Abstract
Objectives
Emotional stress may disproportionally affect young women with ischemic heart disease. We sought to examine whether mental stress-induced myocardial ischemia (MSIMI), but not exercise-induced ischemia, is more common in young women with previous myocardial infarction (MI) than men.
Methods
We studied 98 post-MI patients (49 women and 49 men) aged 38-60 years. Women and men were matched for age, MI type, and months since MI. Patients underwent [99mTc]sestamibi perfusion imaging at rest, after mental stress, and after exercise/pharmacological stress. Perfusion defect scores were obtained with observer-independent software. A summed difference score (SDS), the difference between stress and rest scores, was used to quantify ischemia under both stress conditions.
Results
Women aged 50 or younger, but not older women, showed a more adverse psychosocial profile than age-matched men, but did not differ for conventional risk factors and tended to have less angiographic coronary artery disease (CAD). Compared with age-matched men, women aged 50 or younger exhibited a higher SDS with mental stress (3.1 vs. 1.5, p=0.029) and had twice the rate of MSIMI (SDS ≥3), 52% vs. 25%, while ischemia with physical stress did not differ (36% vs 25%). In older patients there were no sex differences in MSIMI. The higher prevalence of MSIMI in young women persisted when adjusting for sociodemographic and lifestyle factors, CAD severity and depression.
Conclusions
MSIMI post-MI is more common in women aged 50 or younger compared to age-matched men. These sex differences are not observed in post-MI patients who are older than 50 years.
Keywords: cardiovascular diseases, stress, ischemia, gender
INTRODUCTION
Growing evidence supports differences in the pathophysiology, clinical presentation, and prognosis of ischemic heart disease (IHD) between women and men; yet much remains to be learned about the unique characteristics of IHD in women (1,2). Although IHD mortality rates have been decreasing in the United States, a disturbing trend is emerging for a lower decline among young women (3). Notably, young women also show higher mortality and complication rates after an acute myocardial infarction (MI) compared with men of similar age, a difference not seen in older patients (4-8). The excess mortality in young women after MI is independent of coronary risk factors, comorbidities, and treatments, and occurs despite the fact that women have less coronary atherosclerosis and smaller infarcts. The pathophysiology of these findings is unexplained, and young women with IHD remain understudied. We hypothesized that a factor largely ignored in previous literature, emotional stress, might be implicated.
The cardiovascular system is deemed susceptible to emotional stress, and young women appear to be especially vulnerable to psychosocial risk factors (9-12). Direct evidence that emotional stress affects disproportionally young women with IHD is limited, however, partly due to methodological limitations in the assessment and quantification of stress. Myocardial ischemia can be induced by emotional stress in the laboratory in approximately one third to half of patients with IHD (13,14). This phenomenon, commonly referred to as “mental stress-induced myocardial ischemia,” or MSIMI, has a prognostic value similar to that of exercise or pharmacologically-induced ischemia, but is typically painless, occurs at lower levels of oxygen demand than exercise induced ischemia, and is not related to severity of coronary artery disease (CAD) or previous revascularization (14-16). Thus, MSIMI is likely to reflect altered pathophysiological responses to emotional stress rather than disease severity, and may provide a useful model for comparing the effects of emotional stress on IHD between women and men. Accordingly, in a study of young and middle-aged post-MI patients, we investigated whether MSIMI is more frequently in young women than in age-matched men.
METHODS
Participants
Study participants were recruited from the pool of patients admitted with a confirmed diagnosis of MI at Emory-affiliated hospitals. Eligible patients were 18 to 59 years of age at the time of screening and had a documented history of MI within the past 6 months. Women and men were matched by age (within 2 years), MI type (ST-elevation MI or non-ST-elevation MI), and time since the index MI (within 2 months). The diagnosis of MI was verified by medical record review based on standard criteria of troponin level increase and ECG changes (17). Subjects were excluded if they had unstable angina or acute MI within the past week, or a severe comorbid medical or psychiatric disorder that could interfere with study results, such as cancer, renal failure, current alcohol or substance abuse or schizophrenia. Subjects were also excluded if they weighed over 400 lbs (due to limits on the weight bearing of the nuclear stress test equipment), if they were pregnant or breastfeeding, or if they were currently using postmenopausal hormone therapy or psychotropic medications other than antidepressants. Finally, patients were excluded if they were unable to exercise on a treadmill, based on a score <5 METs on the Duke Activity Status Index (DASI), which identifies patients who cannot exercise to heart rate targets (18). We enrolled all consecutive women who were eligible based on our inclusion and exclusion criteria and agreed to participate. Once we enrolled a woman, we selected the next male patient on our list who met the matching criteria.
Study Design
Study participants underwent two one-day single-photon emission computed tomography (SPECT) imaging studies, one with mental stress and one with exercise or pharmacological stress, within one week of each other; the order of the two sessions was balanced. All testing was done in the morning after an overnight fast, and anti-ischemic medications were held for 24 hours prior to testing. Sociodemographic and psychosocial data were collected at the first visit prior to cardiac testing. At the end of the study protocol, medical records were abstracted for clinical information, including catheterization data. The study protocol was approved by the Emory University Institutional Review Board, and informed consent was obtained from all participants.
Mental Stress Procedure
Initially, patients rested for 30 minutes in a quiet, dimly lit, temperature-controlled room. At the end of the resting period, mental stress was induced by a standardized public speaking task as previously described (19). Patients were asked to imagine a real-life stressful situation, such as a close relative been mistreated in a nursing home, and asked to make up a realistic story around this scenario. They were given two minutes to prepare a statement and then three minutes to present it in front of a video camera and an audience wearing white coats. Participants were told that their speech would be evaluated by the laboratory staff for content, quality and duration. Blood pressure and heart rate were recorded at five minute intervals during the resting phase and at one minute intervals during and after the mental stress task. The rate-pressure product was calculated as peak systolic blood pressure X peak heart rate. Subjective ratings of distress were also obtained at baseline and after the mental stress test with the Subjective Units of Distress Scale (20) on a linear scale of 0 to 100, with 100 being the highest level of distress. We also obtained visual analog ratings of nervousness, anxiety, fear and anger with a scale of 0-4, with 4 being extreme. At the end of the test, patients were debriefed.
Myocardial Perfusion Imaging
Participants underwent three SPECT myocardial perfusion imaging scans following injection of sestamibi radiolabelled with Technetium-99m ([99mTc]), at rest, during mental stress, and during “physical stress,” the latter including primarily exercise stress. However, 16 patients, 10 men and 6 women, were unable to reach the target heart rate despite scoring ≥ 5 METs on the DASI and underwent a pharmacological stress test instead. Testing was done in two separate days (to prevent the effects of one stress to contaminate the results of the following one) up to one week apart on a dedicated ultra-fast solid-state camera (Discovery NM 530c) without attenuation correction (21). Only one resting scan was performed with myocardial perfusion images acquired after the injection of 8-15 mCi of [99mTc] sestamibi according to body weight. The stress scan (either mental or physical) followed at least 2 hours later and 20-30 mCi of [99mTc] sestamibi were used for this phase.
On the mental stress day, [99mTc]sestamibi was injected one minute after the onset of the public speech task. This timing is in accordance with commonly employed mental stress protocols with myocardial perfusion imaging (22,23). It reflects the fact that maximal heart rate, blood pressure, and neurohormonal responses to mental stress increase rapidly at the near onset of the stressful task, and ischemic abnormalities are induced relatively quickly during this process (19,24). On the exercise stress day, participants underwent a standard Bruce protocol with exercise target set at 85% of maximum predicted heart rate based on the patient's sex and age. [99mTc]sestamibi was injected at peak exertion. Stress images were acquired 45-60 minutes later using previously described methodology (25). The ECG, blood pressure and heart rate were continuously monitored during the procedure. For patients undergoing a pharmacological stress test, 0.4 mg of regadenoson (Abbott, Chicago, IL), an adenosine receptor agonist, were administered intravenously in approximately 10 seconds, and [99mTc] sestamibi was injected right after regadenoson. SPECT images were then obtained as described above.
