve spatial resolution, but they would also exacerbate the problem of fMRI-related artifacts. Further improvement of EEG/fMRI methodology will be needed to be able to probe specific thalamic nuclei or to provide better data for temporal analysis of the functional images. Nevertheless, EEG/fMRI has provided us with new insights and helped us to redefine the pathophysiology of GGEs.
KEY QUESTIONS.
How has EEG/fMRI contributed to our understanding of the origins of the GSWDs and seizures in GGEs?
Can EEG/fMRI be used to assess the contribution of specific nuclei within the thalamus to generation and propagation of GSWDs?
What are the effects of GGEs and GSWDs on resting state and resting state connectivity?
Can fMRI be used to constrain source reconstruction of simultaneous EEG in order to further investigate the anatomical underpinnings of GSWDs generators?
KEY QUESTIONS ANSWERED.
-
How has EEG/fMRI contributed to our understanding of the origins of the GSWDs and seizures in GGEs?
The majority of studies utilizing EEG/fMRI has provided evidence supporting of the cortical sources of the epileptiform discharges in patients with GGEs. Barring their limitations, the largest studies of patients with GGEs showed temporal evolution of the fMRI signals with initial cortical changes (usually frontal) and later spread to the thalamus which appears to be necessary for seizure maintenance.
-
Can EEG/fMRI be used to assess the contribution of specific nuclei within the thalamus to generation and propagation of GSWDs?
Unfortunately, we are not there yet. Several attempts have been made to assess the contributions of individual thalamic structures to the generation or maintenance of epileptiform discharges and seizures in GGEs but poor spatial resolution of the fMRI signals prevents us from making conclusive inferences. Efforts are underway to improve spatial resolution of the EEG/fMRI images collected in patients with GGEs.
-
What are the effects of GGEs and GSWDs on resting state and resting state connectivity?
It appears that the effects are widespread and dependent on multiple factors including medication response and the presence or absence of epileptiform discharges. Additional studies will need to investigate whether longitudinal changes in seizure control are associated with changes in brain connectivity and investigate the effects of specific antiepileptic drugs on the resting state and brain connectivity.
-
Can fMRI be used to constrain source reconstruction of simultaneous EEG in order to further investigate the anatomical underpinnings of GSWD generators?
While several studies appear to address this question in patients with focal onset epilepsies and there is no clear reason why this could not be done in patients with GGEs, to the best of our knowledge such studies have not been performed to date.
Highlights.
Majority of EEG/fMRI studies support cortical source of GSWDs in GGEs
Improved spatial resolution of fMRI is needed to visualize thalamic nuclei in GGEs
Structural and functional connectivity are variably affected by GSWDs in GGEs
fMRI could be used to constrain EEG analysis in GGEs
Acknowledgments
Study support:
The study was supported in part by T32 GM063483 (BK) and in part by K23 NS052468 (JPS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Berg AT, Berkovic SF, Brodie MJ, Buchhalter J, Cross JH, van Emde Boas W, et al. Revised terminology and concepts for organization of seizures and epilepsies: report of the ILAE Commission on Classification and Terminology, 2005–2009. Epilepsia. 2010;51(4):676–85. doi: 10.1111/j.1528-1167.2010.02522.x. Epub 2010/03/04. [DOI] [PubMed] [Google Scholar]
- 2.Lombroso CT. Consistent EEG focalities detected in subjects with primary generalized epilepsies monitored for two decades. Epilepsia. 1997;38(7):797–812. doi: 10.1111/j.1528-1157.1997.tb01467.x. Epub 1997/07/01. [DOI] [PubMed] [Google Scholar]
- 3.Nicolson A, Appleton RE, Chadwick DW, Smith DF. The relationship between treatment with valproate, lamotrigine, and topiramate and the prognosis of the idiopathic generalised epilepsies. J Neurol Neurosurg Psychiatry. 2004;75(1):75–9. Epub 2004/01/07. [PMC free article] [PubMed] [Google Scholar]
- 4.Szaflarski JP, Lindsell CJ, Zakaria T, Banks C, Privitera MD. Seizure control in patients with idiopathic generalized epilepsies: EEG determinants of medication response. Epilepsy Behav. 2010;17(4):525–30. doi: 10.1016/j.yebeh.2010.02.005. Epub 2010/03/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wolf P, Inoue Y. Therapeutic response of absence seizures in patients of an epilepsy clinic for adolescents and adults. J Neurol. 1984;231(4):225–9. doi: 10.1007/BF00313944. Epub 1984/01/01. [DOI] [PubMed] [Google Scholar]
- 6.Gloor P. Generalized cortico-reticular epilepsies. Some considerations on the pathophysiology of generalized bilaterally synchronous spike and wave discharge. Epilepsia. 1968;9(3):249–63. doi: 10.1111/j.1528-1157.1968.tb04624.x. Epub 1968/09/01. [DOI] [PubMed] [Google Scholar]
- 7.Meeren H, van Luijtelaar G, Lopes da Silva F, Coenen A. Evolving concepts on the pathophysiology of absence seizures: the cortical focus theory. Arch Neurol. 2005;62(3):371–6. doi: 10.1001/archneur.62.3.371. Epub 2005/03/16. [DOI] [PubMed] [Google Scholar]
- 8.Bennett FE. Intracarotid and intravertebral metrazol in petit mal epilepsy. Neurology. 1953;3(9):668–73. doi: 10.1212/wnl.3.9.668. Epub 1953/09/01. [DOI] [PubMed] [Google Scholar]
- 9.Shimizu K, Refsum S, Gibbs FA. Effect on the electrical activity of the brain of intra-arterially and intra-cerebrally injected convulsant and sedative drugs (metrazol and nembutal) Electroencephalogr Clin Neurophysiol. 1952;4(2):141–6. doi: 10.1016/0013-4694(52)90003-5. Epub 1952/05/01. [DOI] [PubMed] [Google Scholar]
- 10.Seidenbecher T, Staak R, Pape HC. Relations between cortical and thalamic cellular activities during absence seizures in rats. Eur J Neurosci. 1998;10(3):1103–12. doi: 10.1046/j.1460-9568.1998.00123.x. Epub 1998/09/30. [DOI] [PubMed] [Google Scholar]
- 11.Penfield W. Epileptic automatism and the centrencephalic integrating system. Res Publ Assoc Res Nerv Ment Dis. 1952;30:513–28. Epub 1952/01/01. [PubMed] [Google Scholar]
- 12.Buzsaki G. The thalamic clock: emergent network properties. Neuroscience. 1991;41(2–3):351–64. doi: 10.1016/0306-4522(91)90332-i. Epub 1991/01/01. [DOI] [PubMed] [Google Scholar]
- 13.Kristiansen K, Courtois G. Rhythmic electrical activity from isolated cerebral cortex. Electroencephalogr Clin Neurophysiol. 1949;1(3):265–72. Epub 1949/08/01. [PubMed] [Google Scholar]
- 14.Yenjun S, Harvey AS, Marini C, Newton MR, King MA, Berkovic SF. EEG in adult-onset idiopathic generalized epilepsy. Epilepsia. 2003;44(2):252–6. doi: 10.1046/j.1528-1157.2003.26402.x. Epub 2003/02/01. [DOI] [PubMed] [Google Scholar]