Myocardial perfusion abnormalities were quantified by means of the Emory Cardiac Toolbox, a software that provides objective quantitative assessment of perfusion with established validity and reproducibility (26,27). Briefly, the three-dimensional tracer uptake distribution in the left ventricle was oriented along the short axis and sampled onto a two-dimensional polar map. An operator-independent summed score, quantifying the extent and severity of perfusion defects across 17 segments of the myocardium at rest and during stress, was computed by the software according to published methodology (27). In each region, defect severity was quantified using a 4-point scale from normal (score=0) to absent perfusion (score=4). The regional severity scoring was then summed up across the 17 myocardial segments yielding a total score. Separate scores were obtained for the rest images (summed rest score, SRS) and the stress images (summed stress score, SSS). A summed difference score (SDS) was obtained by subtracting the rest score from the stress score; in the presence of a reversible defect (or ischemia) the score is positive. This scoring system using automatic quantitative analysis has excellent prognostic value, which is similar to semi-quantitative expert visual analysis (28). For exercise/pharmacological stress, presence of ischemia was defined as a SDS ≥4 (29). A SDS ≥3 is typically used as evidence of MSIMI (16,30). However, the analyses were repeated with the same cut point (SDS≥4) as for physical stress.
Other Measurements
Information on sociodemographic factors was collected using standard questionnaires from population studies. A detailed medical history including medication use was obtained by a research nurse. Weight and height were used to calculate body mass index as weight in kilograms divided by height in meters squared. Blood samples were drawn for the measurements of glucose and a lipid profile after an overnight fast.
Angiographic data were obtained from the coronary angiogram performed in conjunction with the index MI. CAD severity was quantified using the Gensini semi-quantitative angiographic scoring system (31), which takes into account degree of luminal narrowing along with a multiplier for specific coronary tree locations. From the coronary angiogram we also derived the left ventricular ejection fraction.
Behavioral, social, and mental health information was obtained using psychometric instruments with established reliability and validity. Depressive symptoms were assessed with the Beck Depression Inventory-II (BDI-II) (32), a reliable and valid self-report measure of depression which has been widely used in cardiac populations. We also administered the Structured Clinical Interview for DSM IV (SCID) (33) to classify participants based on a lifetime history of major depression and other psychiatric disorders. The Early Trauma Inventory, a reliable and valid measure of childhood traumatic experiences (34), was used to assess physical, emotional, and sexual abuse, and general traumatic events before age 18 years. A total score was computed as a continuous variable. Each domain was also dichotomized based on previously established cutoffs (34). Social and emotional support were measured by means of the Enhancing Recovery in Coronary Heart Disease (ENRICHD) Social Support Inventory (35), a five-item scale assessing both social and emotional support. We also administered the Cohen's Perceived Stress Scale, a 10 item survey of general stress validated in multi-ethnic populations (36), and the State-Trait Anxiety Inventory, a 40-item questionnaire to measure anxiety as an emotional state or a personality trait (37).
Statistical Analysis
Since we were interested in the role of age on sex differences in MSIMI, and because younger and older women appeared different in their risk factor distribution when compared with men, all analyses were stratified in an exploratory fashion by the median/mean age of 50 years. Women and men were compared for demographic, behavioral and clinical characteristics, as well as for hemodynamic changes during mental and physical stress, using t tests for continuous variables and chi-squared tests for categorical variables.
Myocardial perfusion imaging data included the rest perfusion score (SRS), the stress perfusion scores (SSS) and the difference between these two (SDS) for both mental and physical stress. We also examined sex differences in a binary ischemia variable defined as a SDS≥3 for mental stress and SDS ≥4 for physical stress. Results were reanalyzed with a SDS ≥4 for both conditions.
Multiple linear regression models were used to assess differences in stress perfusion scores between women and men adjusting for possible confounding factors. The SDS, which quantifies ischemia, was our main outcome of interest. Since the SDS for both mental and physical stress was highly skewed, while the SSS was approximately normally distributed, we used the SSS scores as dependent variables while adjusting for the rest score (SRS). Because of the mathematical relationship between these scores, the coefficient from a model with SSS as dependent variable, adjusted for SRS, is identical to that from a model where the dependent variable is the difference score (SDS) adjusted for SRS. This strategy allowed us to obtain non-biased standard errors and p values for the relationship between sex and mental/physical SDS. In a series of cumulative hierarchical models, we adjusted for a set of factors that were considered a priori as either possible confounding factors or mediators of the relationships under study. Adjustment factors included sociodemographic and lifestyle characteristics (race, income below poverty level, and current cigarette smoking), CAD severity (Gensini angiographic score and left ventricular ejection fraction), and depressive symptoms (BDI-II score). In a final model we also adjusted for number of months since the MI, given that we found a slight difference between women and men. Analyses were conducted with and without accounting for clustering given the matched design. Since the results were virtually identical, for simplicity we opted to present unmatched results.
RESULTS
Study Sample
Between July 2009 and April 2012, 129 women met the inclusion criteria of the study; of these, 26 were deemed too ill to participate; 11 lived too far, and 43 declined participation or did not come to the scheduled visit, leaving 49 who were enrolled. Overall, 139 men were eligible based on study inclusion criteria and matching algorithm with enrolled women; 24 were too ill, 12 lived too far, and 54 declined participation or did not keep the appointment, leaving a final sample of 49 women and 49 matched men younger than 60 years. The mean and median age was 50 years, with a range of 38 to 60 years (all patients were younger than 60 years, with the exception of one whose birthday occurred after screening but before study visits); the median time since the MI was 5 months; and 45% had had an ST-elevation MI. The mean left ventricular ejection fraction was 52% and only 6 patients had an ejection fraction <30%. Overall, 55% of the women reported having reached menopause; this proportion was 17% among women age 50 or younger. In the younger group, women differed from men in their sociodemographic and psychosocial profile, while they were more similar to men in the older group (Table 1). They were more often African American, more likely to have an income level below poverty, and to be current smokers. They had higher levels of depressive symptoms and reported more often a history of sexual abuse, although perceived stress and anxiety did not differ. In either age group, however, there were no sex differences in medical history and conventional CAD risk factors, except for the expected higher HDL-cholesterol among women. Medication use was also similar. In the younger age group women were enrolled slightly earlier since the index MI than men (4.3 months vs. 5.2 months). In both age groups women tended to have less severe angiographic CAD than men; overall, the mean Gensini angiographic score was 30.2 in women and 45.6 in men (p=0.023).
Table 1.
Sex differences in patient characteristics stratified by age.