- 15.Rodin E, Ancheta O. Cerebral electrical fields during petit mal absences. Electroencephalogr Clin Neurophysiol. 1987;66(6):457–66. doi: 10.1016/0013-4694(87)90092-7. Epub 1987/06/01. [DOI] [PubMed] [Google Scholar]
- 16.Holmes MD, Brown M, Tucker DM. Are “generalized” seizures truly generalized? Evidence of localized mesial frontal and frontopolar discharges in absence. Epilepsia. 2004;45(12):1568–79. doi: 10.1111/j.0013-9580.2004.23204.x. Epub 2004/12/02. [DOI] [PubMed] [Google Scholar]
- 17.Westmijse I, Ossenblok P, Gunning B, van Luijtelaar G. Onset and propagation of spike and slow wave discharges in human absence epilepsy: A MEG study. Epilepsia. 2009 doi: 10.1111/j.1528-1167.2009.02162.x. Epub 2009/06/13. [DOI] [PubMed] [Google Scholar]
- 18.Engel J, editor. Surgical treatment of the epilepsies. New York: Raven Press; 1993. [Google Scholar]
- 19.Niedermeyer E, Laws ER, Walker AE, Blumer D, Ray CD. The contribution of scalp and depth EEG findings to the surgical treatment of temporal lobe epilepsy. Electroencephalogr Clin Neurophysiol. 1969;26(1):110. Epub 1969/01/01. [PubMed] [Google Scholar]
- 20.Williams D. A study of thalamic and cortical rhythms in petit mal. Brain. 1953;76(1):50–69. doi: 10.1093/brain/76.1.50. Epub 1953/03/01. [DOI] [PubMed] [Google Scholar]
- 21.Gotman J. Epileptic networks studied with EEG-fMRI. Epilepsia. 2008;49 (Suppl 3):42–51. doi: 10.1111/j.1528-1167.2008.01509.x. Epub 2008/05/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gotman J, Kobayashi E, Bagshaw AP, Benar CG, Dubeau F. Combining EEG and fMRI: a multimodal tool for epilepsy research. J Magn Reson Imaging. 2006;23(6):906–20. doi: 10.1002/jmri.20577. [DOI] [PubMed] [Google Scholar]
- 23.Aghakhani Y, Bagshaw AP, Benar CG, Hawco C, Andermann F, Dubeau F, et al. fMRI activation during spike and wave discharges in idiopathic generalized epilepsy. Brain. 2004;127(Pt 5):1127–44. doi: 10.1093/brain/awh136. [DOI] [PubMed] [Google Scholar]
- 24.Benuzzi F, Mirandola L, Pugnaghi M, Farinelli V, Tassinari CA, Capovilla G, et al. Increased cortical BOLD signal anticipates generalized spike and wave discharges in adolescents and adults with idiopathic generalized epilepsies. Epilepsia. 2012;53(4):622–30. doi: 10.1111/j.1528-1167.2011.03385.x. Epub 2012/01/17. [DOI] [PubMed] [Google Scholar]
- 25.Berman R, Negishi M, Vestal M, Spann M, Chung MH, Bai X, et al. Simultaneous EEG, fMRI, and behavior in typical childhood absence seizures. Epilepsia. 2010;51(10):2011–22. doi: 10.1111/j.1528-1167.2010.02652.x. Epub 2010/07/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hamandi K, Salek-Haddadi A, Laufs H, Liston A, Friston K, Fish DR, et al. EEG-fMRI of idiopathic and secondarily generalized epilepsies. Neuroimage. 2006;31(4):1700–10. doi: 10.1016/j.neuroimage.2006.02.016. Epub 2006/04/21. [DOI] [PubMed] [Google Scholar]
- 27.Laufs H, Lengler U, Hamandi K, Kleinschmidt A, Krakow K. Linking generalized spike-and-wave discharges and resting state brain activity by using EEG/fMRI in a patient with absence seizures. Epilepsia. 2006;47(2):444–8. doi: 10.1111/j.1528-1167.2006.00443.x. Epub 2006/02/28. [DOI] [PubMed] [Google Scholar]
- 28.Moeller F, Siebner HR, Wolff S, Muhle H, Granert O, Jansen O, et al. Simultaneous EEG-fMRI in drug-naive children with newly diagnosed absence epilepsy. Epilepsia. 2008;49(9):1510–9. doi: 10.1111/j.1528-1167.2008.01626.x. Epub 2008/04/26. [DOI] [PubMed] [Google Scholar]
- 29.Salek-Haddadi A, Lemieux L, Merschhemke M, Friston KJ, Duncan JS, Fish DR. Functional magnetic resonance imaging of human absence seizures. Ann Neurol. 2003;53(5):663–7. doi: 10.1002/ana.10586. [DOI] [PubMed] [Google Scholar]
- 30.Szaflarski JP, DiFrancesco M, Hirschauer T, Banks C, Privitera MD, Gotman J, et al. Cortical and subcortical contributions to absence seizure onset examined with EEG/fMRI. Epilepsy Behav. 2010;18(4):404–13. doi: 10.1016/j.yebeh.2010.05.009. Epub 2010/06/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Szaflarski JP, Kay B, Gotman J, Privitera MD, Holland SK. The relationship between the localization of the generalized spike and wave discharge generators and the response to valproate. Epilepsia. 2013;54(3):471–80. doi: 10.1111/epi.12062. Epub 2013/01/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tyvaert L, Chassagnon S, Sadikot A, Levan P, Dubeau F, Gotman J. Thalamic nuclei activity in idiopathic generalized epilepsy: An EEG-fMRI study. Neurology. 2009;73(23):2018–22. doi: 10.1212/WNL.0b013e3181c55d02. Epub 2009/12/10. [DOI] [PubMed] [Google Scholar]
- 33.Bai X, Vestal M, Berman R, Negishi M, Spann M, Vega C, et al. Dynamic time course of typical childhood absence seizures: EEG, behavior, and functional magnetic resonance imaging. J Neurosci. 2010;30(17):5884–93. doi: 10.1523/JNEUROSCI.5101-09.2010. Epub 2010/04/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moeller F, Siebner HR, Wolff S, Muhle H, Boor R, Granert O, et al. Changes in activity of striato-thalamo-cortical network precede generalized spike wave discharges. Neuroimage. 2008;39(4):1839–49. doi: 10.1016/j.neuroimage.2007.10.058. Epub 2007/12/18. [DOI] [PubMed] [Google Scholar]
- 35.Pillay N, Archer JS, Badawy RA, Flanagan DF, Berkovic SF, Jackson G. Networks underlying paroxysmal fast activity and slow spike and wave in Lennox-Gastaut syndrome. Neurology. 2013;81(7):665–73. doi: 10.1212/WNL.0b013e3182a08f6a. Epub 2013/07/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Szaflarski JP, Allendorfer JB. Topiramate and its effect on fMRI of language in patients with right or left temporal lobe epilepsy. Epilepsy Behav. 2012 doi: 10.1016/j.yebeh.2012.02.022. Epub 2012/04/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gotman J, Grova C, Bagshaw A, Kobayashi E, Aghakhani Y, Dubeau F. Generalized epileptic discharges show thalamocortical activation and suspension of the default state of the brain. Proc Natl Acad Sci U S A. 2005;102(42):15236–40. doi: 10.1073/pnas.0504935102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li Q, Luo C, Yang T, Yao Z, He L, Liu L, et al. EEG-fMRI study on the interictal and ictal generalized spike-wave discharges in patients with childhood absence epilepsy. Epilepsy Res. 2009;87(2–3):160–8. doi: 10.1016/j.eplepsyres.2009.08.018. Epub 2009/10/20. [DOI] [PubMed] [Google Scholar]
- 39.Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102(27):9673–8. doi: 10.1073/pnas.0504136102. Epub 2005/06/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morgan VL, Gore JC, Szaflarski JP. Temporal clustering analysis: what does it tell us about the resting state of the brain? Med Sci Monit. 2008;14(7):CR345–52. Epub 2008/07/02. [PMC free article] [PubMed] [Google Scholar]