| Age ≤50 yrs |
Age >50 yrs |
|||||||
|---|---|---|---|---|---|---|---|---|
| Women (N=24) | Men (N=25) | % Difference | p | Women (N=25) | Men (N=24) | % Difference | p | |
|
Demographic and Lifestyle Factors
| ||||||||
| Age, years | 45.2 (4.1) | 45.9 (3.4) | −1.6 | 0.51 | 55.3 (3.0) | 55.3 (2.5) | 0.05 | 0.97 |
| Black Race, % | 70.8 | 40.0 | 77.0 | 0.030 | 60.0 | 45.8 | 31.0 | 0.32 |
| Married, % | 33.3 | 52.0 | −36.0 | 0.19 | 40.0 | 37.5 | 6.7 | 0.86 |
| Education, total school years | 12.9 (2.4) | 13.5 (3.2) | −4.5 | 0.46 | 13.9 (3.1) | 14.8 (3.6) | −5. 9 | 0.36 |
| Income below poverty level (≤$20,000), % | 58.3 | 20.8 | 180.3 | 0.008 | 20.8 | 29.2 | −28.8 | 0.50 |
| Current smoking % | 41.7 | 16.0 | 160.6 | 0.047 | 28.0 | 29.2 | −4.1 | 0.93 |
|
Medical History and CHD Risk Factors | ||||||||
| ST-elevation MI, % | 45.8 | 48.0 | −4.6 | 0.88 | 44.0 | 41.7 | 5.5 | 0.88 |
| Time since MI (months) | 4.3 | 5.2 | −17% | 0.01 | 4.6 | 5.0 | −8% | 0.29 |
| Angina in past 4 weeks, % | 62.5 | 44.0 | 42.0 | 0.19 | 68.0 | 62.5 | 8.8 | 0.67 |
| Hypertension, % | 66.7 | 62.5 | 6.7 | 0.76 | 72.0 | 75.0 | −4.0 | 0.81 |
| Hyperlipidemia, % | 66.7 | 70.8 | −5.8 | 0.75 | 80.0 | 75.0 | 6.7 | 0.67 |
| Diabetes, % | 25.0 | 16.7 | 49.7 | 0.72 | 16.0 | 25.0 | −36.0 | 0.50 |
| Previous revascularization, % | 75.0 | 92.0 | −18.5 | 0.12 | 80.0 | 95.8 | −16.5 | 0.091 |
| BMI | 32.36 (7.47) | 31.30 (4.86) | 3.4 | 0.56 | 31.03 (6.30) | 29.21 (6.61) | 6.2 | 0.33 |
| BMI ≤30, % | 50.0 | 58.3 | −14.2 | 0.56 | 48.0 | 33.3 | 44.4 | 0.30 |
| Triglycerides, mg/dL | 117.3 (70.3) | 153.4 (108.6) | −23.5 | 0.18 | 129.3 (132.9) | 129.9 (76.6) | −0.5 | 0.97 |
| HDL-Cholesterol, mg/dL | 50.3 (14.3) | 41.1 (8.7) | 22.2 | 0.014 | 53.5 (15.0) | 44.2 (11.0) | 21.1 | 0.019 |
| LDL-cholesterol, mg/dL | 94.1 (35.8) | 95.6 (33.3) | −1.5 | 0.89 | 92.8 (30.5) | 87.4 (35.5) | 6.2 | 0.58 |
|
Coronary Angiography Data (at time of MI) | ||||||||
| Gensini score | 26.2 (23.8) | 40.0 (38.3) | −34.4 | 0.14 | 34.0 (26.2) | 51.5 (40.7) | −34.0 | 0.082 |
| Left ventricular ejection fraction | 52.0 (8.2) | 53.9 (8.4) | −3.6 | 0.45 | 53.5 (10.6) | 47.6 (14.3) | 12.5 | 0.11 |
|
Psychosocial Factors | ||||||||
| Lifetime history of major depression, % | 50.0 | 30.4 | 64.5 | 0.17 | 44.0 | 25.0 | 76.0 | 0.16 |
| Lifetime history of substance abuse, % | 54.2 | 52.0 | 4.2 | 0.88 | 60.0 | 54.2 | 10.7 | 0.68 |
| Beck Depression Inventory | 13.9 (9.5) | 8.0 (6.5) | 73.4 | 0.015 | 12.1 (10.3) | 10.9 (7.0) | 10.7 | 0.64 |
|
Early Trauma inventory | ||||||||
| Total score | 7.7 (5.8) | 6.9 (5.3) | 12.0 | 0.60 | 7.7 (6.0) | 7.4 (4.2) | 4.1 | 0.84 |
| Physical abuse, % | 25.0 | 40.0 | −37.5 | 0.26 | 20.0 | 54.2 | −63.1 | 0.013 |
| Emotional abuse, % | 20.8 | 28.0 | −25.7 | 0.56 | 36.0 | 16.7 | 116 | 0.12 |
| Sexual abuse, % | 50.0 | 24.0 | 108 | 0.059 | 36.0 | 20.8 | 73.1 | 0.24 |
| General trauma, % | 50.0 | 32.0 | 56.3 | 0.20 | 52.0 | 45.8 | 13.5 | 0.66 |
| Cohen's Perceived stress scale | 15.4 (6.1) | 15.0 (8.3) | 2.5 | 0.86 | 17.8 (7.2) | 16.2 (8.7) | 10.1 | 0.48 |
| State-Trait Anxiety Inventory | ||||||||
| State | 39.1 (11.1) | 35.8 (11.2) | 9.1 | 0.32 | 41.1 (11.5) | 35.1 (11.2) | 17.1 | 0.071 |
| Trait | 39.3 (10.6) | 38.8 (10.5) | 1.5 | 0.85 | 39.9 (10.8) | 38.0 (11.6) | 5.0 | 0.55 |
| ENRICHD Social Support scale | 27.5 (4.1) | 27.5 (5.8) | −0.1 | 0.99 | 23.8 (7.8) | 25.6 (7.7) | −7.3 | 0.40 |
|
Medications, % | ||||||||
| Statins | 95.8 | 79.2 | 21.0 | 0.081 | 84.0 | 91.7 | −8.4 | 0.41 |
| Beta blockers | 91.7 | 87.5 | 4.8 | 1.00 | 84.0 | 87.5 | −4.0 | >.99 |
| ACE-Inhibitors | 54.2 | 54.2 | 0 | 0.99 | 48.0 | 62.5 | −30.2 | 0.31 |
| Aspirin | 91.7 | 83.3 | 10.1 | 0.39 | 92.0 | 83.3 | 10.4 | 0.35 |
All values are means (standard deviation), unless otherwise specified.
Hemodynamic Measurements
As shown in Table 2, both women and men achieved adequate hemodynamic responses to mental stress and physical stress, which were similar across sex and age groups. Similarly, all groups showed comparable increases in subjective ratings of distress with mental stress.
Table 2.
Hemodynamic and subjective distress measures during stress testing according to sex and age.
| Age ≤50 yrs | Age >50 yrs | |||||
|---|---|---|---|---|---|---|
| Women (N=24) | Men (N=25) | p | Women (N=25) | Men (N=24) | p | |
| Mental Stress | ||||||
| Systolic Blood Pressure (mm Hg) | ||||||
| Rest, minimum | 118.4 (17.7) | 125.3 (16.3) | 0.17 | 123.0 (17.3) | 124.4 (16.6) | 0.78 |
| Stress, maximum | 162.3 (25.2) | 171.3 (28.0) | 0.25 | 170.3 (31.3) | 175.0 (30.9) | 0.60 |
| Change | 48.0 (30.5) | 46.0 (19.4) | 0.79 | 47.3 (22.5) | 50.6 (21.2) | 0.60 |
| Diastolic Blood Pressure (mm Hg) | ||||||
| Rest, minimum | 72.5 (12. 6) | 77.5 (10.9) | 0.15 | 71.4 (8.8) | 77.7 (8.9) | 0.016 |
| Stress, maximum | 102.2 (14.9) | 107.0 (17.7) | 0.32 | 100.9 (15.1) | 109.3 (18.7) | 0.088 |
| Change | 29. 7 (12.9) | 29.5 (15.2) | 0.98 | 29.4 (11.6) | 31.6 (14.5) | 0.57 |
| Heart rate (beats/min) | ||||||
| Rest, minimum | 64.5 (8.5) | 59.8 (11.3) | 0.12 | 59.2 (9.0) | 59.5 (10.6) | 0.93 |
| Stress, maximum | 91.3 (15.3) | 88.0 (17.8) | 0.50 | 85.0 (16.7) | 92.1 (27.5) | 0.28 |
| Change | 26.7 (14.9) | 28.2(18.5) | 0.76 | 25.7 (12.7) | 32.6 (23.8) | 0.22 |
| Rate-pressure product during stress | 15,031 (4262) | 15,182 (4,305) | 0.90 | 14,596 (4,401) | 16,464 (7,033) | 0.27 |
| Subjective Ratings of Distress | ||||||
| Subjective Units of Distress Scale (0-100) | ||||||
| Baseline | 28.2 (32.7) | 10.4 (15.7) | 0.022 | 21.0 (29.0) | 21.4 (26.4) | 0.96 |