- 41.Raichle ME, Snyder AZ. A default mode of brain function: a brief history of an evolving idea. Neuroimage. 2007;37(4):1083–90. doi: 10.1016/j.neuroimage.2007.02.041. discussion 97–9. [DOI] [PubMed] [Google Scholar]
- 42.Vanhaudenhuyse A, Noirhomme Q, Tshibanda LJ, Bruno MA, Boveroux P, Schnakers C, et al. Default network connectivity reflects the level of consciousness in non-communicative brain-damaged patients. Brain. 2010;133(Pt 1):161–71. doi: 10.1093/brain/awp313. Epub 2009/12/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Blumenfeld H. Impaired consciousness in epilepsy. Lancet Neurol. 2012;11(9):814–26. doi: 10.1016/S1474-4422(12)70188-6. Epub 2012/08/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vaudano AE, Laufs H, Kiebel SJ, Carmichael DW, Hamandi K, Guye M, et al. Causal hierarchy within the thalamo-cortical network in spike and wave discharges. PloS one. 2009;4(8):e6475. doi: 10.1371/journal.pone.0006475. Epub 2009/08/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Blumenfeld H. The thalamus and seizures. Arch Neurol. 2002;59(1):135–7. doi: 10.1001/archneur.59.1.135. [DOI] [PubMed] [Google Scholar]
- 46.Herrero MT, Barcia C, Navarro JM. Functional anatomy of thalamus and basal ganglia. Child’s nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 2002;18(8):386–404. doi: 10.1007/s00381-002-0604-1. Epub 2002/08/23. [DOI] [PubMed] [Google Scholar]
- 47.Betting LE, Mory SB, Li LM, Lopes-Cendes I, Guerreiro MM, Guerreiro CA, et al. Voxel-based morphometry in patients with idiopathic generalized epilepsies. Neuroimage. 2006;32(2):498–502. doi: 10.1016/j.neuroimage.2006.04.174. Epub 2006/05/17. [DOI] [PubMed] [Google Scholar]
- 48.O’Muircheartaigh J, Vollmar C, Barker GJ, Kumari V, Symms MR, Thompson P, et al. Abnormal thalamocortical structural and functional connectivity in juvenile myoclonic epilepsy. Brain. 2012;135(Pt 12):3635–44. doi: 10.1093/brain/aws296. Epub 2012/12/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Friston K, Holmes A, Worsley K, Poline J-P, Frith C, Frackowiak R. Statistical Parametric Maps in Functional Imaging: A General Linear Approach. Hum Brain Mapp. 1994;2(4):189–210. [Google Scholar]
- 50.Molloy EK, Meyerand ME, Birn RM. The influence of spatial resolution and smoothing on the detectability of resting-state and task fMRI. Neuroimage. 2013 doi: 10.1016/j.neuroimage.2013.09.001. Epub 2013/09/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bowman FD, Guo Y, Derado G. Statistical approaches to functional neuroimaging data. Neuroimaging clinics of North America. 2007;17(4):441–58. viii. doi: 10.1016/j.nic.2007.09.002. Epub 2007/11/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nichols TE, Holmes AP. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp. 2002;15(1):1–25. doi: 10.1002/hbm.1058. Epub 2001/12/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lenglet C, Abosch A, Yacoub E, De Martino F, Sapiro G, Harel N. Comprehensive in vivo mapping of the human basal ganglia and thalamic connectome in individuals using 7T MRI. PloS one. 2012;7(1):e29153. doi: 10.1371/journal.pone.0029153. Epub 2012/01/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Menon RS, Goodyear BG. Submillimeter functional localization in human striate cortex using BOLD contrast at 4 Tesla: implications for the vascular point-spread function. Magn Reson Med. 1999;41(2):230–5. doi: 10.1002/(sici)1522-2594(199902)41:2<230::aid-mrm3>3.0.co;2-o. Epub 1999/03/18. [DOI] [PubMed] [Google Scholar]
- 55.Yacoub E, Harel N, Ugurbil K. High-field fMRI unveils orientation columns in humans. Proc Natl Acad Sci U S A. 2008;105(30):10607–12. doi: 10.1073/pnas.0804110105. Epub 2008/07/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Metzger CD, Eckert U, Steiner J, Sartorius A, Buchmann JE, Stadler J, et al. High field FMRI reveals thalamocortical integration of segregated cognitive and emotional processing in mediodorsal and intralaminar thalamic nuclei. Frontiers in neuroanatomy. 2010;4:138. doi: 10.3389/fnana.2010.00138. Epub 2010/11/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Neuner I, Arrubla J, Felder J, Shah NJ. Simultaneous EEG-fMRI acquisition at low, high and ultra-high magnetic fields up to 9.4T: Perspectives and challenges. Neuroimage. 2013 doi: 10.1016/j.neuroimage.2013.06.048. Epub 2013/06/26. [DOI] [PubMed] [Google Scholar]
- 58.Moeller F, Maneshi M, Pittau F, Gholipour T, Bellec P, Dubeau F, et al. Functional connectivity in patients with idiopathic generalized epilepsy. Epilepsia. 2011;52(3):515–22. doi: 10.1111/j.1528-1167.2010.02938.x. Epub 2011/01/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang Z, Zhang Z, Jiao Q, Liao W, Chen G, Sun K, et al. Impairments of thalamic nuclei in idiopathic generalized epilepsy revealed by a study combining morphological and functional connectivity MRI. PloS one. 2012;7(7):e39701. doi: 10.1371/journal.pone.0039701. Epub 2012/07/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kay B, Holland SK, Privitera M, Szaflarski JP. Differences in Paracingulate Connectivity Associated with Epileptiform Discharges and Uncontrolled Seizures in Idiopathic Generalized Epilepsy. Epilepsia. 2013 doi: 10.1111/epi.12486. in print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang Z, Liao W, Chen H, Mantini D, Ding JR, Xu Q, et al. Altered functional-structural coupling of large-scale brain networks in idiopathic generalized epilepsy. Brain. 2011;134(Pt 10):2912–28. doi: 10.1093/brain/awr223. Epub 2011/10/07. [DOI] [PubMed] [Google Scholar]
- 62.Greicius MD, Supekar K, Menon V, Dougherty RF. Resting-state functional connectivity reflects structural connectivity in the default mode network. Cereb Cortex. 2009;19(1):72–8. doi: 10.1093/cercor/bhn059. Epub 2008/04/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Luo C, Li Q, Lai Y, Xia Y, Qin Y, Liao W, et al. Altered functional connectivity in default mode network in absence epilepsy: a resting-state fMRI study. Hum Brain Mapp. 2011;32(3):438–49. doi: 10.1002/hbm.21034. Epub 2011/02/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.McGill ML, Devinsky O, Kelly C, Milham M, Castellanos FX, Quinn BT, et al. Default mode network abnormalities in idiopathic generalized epilepsy. Epilepsy Behav. 2012;23(3):353–9. doi: 10.1016/j.yebeh.2012.01.013. Epub 2012/03/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kay BP, Difrancesco M, Privitera M, Gotman J, Holland SK, Szaflarski JP. Reduced default mode network connectivity in treatment-resistant idiopathic generalized epilepsy. Epilepsia. 2013 doi: 10.1111/epi.12057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Luo C, Li Q, Xia Y, Lei X, Xue K, Yao Z, et al. Resting state basal ganglia network in idiopathic generalized epilepsy. Hum Brain Mapp. 2012;33(6):1279–94. doi: 10.1002/hbm.21286. Epub 2011/04/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Iqbal N, Caswell HL, Hare DJ, Pilkington O, Mercer S, Duncan S. Neuropsychological profiles of patients with juvenile myoclonic epilepsy and their siblings: a preliminary controlled experimental video-EEG case series. Epilepsy Behav. 2009;14(3):516–21. doi: 10.1016/j.yebeh.2008.12.025. Epub 2009/01/27. [DOI] [PubMed] [Google Scholar]