| Post-stress | 38.5 (32.3) | 30.2 (29.4) | 0.36 | 37.4 (32.9) | 32.9 (26.3) | 0.60 |
| Change | 10.3 (29.3) | 21.1 (25.6) | 0.18 | 16.4 (44.5) | 11.5 (33.2) | 0.67 |
| Visual Analog Scales (0-4) | ||||||
| Nervousness | ||||||
| Baseline | 1.04 (1.36) | 0.73 (1.26) | 0.41 | 0.96 (1.08) | 0.88 (0.93) | 0.77 |
| Post-stress | 1.54 (1.53) | 1.21 (1.34) | 0.43 | 1.58 (1.45) | 1.31 (1.27) | 0.49 |
| Change | 0.46 (1.54) | 0.48 (1.60) | 0.96 | 0.62 (1.24) | 0.44 (1.43) | 0.64 |
| Anxiety | ||||||
| Baseline | 1.42 (1.50) | 0.90 (1.27) | 0.20 | 1.50 (1.33) | 1.19 (1.22) | 0.40 |
| Post-stress | 1.67 (1.40) | 1.31 (1.25) | 0.36 | 1.70 (1.41) | 1.50 (1.35) | 0.62 |
| Change | 0.25 (1.54) | 0.42 (1.61) | 0.71 | 0.20 (1.66) | 0.31 (1.77) | 0.81 |
| Fear | ||||||
| Baseline | 0.21 (0.66) | 0.21 (0.83) | 0.99 | 0.35 (0.54) | 0.25 (0.61) | 0.55 |
| Post-stress | 0.65 (1.27) | 0.54 (1.01) | 0.75 | 0.58 (1.08) | 0.67 (0.92) | 0.76 |
| Change | 0.44 (1.03) | 0.33 (1.33) | 0.76 | 0.23 (0.86) | 0.42 (1.10) | 0.52 |
| Anger | ||||||
| Baseline | 0.29 (0.99) | 0.04 (0.20) | 0.24 | 0.10 (0.41) | 0.29 (0.81) | 0.30 |
| Post-stress | 0.65 (1.34) | 0.25 (0.66) | 0.20 | 0.63 (1.09) | 0.71 (1.20) | 0.81 |
| Change | 0.36 (1.52) | 0.21 (0.71) | 0.66 | 0.52 (1.06) | 0.42 (1.10) | 0.73 |
| Physical Stress | ||||||
|---|---|---|---|---|---|---|
| Systolic Blood Pressure (mm Hg) | ||||||
| Rest, minimum | 116.9 (15.7) | 124.4 (14.6) | 0.088 | 118.8 (20.6) | 126.2 (17.3) | 0.18 |
| Stress, maximum | 174.2 (27.8) | 191.1 (35.0) | 0.070 | 189.0 (32.3) | 195.0 (25.6) | 0.48 |
| Change | 57.3 (26.3) | 66.9 (28.5) | 0.23 | 70.2 (36.5) | 68.8 (22.2) | 0.87 |
| Diastolic Blood Pressure (mm Hg) | ||||||
| Rest, minimum | 73.2 (10.2) | 79.4 (11.0) | 0.045 | 71.0 (8.5) | 78.2 (10.5) | 0.012 |
| Stress, maximum | 90.2 (17.5) | 97.5 (17.7) | 0.16 | 90.7 (16.1) | 96.8 (16.9) | 0.21 |
| Change | 17.0 (14.9) | 18.3 (16.8) | 0.78 | 19.8 (16.6) | 18.6 (13.2) | 0.79 |
| Heart rate (beats/min) | ||||||
| Rest, minimum | 66.5 (8.8) | 63.6 (12.0) | 0.34 | 64.0 (10.8) | 63.1 (11.3) | 0.77 |
| Stress, maximum | 153.5 (22.8) | 149.4 (23.0) | 0.53 | 147.8 (27.1) | 144.5 (20.2) | 0.64 |
| Change | 87.0 (23.0) | 85.8 (26.6) | 0.86 | 85.8 (24.1) | 81.4 (22.0) | 0.51 |
| Rate-pressure product during stress | 27,009 (6,852) | 28,965 (8,501) | 0.38 | 28,210 (7,988) | 28,318 (5,776) | 0.96 |
All values are means (standard deviation).
* Difference in ratings posttest-pretest. Ratings were obtained only for mental stress. A positive value indicates more distress after mental stress.
Myocardial Perfusion
Three patients, 1 woman and 2 men, had missing myocardial perfusion data for both mental stress and physical stress, and were excluded from the perfusion imaging analyses. One woman ≤50 years had missing physical stress perfusion data, and another woman >50 years had missing mental stress perfusion data. The 16 patients who underwent pharmacological stress had a rate of inducible ischemia (43.8%) that was not significantly different from all the other patients who underwent exercise treadmill stress (32.9%, p=0.41).
Overall, men tended to have more resting (fixed) perfusion defects (median SRS, 7.5 vs. 5.0, p=0.037) and more perfusion defects with physical stress (median SSS, 9.5 vs. 8.0, p=0.072), while women tended to have more reversible defects during mental stress, suggestive of MSIMI (median SDS, 2.0 vs. 1.0, p=0.17). These differences, however, were more marked in patients of age ≤50 (Table 3). In this group, the SDS with mental stress was significantly higher in women than in men (3.1 vs. 1.5, p=0.029), while there were no sex differences among patients older than 50 years. In contrast, the SDS with exercise or pharmacological stress tended to be higher in older men. Figure 1 shows a representative case to illustrate our method.
Table 3.
Sex differences in myocardial perfusion imaging data according to age group.
| Age ≤50 yrs | Age >50 yrs | |||||
|---|---|---|---|---|---|---|
| Rest | ||||||
| Women (N=23) | Men (N=24) | P* | Women (N=25) | Men (N=22) | P* | |
| Summed Rest Score | ||||||
| Mean (SD) | 5.7 (4.8) | 9.2 (6.0) | 0.033 | 6.9 (5.9) | 8.0 (5.4) | 0.50 |
| Median (Q1, Q3) | 5.0 (1.0, 10.0) | 8.0 (5.5, 10.5) | 0.046 | 4.0 (2.0, 11.0) | 6.5 (4.0, 13.0) | 0.34 |
| Mental Stress | ||||||
|---|---|---|---|---|---|---|
| Women (N=23) | Men (N=24) | p | Women (N=24) | Men (N=22) | p | |
| Summed Stress Score | ||||||
| Mean (SD) | 8.9 (5.2) | 10.8 (6.3) | 0.26 | 9.1 (6.6) | 10.4 (6.2) | 0.49 |
| Median (Q1, Q3) | 9.0 (5.0, 13.0) | 9.5 (8.0, 12.0) | 0.46 | 8.0 (4.0, 14.0) | 9.0 (6.0, 14.0) | 0.46 |
| Summed Difference Score | ||||||
| Mean (SD) | 3.1 (2.9) | 1.5 (1.8) | 0.029 | 2.2 (2.5) | 2.4 (3.3) | 0.82 |
| Median (Q1, Q3) | 3.0 (0.0, 5.0) | 1.0 (0.0, 2.5) | 0.053 | 1.5 (0.0, 3.5) | 1.0 (0.0, 3.0) | 0.91 |
| Physical Stress | ||||||
|---|---|---|---|---|---|---|
| Women (N=22) | Men (N=24) | p | Women (N=25) | Men (N=22) | p | |
| Summed Stress Score | ||||||
| Mean (SD) | 8.7 (5.4) | 11.6 (7.4) | 0.14 | 9.2 (6.1) | 11.6 (5.4) | 0.17 |
| Median (Q1, Q3) | 7.0 (5.0, 14.0) | 9.0 (7.5, 12.0) | 0.20 | 8.0 (4.0, 14.0) | 10.0 (8.0, 15.0) | 0.19 |
| Summed Difference Score | ||||||
| Mean (SD) | 2.9 (3.3) | 2.3 (3.1) | 0.57 | 2.3 (3.7) | 3.5 (2.9) | 0.21 |
| Median (Q1, Q3) | 2.0 (0.0, 4.0) | 1.0 (0.0, 3.5) | 0.47 | 0 (0.0, 3.0) | 4.0 (0.0, 6.0) | 0.10 |
SD: Standard deviation; Q1: quartile 1; Q3: quartile 3.
The two-tailed Student's t test was used to compare the means, and the Wilcoxon Mann-Whitney test was used to compare the medians.
Figure 1.
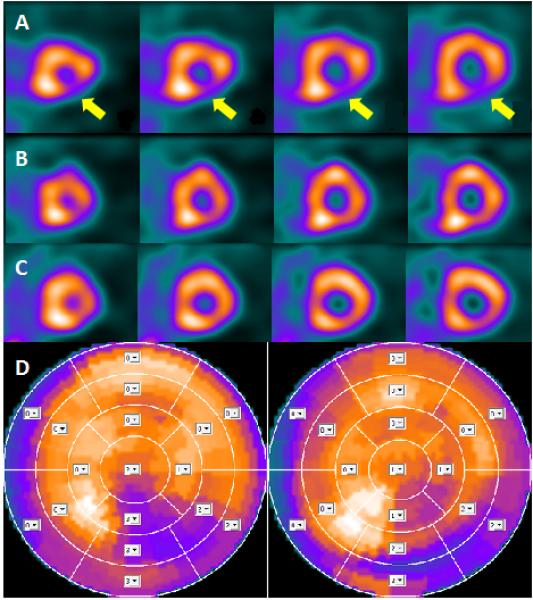
Example of mental and physical stress myocardial perfusion imaging in one of the women enrolled in the study. The horizontal short axis slices in row A were obtained after mental stress and show a clear perfusion defect in the infero-lateral wall of the left ventricle (yellow arrows). The images in row B were obtained after physical stress; the perfusion defect is much smaller and barely visible. The images in row C were obtained at rest and are very similar to those obtained after physical stress test (row B). The bull's eyes in row D show the infero-lateral perfusion defect after mental stress on the left and after physical stress on the right. The sum stress score was 16 for the mental stress and 11 for the physical stress.
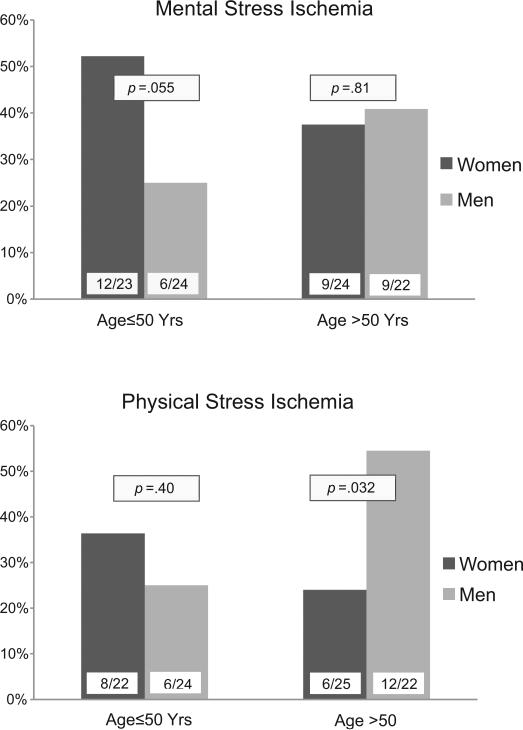
When myocardial ischemia was examined as a categorical variable, results were similar (Figure 2). Women ≤50 years of age had twice the rate of ischemia with mental stress (defined as a SDS score ≥3) than age-matched men, 52% vs. 25%, and had higher rates of MSIMI than any of the other sex and age strata, although the sex-age interaction did not reach statistical significance (p=0.13). In contrast, ischemia with physical stress did not differ between women and men aged ≤50 years (36% vs 25%), and men older than 50 years had higher rates of exercise or pharmacological stress ischemia than any of the other strata (p=0.049 for the sexage interaction). Results remained similar when only patients with complete data for both mental and physical stress ischemia were compared (2 women excluded). In this group, the rates of MSIMI among patients ≤50 years were 54.6% in women and 25.0% in men (p=0.040), and the rates of physical stress ischemia in the older group were 25.0% in women and 54.6 in men (p=0.040). Rates in the other groups did not change. Sex differences also remained similar when a cut point of ≥4 in the SDS was used as definition of MSIMI. With this alternative definition, 35% (n=8) younger women and 12% (n=3) younger men had ischemia; corresponding rates among older patients were 25% (n=6) and 23% (n=5).
Figure 2.
Sex differences in mental stress ischemia (SDS ≥3) and physical stress ischemia (SDS ≥4) according to age group. Numbers at the bottom of each bar are number of patients of ischemia over the total sample in each group.
The association between patients’ sex and SDS with mental stress, stratified by age, was examined after adjusting for sociodemographic and lifestyle factors, disease severity and depression in a series of nested models (Table 4). In all models, women 50 years of age or younger continued to show significantly more ischemic perfusion defects with mental stress than age-matched men. In the final model, women age ≤50 had on average a mental stress SDS that was 2.5 points higher than men, while there was no difference in physical stress SDS. In contrast, among patients older than 50 years, the SDS with mental stress was similar in women and men, and there was a tendency for women to have less ischemia with physical stress than men. Given the differences found in history of sexual abuse between young women and men, this factor was added to the final model; the results, however, were unchanged.
Table 4.
Association between sex and SDS, adjusted for SRS, with mental stress and physical stress according to age group.
| Age ≤50 yrs |
Age >50 yrs |
|||||
|---|---|---|---|---|---|---|
| β * | 95% CI | p | β * | 95% CI | p | |
| Mental Stress | ||||||
| Model 1: Only adjusted for SRS | 1.48 | 0.004 - 2.96 | 0.049 | −0.22 | −2.00 - 1.56 | 0.81 |
| Model 2: Also adjusted for demographic and lifestyle factors† | 1.76 | 0.10 - 3.41 | 0.038 | −0.04 | −1.82 - 1.73 | 0.96 |
| Model 3: Adjusted for the above plus disease severity‡ | 2.30 | 0.42 - 4.17 | 0.018 | −0.24 | −2.13 - 1.66 | 0.80 |
| Model 4: Adjusted for the above plus depressive symptoms (BDI-II score) | 2.51 | 0.44 - 4.58 | 0.019 | −0.28 | −1.86 - 1.29 | 0.72 |
| Model 5: Adjusted for the above plus time since the MI | 2.49 | 0.17 - 4.82 | 0.036 | −0.61 | −2.17 - 0.94 | 0.43 |
| Physical Stress | ||||||
|---|---|---|---|---|---|---|
| Model 1: Only adjusted for SRS | 0.64 | −1.36 - 2.64 | 0.52 | −1.41 | −3.33 - 0.51 | 0.14 |
| Model 2: Also adjusted for demographic and lifestyle factors† | 0.88 | −1.37 - 3.14 | 0.43 | −1.67 | −3.67 - 0.34 | 0.10 |
| Model 3: Adjusted for the above plus disease severity‡ | 0.78 | −2.02 - 3.57 | 0.58 | −2.06 | −4.19 - 0.06 | 0.057 |
| Model 4: Adjusted for the above plus depressive symptoms (BDI-II score) | 0.86 | −2.24 - 3.95 | 0.58 | −2.06 | −4.21 - 0.08 | 0.058 |
| Model 5: Adjusted for the above plus time since the MI | 0.10 | −3.31 - 3.51 | 0.95 | −2.44 | −4.56 - -0.31 | 0.026 |
CI: confidence interval; BDI: beck Depression Inventory. SDS: Summed Difference Score. SRS: Summed Rest Score.
The β coefficient expresses the number of SDS score points for women compared with men for each condition and age group. Each model was constructed with SSS as dependent variable adjusting for SRS. The coefficient from these models is identical to that from models where the dependent variable is the difference or ischemia score (SDS) adjusted for SRS.
Race (black vs. non-black), income below poverty level, and current cigarette smoking.
Gensini angiographic score and left ventricular ejection fraction.
DISCUSSION
In a sample of young and middle-aged survivors of acute MI, we found that MSIMI, but not exercise or pharmacologically-induced ischemia, is more common in women 50 years or younger compared with age-matched men. Women in this group had about twice the rate of MSIMI compared with men, and their SDS with mental stress was on average twice as high as men. We obtained these results despite the fact that women had less perfusion abnormalities at rest and less obstructive CAD; they also did not differ from men in most traditional CAD risk factors. Since MSIMI is associated with increased mortality in patients with IHD (13,14), our study is the first to uncover a potential mechanism that could explain the worse prognosis of younger women after an acute MI compared with men of the same age (4-8). Because MSIMI correlates with ischemia in daily life (15), these results also suggest that MSIMI could be implicated as a triggering factor of acute coronary events more often in young women than in other groups.