- 68.Wandschneider B, Kopp UA, Kliegel M, Stephani U, Kurlemann G, Janz D, et al. Prospective memory in patients with juvenile myoclonic epilepsy and their healthy siblings. Neurology. 2010;75(24):2161–7. doi: 10.1212/WNL.0b013e318202010a. Epub 2010/11/05. [DOI] [PubMed] [Google Scholar]
- 69.Killory BD, Bai X, Negishi M, Vega C, Spann MN, Vestal M, et al. Impaired attention and network connectivity in childhood absence epilepsy. Neuroimage. 2011;56(4):2209–17. doi: 10.1016/j.neuroimage.2011.03.036. Epub 2011/03/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Maneshi M, Moeller F, Fahoum F, Gotman J, Grova C. Resting-state connectivity of the sustained attention network correlates with disease duration in idiopathic generalized epilepsy. PloS one. 2012;7(12):e50359. doi: 10.1371/journal.pone.0050359. Epub 2012/12/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kim SG, Richter W, Ugurbil K. Limitations of temporal resolution in functional MRI. Magn Reson Med. 1997;37(4):631–6. doi: 10.1002/mrm.1910370427. Epub 1997/04/01. [DOI] [PubMed] [Google Scholar]
- 72.Boor R, Jacobs J, Hinzmann A, Bauermann T, Scherg M, Boor S, et al. Combined spike-related functional MRI and multiple source analysis in the non-invasive spike localization of benign rolandic epilepsy. Clin Neurophysiol. 2007;118(4):901–9. doi: 10.1016/j.clinph.2006.11.272. Epub 2007/02/24. [DOI] [PubMed] [Google Scholar]
- 73.Sperli F, Spinelli L, Seeck M, Kurian M, Michel CM, Lantz G. EEG source imaging in pediatric epilepsy surgery: a new perspective in presurgical workup. Epilepsia. 2006;47(6):981–90. doi: 10.1111/j.1528-1167.2006.00550.x. Epub 2006/07/11. [DOI] [PubMed] [Google Scholar]
- 74.Groening K, Brodbeck V, Moeller F, Wolff S, van Baalen A, Michel CM, et al. Combination of EEG-fMRI and EEG source analysis improves interpretation of spike-associated activation networks in paediatric pharmacoresistant focal epilepsies. Neuroimage. 2009;46(3):827–33. doi: 10.1016/j.neuroimage.2009.02.026. Epub 2009/03/07. [DOI] [PubMed] [Google Scholar]
- 75.Zijlmans M, Huiskamp G, Hersevoort M, Seppenwoolde JH, van Huffelen AC, Leijten FS. EEG-fMRI in the preoperative work-up for epilepsy surgery. Brain. 2007;130(Pt 9):2343–53. doi: 10.1093/brain/awm141. Epub 2007/06/26. [DOI] [PubMed] [Google Scholar]
- 76.Vulliemoz S, Thornton R, Rodionov R, Carmichael DW, Guye M, Lhatoo S, et al. The spatio-temporal mapping of epileptic networks: combination of EEG-fMRI and EEG source imaging. Neuroimage. 2009;46(3):834–43. doi: 10.1016/j.neuroimage.2009.01.070. Epub 2009/05/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pflieger M, Greenblatt R. Nonlinear Analysis of Multimodal Dynamic Brain Imaging Data. International Journal of Bioelectromagnetism. 2001;3(1) [Google Scholar]
- 78.Benar CG, Grova C, Kobayashi E, Bagshaw AP, Aghakhani Y, Dubeau F, et al. EEG-fMRI of epileptic spikes: concordance with EEG source localization and intracranial EEG. Neuroimage. 2006;30(4):1161–70. doi: 10.1016/j.neuroimage.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 79.Vulliemoz S, Carmichael DW, Rosenkranz K, Diehl B, Rodionov R, Walker MC, et al. Simultaneous intracranial EEG and fMRI of interictal epileptic discharges in humans. Neuroimage. 2011;54(1):182–90. doi: 10.1016/j.neuroimage.2010.08.004. Epub 2010/08/17. [DOI] [PubMed] [Google Scholar]