Previous studies of MSIMI included broadly defined cardiac patients and predominantly men and older patients. When women were included, the sample was often too small to be examined separately. These issues may be responsible for the heterogeneous results reported. In a study of 58 IHD patients (6 women), women showed a larger -but only borderline significant- ejection fraction reduction with mental stress than men, an effect not seen among normal controls (38). Another study of stable, broadly defined cardiac patients found no significant sex differences in myocardial perfusion abnormalities in response to mental stress (30). However, only about 1/4 of the patients had a previous MI, and the age ranged from 39 to 83 years. In a recent study where MSIMI was assessed by echocardiography, women had nearly two times higher odds of MSIMI than men, although the difference was not significant in multivariable analysis (39). Our investigation differs from these studies by focusing on a young post-MI population, which was specifically chosen based on sex differences in outcome described in the literature. In this population, we were able to uncover an important difference in MSIMI between young women and men.
It is unclear why myocardial ischemia with mental stress is more common in women, specifically younger women. This finding was not due to more pronounced hemodynamic responses to stress in female patients, as hemodynamic data were similar in women and men, consistent with previous studies (30). A possible mechanism is a higher burden of psychosocial risk factors in women. We did find that younger women with MI were more often poor, of minority race, with a history of sexual abuse and with higher levels of depressive symptoms, in agreement with previous data (4,9). These factors, however, did not explain sex differences in MSIMI. The high proportion of black patients in our young sample, particularly among women aged 50 and younger, is not surprising. MI occurs at a younger age in Blacks than in Whites, especially among women (40). As a result, black patients hospitalized for MI are much younger than their white counterparts, particularly black women, and thus black women are over-represented among young post-MI patients (4,41).
Another possible explanation for our findings is that emotional stress is a more powerful risk factor for IHD in young women than similarly aged men. In support of this hypothesis, two recent follow-up studies of young community samples (less than 40 years old) found a dramatic increase in risk of IHD due to depression in young women, which was higher than in men (11,12). Similarly, severe childhood adversities such as physical and sexual abuse are independent risk factors for the incidence of IHD among women (42), and, among participants younger than 55 years, early life stressors are stronger predictors of cardiovascular events in women than in men (10). Although these studies have not examined mental stress in the laboratory, they do suggest a profound role of emotional factors on cardiovascular risk in young women. The high prevalence of depression and sexual abuse among women in our sample, particularly those 50 years and younger, likely reflects the fact that these factors are powerful predictors for premature heart disease in young women (10-12).
Women may be more susceptible to MSIMI through pathophysiological mechanisms that involve endothelial dysfunction and microvascular disease. Abnormal coronary reactivity and microvascular dysfunction have long been implicated as female-specific mechanisms of IHD and prognostic factors (2). Women have less obstructive coronary artery disease than men along the entire spectrum of acute coronary syndromes and across all age groups (43), and coronary microvascular dysfunction is reported to be common in women in the setting of chest pain without significant coronary obstruction (44,45). Although no data are available for IHD more broadly, it is possible that abnormal coronary vasoconstriction and microvascular dysfunction play a larger role in the pathophysiology of acute coronary syndromes among women than men, and, consequently, are more prevalent among female post-MI patients than male counterparts. In parallel, evidence suggests a significant role of vasomotor tone in MSIMI, possibly through sympathetic nervous system activation via adrenergic receptors in the vascular system. During mental stress, paradoxical vasoconstriction or reduced blood flow in coronary vessels (46,47), and peripheral endothelial dysfunction that lasts up to four hours (48) have been described. Furthermore, patients with IHD who develop MSIMI show an increase in systemic vascular resistance through peripheral vasoconstriction (19,49), suggesting that a rise in afterload plays a role in MSIMI. As a whole, these observations point to a prominent role of endothelial and microvascular dysfunction in MSIMI, which could explain a female predominance of this phenomenon in post-MI patients.
A limitation of our study is the use of a single mental stress task. The reason for conducting only one task is due to our assessment of ischemia with [99mTc]sestamibi perfusion imaging with SPECT. An important advantage of this method is that [99mTc]sestamibi, once injected during mental stress, is trapped in the myocyte, and thus it provides a “snapshot” of perfusion at the time of stress, even though scanning occurs later. A disadvantage, however, is that only one stress task is feasible with this method. On the other hand, while the use of only one task can be seen as a limitation, the protracted effects of mental stress on vascular regulation described above raise questions on the validity of using multiple mental stress tasks in a single session.
Our study included post-MI patients with relatively preserved functional capacity, since they all scored ≥5 METs on the DASI by design. As a result, the majority of our sample had a normal ejection fraction. Whether our results are applicable to post-MI patients with abnormal ventricular function remains to be established. The main limitation of this study, however, is its small sample that limited the power of our analyses and caused wide confidence intervals. In addition, the age range up to age 60 was narrow. As a result, the sex by age interaction for MSIMI was not statistically significant despite large numerical differences in the coefficients. Therefore, our results need to be replicated in larger studies with a broader age distribution. Furthermore, because of the small sample size, the absolute number of patients with myocardial ischemia was small. Yet women and men were matched on a number of important factors, and there were few differences in baseline risk factors and clinical features by sex, supporting the validity of our findings. Young women with MI are under-represented in clinical studies in part because their risk of MI is relatively low. Yet about 10,000 women younger than 45 years are diagnosed with an MI each year in the United States (50), and their hospital mortality is more than twice that of men of similar age (4). The study of this group is especially informative in uncovering women-specific pathways of risk, and likely to provide novel opportunities for prevention.
In conclusion, we report that myocardial ischemia due to emotional stress is more common in young women after an MI than in age-matched men. Future studies should evaluate whether MSIMI plays a role in the prognosis and perhaps the etiology of IHD in young women, and whether this phenomenon helps explain sex differences in outcome previously observed in young post-MI patients. If our results are confirmed by larger studies, interventions specifically designed to address women's stressors and treat MSIMI could be helpful to reduce the risk of cardiovascular mortality in women.
Acknowledgments
Sources of Funding: This work was supported by the National Institutes of Health (R21HL093665, R21HL093665-01A1S1, R01 HL109413, K24HL077506, and K24 MH076955). The sponsors of this study had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
ABBREVIATION LIST
- BDI
Beck Depression Inventory
- CAD
Coronary Artery Disease
- DASI
Duke Activity Status Index
- IHD
Ischemic Heart Disease
- MI
Myocardial Infarction
- MSIMI
Mental Stress-Induced Myocardial Ischemia
- SPECT
Single-Photon Emission Computed Tomography
- SDS
Summed Difference Score
- SRS
Summed Rest Score
- SSS
Summed Stress Score
Footnotes
Conflicts of Interest
None of the authors report conflict of interest relevant to this article.
REFERENCES
- 1.Vaccarino V. Ischemic heart disease in women: many questions, few facts. Circ Cardiovasc Qual Outcomes. 2010;3:111–5. doi: 10.1161/CIRCOUTCOMES.109.925313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shaw LJ, Bugiardini R, Merz CNB. Women and ischemic heart disease: evolving knowledge. J. Am. Coll. Cardiol. 2009;54:1561–1575. doi: 10.1016/j.jacc.2009.04.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ford ES, Capewell S. Coronary heart disease mortality among young adults in the U.S. from 1980 through 2002: concealed leveling of mortality rates. J. Am. Coll. Cardiol. 2007;50:2128–32. doi: 10.1016/j.jacc.2007.05.056. [DOI] [PubMed] [Google Scholar]
- 4.Vaccarino V, Parsons L, Every NR, Barron HV, Krumholz HM. Sex-based differences in early mortality after myocardial infarction. National Registry of Myocardial Infarction 2 Participants. N. Engl. J. Med. 1999;341:217–25. doi: 10.1056/NEJM199907223410401. [DOI] [PubMed] [Google Scholar]
- 5.Vaccarino V, Krumholz HM, Yarzebski J, Gore JM, Goldberg RJ. Sex differences in 2-year mortality after hospital discharge for myocardial infarction. Ann. Intern. Med. 2001;134:173–81. doi: 10.7326/0003-4819-134-3-200102060-00007. [DOI] [PubMed] [Google Scholar]
- 6.Rosengren A, Spetz CL, Koster M, Hammar N, Alfredsson L, Rosen M. Sex differences in survival after myocardial infarction in Sweden. Data from the Swedish National Acute Myocardial Infarction register. Eur. Heart J. 2001;22:314–322. doi: 10.1053/euhj.2000.2368. [DOI] [PubMed] [Google Scholar]
- 7.Andrikopoulos GK, Tzeis SE, Pipilis AG, Richter DJ, Kappos KG, Stefanadis CI, Toutouzas PK, Chimonas ET. Younger age potentiates post myocardial infarction survival disadvantage of women. Int. J. Cardiol. 2006;108:320–5. doi: 10.1016/j.ijcard.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 8.Koek HL, de Bruin A, Gast F, Gevers E, Kardaun JWPF, Reitsma JB, Grobbee DE, Bots ML. Short- and long-term prognosis after acute myocardial infarction in men versus women. Am. J. Cardiol. 2006;98:993–999. doi: 10.1016/j.amjcard.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 9.Mallik S, Spertus JA, Reid KJ, Krumholz HM, Rumsfeld JS, Weintraub WS, Agarwal P, Santra M, Bidyasar S, Lichtman JH, Wenger NK, Vaccarino V. Depressive symptoms after acute myocardial infarction: evidence for highest rates in younger women. Arch. Intern. Med. 2006;166:876–83. doi: 10.1001/archinte.166.8.876. [DOI] [PubMed] [Google Scholar]
- 10.Korkeila J, Vahtera J, Korkeila K, Kivimaki M, Sumanen M, Koskenvuo K, Koskenvuo M. Childhood adversities as predictors of incident coronary heart disease and cerebrovascular disease. Heart. 2010;96:298–303. doi: 10.1136/hrt.2009.188250. [DOI] [PubMed] [Google Scholar]
- 11.Shah AJ, Veledar E, Hong Y, Bremner JD, Vaccarino V. Depression and history of attempted suicide as risk factors for heart disease mortality in young individuals. Arch. Gen. Psychiatry. 2011;68:1135–42. doi: 10.1001/archgenpsychiatry.2011.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wyman L, Crum RM, Celentano D. Depressed mood and cause-specific mortality: a 40-year general community assessment. Ann. Epidemiol. 2012;22:638–43. doi: 10.1016/j.annepidem.2012.06.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Strike PC, Steptoe A. Systematic review of mental stress-induced myocardial ischaemia. Eur. Heart J. 2003;24:690–703. doi: 10.1016/s0195-668x(02)00615-2. [DOI] [PubMed] [Google Scholar]
- 14.Holmes SD, Krantz DS, Rogers H, Gottdiener J, Contrada RJ. Mental stress and coronary artery disease: a multidisciplinary guide. Prog. Cardiovasc. Dis. 2006;49:106–122. doi: 10.1016/j.pcad.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 15.Blumenthal JA, Jiang W, Waugh RA, Frid DJ, Morris JJ, Coleman RE, Hanson M, Babyak M, Thyrum ET, Krantz DS, O'Connor C. Mental stress-induced ischemia in the laboratory and ambulatory ischemia during daily life. Association and hemodynamic features. Circulation. 1995;92:2102–8. doi: 10.1161/01.cir.92.8.2102. [DOI] [PubMed] [Google Scholar]
- 16.Ramachandruni S, Fillingim RB, McGorray SP, Schmalfuss CM, Cooper GR, Schofield RS, Sheps DS. Mental stress provokes ischemia in coronary artery disease subjects without exercise- or adenosine-induced ischemia. J. Am. Coll. Cardiol. 2006;47:987–91. doi: 10.1016/j.jacc.2005.10.051. [DOI] [PubMed] [Google Scholar]
- 17.Thygesen K, Alpert JS, White HD. Universal definition of myocardial infarction. J. Am. Coll. Cardiol. 2007;50:2173–2195. doi: 10.1016/j.jacc.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 18.Shaw LJ, Olson MB, Kip K, Kelsey SF, Johnson BD, Mark DB, Reis SE, Mankad S, Rogers WJ, Pohost GM, Arant CB, Wessel TR, Chaitman BR, Sopko G, Handberg E, Pepine CJ, Bairey Merz CN. The value of estimated functional capacity in estimating outcome: results from the NHBLI-Sponsored Women's Ischemia Syndrome Evaluation (WISE) Study. J. Am. Coll. Cardiol. 2006;47:S36–S43. doi: 10.1016/j.jacc.2005.03.080. [DOI] [PubMed] [Google Scholar]
- 19.Goldberg AD, Becker LC, Bonsall R, Cohen JD, Ketterer MW, Kaufman PG, Krantz DS, Light KC, McMahon RP, Noreuil T, Pepine CJ, Raczynski J, Stone PH, Strother D, Taylor H, Sheps DS. Ischemic, hemodynamic, and neurohormonal responses to mental and exercise stress. Experience from the Psychophysiological Investigations of Myocardial Ischemia Study (PIMI). Circulation. 1996;94:2402–9. doi: 10.1161/01.cir.94.10.2402. [DOI] [PubMed] [Google Scholar]
- 20.Wolpe J. The practice of behavior therapy. New York Pergamon Press; New York: 1969. [Google Scholar]
- 21.Esteves FP, Raggi P, Folks RD, Keidar Z, Askew JW, Rispler S, O'Connor MK, Verdes L, Garcia EV. Novel solid-state-detector dedicated cardiac camera for fast myocardial perfusion imaging: multicenter comparison with standard dual detector cameras. J. Nucl. Cardiol. 2009;16:927–34. doi: 10.1007/s12350-009-9137-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hassan M, York KM, Li Q, Lucey DG, Fillingim RB, Sheps DS. Variability of myocardial ischemic responses to mental versus exercise or adenosine stress in patients with coronary artery disease. J. Nucl. Cardiol. 2008;15:518–25. doi: 10.1016/j.nuclcard.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burg MM, Graeber B, Vashist A, Collins D, Earley C, Liu J, Lampert R, Soufer R. Noninvasive detection of risk for emotion-provoked myocardial ischemia. Psychosom. Med. 2009;71:14–20. doi: 10.1097/PSY.0b013e318187c035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.LaVeau PJ, Rozanski A, Krantz DS, Cornell CE, Cattanach L, Zaret BL, Wackers FJ. Transient left ventricular dysfunction during provocative mental stress in patients with coronary artery disease. Am. Heart J. 1989;118:1–8. doi: 10.1016/0002-8703(89)90064-1. [DOI] [PubMed] [Google Scholar]
- 25.Walkers F, Soufer R, Zaret BL. Nuclear cardiology. In: Braunwald E, Zipes D, Libby P, editors. Heart Disease: A Text Book of Cardiovascular Medicine. W.B. Saunders; Philadelphia, Pennsylvania: 2000. pp. 273–323. [Google Scholar]