- 80.Grova C, Daunizeau J, Kobayashi E, Bagshaw AP, Lina JM, Dubeau F, et al. Concordance between distributed EEG source localization and simultaneous EEG-fMRI studies of epileptic spikes. Neuroimage. 2008;39(2):755–74. doi: 10.1016/j.neuroimage.2007.08.020. Epub 2007/10/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Seeck M, Lazeyras F, Michel CM, Blanke O, Gericke CA, Ives J, et al. Non-invasive epileptic focus localization using EEG-triggered functional MRI and electromagnetic tomography. Electroencephalogr Clin Neurophysiol. 1998;106(6):508–12. doi: 10.1016/s0013-4694(98)00017-0. [DOI] [PubMed] [Google Scholar]
- 82.Mulert C, Jager L, Schmitt R, Bussfeld P, Pogarell O, Moller HJ, et al. Integration of fMRI and simultaneous EEG: towards a comprehensive understanding of localization and time-course of brain activity in target detection. Neuroimage. 2004;22(1):83–94. doi: 10.1016/j.neuroimage.2003.10.051. Epub 2004/04/28. [DOI] [PubMed] [Google Scholar]
- 83.Lemieux L, Krakow K, Fish DR. Comparison of spike-triggered functional MRI BOLD activation and EEG dipole model localization. Neuroimage. 2001;14(5):1097–104. doi: 10.1006/nimg.2001.0896. Epub 2001/11/08. [DOI] [PubMed] [Google Scholar]
- 84.Brookes MJ, Vrba J, Mullinger KJ, Geirsdottir GB, Yan WX, Stevenson CM, et al. Source localisation in concurrent EEG/fMRI: applications at 7T. Neuroimage. 2009;45(2):440–52. doi: 10.1016/j.neuroimage.2008.10.047. Epub 2008/12/04. [DOI] [PubMed] [Google Scholar]
- 85.Vulliemoz S, Rodionov R, Carmichael DW, Thornton R, Guye M, Lhatoo SD, et al. Continuous EEG source imaging enhances analysis of EEG-fMRI in focal epilepsy. Neuroimage. 2010;49(4):3219–29. doi: 10.1016/j.neuroimage.2009.11.055. Epub 2009/12/02. [DOI] [PubMed] [Google Scholar]
- 86.Ahlfors SP, Simpson GV. Geometrical interpretation of fMRI-guided MEG/EEG inverse estimates. Neuroimage. 2004;22(1):323–32. doi: 10.1016/j.neuroimage.2003.12.044. Epub 2004/04/28. [DOI] [PubMed] [Google Scholar]
- 87.Babiloni F, Babiloni C, Carducci F, Romani GL, Rossini PM, Angelone LM, et al. Multimodal integration of high-resolution EEG and functional magnetic resonance imaging data: a simulation study. Neuroimage. 2003;19(1):1–15. doi: 10.1016/s1053-8119(03)00052-1. Epub 2003/06/05. [DOI] [PubMed] [Google Scholar]
- 88.Bagshaw AP, Kobayashi E, Dubeau F, Pike GB, Gotman J. Correspondence between EEG-fMRI and EEG dipole localisation of interictal discharges in focal epilepsy. Neuroimage. 2006;30(2):417–25. doi: 10.1016/j.neuroimage.2005.09.033. [DOI] [PubMed] [Google Scholar]
- 89.Daunizeau J, Grova C, Marrelec G, Mattout J, Jbabdi S, Pelegrini-Issac M, et al. Symmetrical event-related EEG/fMRI information fusion in a variational Bayesian framework. Neuroimage. 2007;36(1):69–87. doi: 10.1016/j.neuroimage.2007.01.044. Epub 2007/04/06. [DOI] [PubMed] [Google Scholar]
- 90.Kay BP, DiFrancesco MW, Privitera MD, Gotman J, Holland SK, Szaflarski JP. Reduced default mode network connectivity in treatment-resistant idiopathic generalized epilepsy. Epilepsia. 2013;54(3):461–70. doi: 10.1111/epi.12057. Epub 2013/01/09. [DOI] [PMC free article] [PubMed] [Google Scholar]