- 26.Garcia EV, Faber TL, Cooke CD, Folks RD, Chen J, Santana C. The increasing role of quantification in clinical nuclear cardiology: the Emory approach. J. Nucl. Cardiol. 2007;14:420–32. doi: 10.1016/j.nuclcard.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 27.Garcia EV, DePuey EG, DePasquale EE. Quantitative planar and tomographic thallium-201 myocardial perfusion imaging. Cardiovasc. Intervent. Radiol. 1987;10:374–83. doi: 10.1007/BF02577348. [DOI] [PubMed] [Google Scholar]
- 28.Berman DS, Kang X, Van Train KF, Lewin HC, Cohen I, Areeda J, Friedman JD, Germano G, Shaw LJ, Hachamovitch R. Comparative prognostic value of automatic quantitative analysis versus semiquantitative visual analysis of exercise myocardial perfusion single-photon emission computed tomography. J. Am. Coll. Cardiol. 1998;32:1987–95. doi: 10.1016/s0735-1097(98)00501-4. [DOI] [PubMed] [Google Scholar]
- 29.Hachamovitch R, Berman DS, Shaw LJ, Kiat H, Cohen I, Cabico JA, Friedman J, Diamond GA. Incremental prognostic value of myocardial perfusion single photon emission computed tomography for the prediction of cardiac death: differential stratification for risk of cardiac death and myocardial infarction. Circulation. 1998;97:535–43. doi: 10.1161/01.cir.97.6.535. [DOI] [PubMed] [Google Scholar]
- 30.York KM, Hassan M, Li Q, Li H, Fillingim RB, Lucey D, Bestland M, Sheps DS. Do men and women differ on measures of mental stress-induced ischemia? Psychosom. Med. 2007;69:918–22. doi: 10.1097/PSY.0b013e31815a9245. [DOI] [PubMed] [Google Scholar]
- 31.Gensini GG. A more meaningful scoring system for determining the severity of coronary heart disease. Am J Cardiol. 1983;51:606. doi: 10.1016/s0002-9149(83)80105-2. [DOI] [PubMed] [Google Scholar]
- 32.Beck AT, Steer RA, Brown GK. BDI-II. Beck Depression Inventory. Second Edition. The Psychological Corporation; San Antonio, TX: 1996. [Google Scholar]
- 33.First MB, Spitzer RL, Williams JBW, Gibbon M. Structured Clinical Interview for DSM IV-Patient Edition (SCID-P) American Psychiatric Press; Washington, D.C.: 1995. [Google Scholar]
- 34.Bremner JD, Bolus R, Mayer EA. Psychometric properties of the Early Trauma Inventory-Self Report. J. Nerv. Ment. Dis. 2007;195:211–8. doi: 10.1097/01.nmd.0000243824.84651.6c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mitchell PH, Powell L, Blumenthal J, Norten J, Ironson G, Pitula CR, Froelicher ES, Czajkowski S, Youngblood M, Huber M, Berkman LF. A short social support measure for patients recovering from myocardial infarction: the ENRICHD Social Support Inventory. J. Cardpulm. Rehabil. 2003;23:398–403. doi: 10.1097/00008483-200311000-00001. [DOI] [PubMed] [Google Scholar]
- 36.Anonymous The Sixth Report of the Joint National Commitee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Arch. Intern. Med. 1997;157:2413–2446. doi: 10.1001/archinte.157.21.2413. [DOI] [PubMed] [Google Scholar]
- 37.Spielberger CD, Gorsuch RL, Lushene RE. State-Trait Anxiety (STAI) manual. Consulting Psychologists Press; Palo Alto, CA: 1970. [Google Scholar]
- 38.Bairey Merz CN, Kop W, Krantz DS, Helmers KF, Berman DS, Rozanski A. Cardiovascular stress response and coronary artery disease: evidence of an adverse postmenopausal effect in women. Am. Heart J. 1998;135:881–7. doi: 10.1016/s0002-8703(98)70050-x. [DOI] [PubMed] [Google Scholar]
- 39.Jiang W, Samad Z, Boyle S, Becker RC, Williams R, Kuhn C, Ortel TL, Rogers J, Kuchibhatla M, O'Connor C, Velazquez EJ. Prevalence and clinical characteristics of mental stress-induced myocardial ischemia in patients with coronary heart disease. J. Am. Coll. Cardiol. 2013;61:714–22. doi: 10.1016/j.jacc.2012.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jones DW, Chambless LE, Folsom AR, Heiss G, Hutchinson RG, Sharrett AR, Szklo M, Taylor HA., Jr. Risk factors for coronary heart disease in African Americans: the atherosclerosis risk in communities study, 1987-1997. Arch. Intern. Med. 2002;162:2565–71. doi: 10.1001/archinte.162.22.2565. [DOI] [PubMed] [Google Scholar]
- 41.Vaccarino V, Rathore SS, Wenger NK, Frederick PD, Abramson JL, Barron HV, Manhapra A, Mallik S, Krumholz HM. Sex and racial differences in the management of acute myocardial infarction, 1994 through 2002. N. Engl. J. Med. 2005;353:671–82. doi: 10.1056/NEJMsa032214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rich-Edwards JW, Mason S, Rexrode K, Spiegelman D, Hibert E, Kawachi I, Jun HJ, Wright RJ. Physical and sexual abuse in childhood as predictors of early-onset cardiovascular events in women. Circulation. 2012;126:920–7. doi: 10.1161/CIRCULATIONAHA.111.076877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vaccarino V, Badimon L, Corti R, de Wit C, Dorobantu M, Hall A, Koller A, Marzilli M, Pries A, Bugiardini R. Ischaemic heart disease in women: are there sex differences in pathophysiology and risk factors? Position paper from the working group on coronary pathophysiology and microcirculation of the European Society of Cardiology. Cardiovasc. Res. 2011;90:9–17. doi: 10.1093/cvr/cvq394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reis SE, Holubkov R, Smith AJC, Kelsey SF, Sharaf BL, Reichek N, Rogers WJ, Merz CNB, Sopko G, Pepine CJ. Coronary microvascular dysfunction is highly prevalent in women with chest pain in the absence of coronary artery disease: Results from the NHLBI WISE study. Am. Heart J. 2001;141:735–741. doi: 10.1067/mhj.2001.114198. [DOI] [PubMed] [Google Scholar]
- 45.Buchthal SD, den Hollander JA, Merz CN, Rogers WJ, Pepine CJ, Reichek N, Sharaf BL, Reis S, Kelsey SF, Pohost GM. Abnormal myocardial phosphorus-31 nuclear magnetic resonance spectroscopy in women with chest pain but normal coronary angiograms. N. Engl. J. Med. 2000;342:829–35. doi: 10.1056/NEJM200003233421201. [DOI] [PubMed] [Google Scholar]
- 46.Arrighi JA, Burg M, Cohen IS, Kao AH, Pfau S, Caulin-Glaser T, Zaret BL, Soufer R. Myocardial blood-flow response during mental stress in patients with coronary artery disease. Lancet. 2000;356:310–311. doi: 10.1016/S0140-6736(00)02510-1. [DOI] [PubMed] [Google Scholar]
- 47.Kop WJ, Krantz DS, Howell RH, Ferguson MA, Papademetriou V, Lu D, Popma JJ, Quigley JF, Vernalis M, Gottdiener JS. Effects of mental stress on coronary epicardial vasomotion and flow velocity in coronary artery disease: relationship with hemodynamic stress responses. J. Am. Coll. Cardiol. 2001;37:1359–66. doi: 10.1016/s0735-1097(01)01136-6. [DOI] [PubMed] [Google Scholar]
- 48.Ghiadoni L, Donald AE, Cropley M, Mullen MJ, Oakley G, Taylor M, O'Connor G, Betteridge J, Klein N, Steptoe A, Deanfield JE. Mental Stress Induces Transient Endothelial Dysfunction in Humans. Circulation. 2000;102:2473–2478. doi: 10.1161/01.cir.102.20.2473. [DOI] [PubMed] [Google Scholar]
- 49.Ramadan R, Sheps D, Esteves F, Zafari AM, Bremner JD, Vaccarino V, Quyyumi AA. Myocardial ischemia during mental stress: role of coronary artery disease burden and vasomotion. J Am Heart Assoc. 2013;2:e000321. doi: 10.1161/JAHA.113.000321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rosamond W, Flegal K, Friday G, Furie K, Go A, Greenlund K, Haase N, Ho M, Howard V, Kissela B, Kittner S, Lloyd-Jones D, McDermott M, Meigs J, Moy C, Nichol G, O'Donnell CJ, Roger V, Rumsfeld J, Sorlie P, Steinberger J, Thom T, Wasserthiel-Smoller S, Hong Y. Heart disease and stroke statistics--2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007;115:e69–171. doi: 10.1161/CIRCULATIONAHA.106.179918. [DOI] [PubMed] [Google Scholar]