Abstract
Objective
To examine the relationship between dairy food intake and semen parameters
Design
Longitudinal study
Setting
Men attending academic medical center fertility clinic in Boston, MA
Patients
155 men
Interventions
None
Main Outcome Measures
total sperm count, sperm concentration, progressive motility, and morphology
Results
Low-fat dairy intake was positively related to sperm concentration and progressive motility. On average, men in the highest quartile of intake (1.22–3.54 servings/day) had 33% (95% confidence interval (CI) 1, 55) higher sperm concentration and 9.3 (95%CI 1.4, 17.2) percentage units higher sperm motility than men in the lowest quartile of intake (≤0.28 servings/day). These associations were primarily explained by intake of low-fat milk. The corresponding results for low-fat milk were 30% (95%CI 1,51) higher sperm concentration and 8.7 (95%CI 3.0, 14.4) percentage units higher sperm motility. Cheese intake was associated with lower sperm concentration among ever smokers. In this group, men in the highest tertile of intake (0.82–2.43 servings/day) had 53.2% (95%CI 9.7, 75.7) lower sperm concentration than men in the lowest tertile of cheese intake (<0.43 servings/day).
Conclusions
Our findings suggest that low-fat dairy intake, particularly low-fat milk, is related to higher sperm concentration and progressive motility, while cheese intake to lower sperm concentration among past or current smokers.
Keywords: infertility, sperm quality, dairy, diet
Introduction
Infertility affects 10–15% of reproductive-aged couples (1, 2). While reproductive abnormalities in the male partner are identified in as many as 58% of the couples evaluated for infertility (3), few risk factors for abnormal semen quality have been identified. Emerging evidence suggests that environmental estrogens may be related to lower semen quality (4). A particularly prevalent exposure route to environmental estrogens is via consumption of dairy foods (5). Because commercial milk is a mixture of milk from cows at different stages of pregnancy (6), dairy products contain detectable amounts of estrogens and other hormones that increase during pregnancy (7, 8) and account for 60–80% of intake of estrogens from foods in Western countries (9). Intake of milk and other dairy products has been related to lower semen quality in some studies (10–12), but not others (13). We have previously reported that intake of full-fat dairy foods is associated with a lower sperm morphology and progressive motility among healthy young men (12). Others have reported higher intake of full-fat dairy products among oligoasthenoteratospermic men (11) and of dairy products in general among asthenospermic (10) men. In addition, full fat dairy foods are an important source of saturated fat which has been previously related to low sperm counts (14, 15). Thus we hypothesized that full-fat dairy products would be related to lower semen quality. We examined this hypothesis among men attending a fertility clinic in Boston, Massachusetts.
Materials and Methods
Study population
Men in subfertile couples presenting for evaluation at the Massachusetts General Hospital (MGH) Fertility Center were invited to participate in an ongoing study of environmental factors and fertility (16). Men from couples using their own gametes for intrauterine insemination or assisted reproductive technologies, aged 18–55, and without a history of vasectomy were eligible. A food frequency questionnaire (FFQ) was introduced in April 2007, and was completed by 188 of the 246 men (76%) recruited through March 2012. Of these, 161 men produced one or more semen samples after the completion of the FFQ. We excluded men with incomplete semen analysis data (n=5) and azoospermic men (n=1). Because diet was assessed once, we also excluded all semen samples (47 samples from 8 men) that were collected more than 18 months after FFQ completion to minimize any influence that misclassification of dairy intake due to true intake changes over time might have on the associations. After exclusions, 155 men with a total of 338 semen samples were included in the analysis; 57 men provided only 1 sample, 51 men provided 2 samples, and 47 men provided 3 or more samples. At enrollment, trained personnel administered a general health questionnaire (asking about demographics, lifestyle, and reproductive disorders such as varicocele and surgical scars) and men completed an anthropometric assessment at the clinic. The study was approved by the Human Subject Committees of the Harvard School of Public Health and the MGH, and informed consent was obtained from all participants.
Semen analysis
Semen samples were obtained on site by masturbation and collected into a sterile plastic container. Men were instructed to abstain from ejaculation for 48hs before producing the sample and to report the specific time of abstinence; 18 men (19 semen samples) did not report their last ejaculation date and were assigned to the most common abstinence time category (2–3 days). Semen samples were liquefied at 37°C for 20 min before analysis. Sperm morphology was determined using Kruger’s strict criteria and results were expressed as percent normal spermatozoa (17). Ejaculate volume was estimated by sample weight assuming a density of 1g/mL. Sperm concentration and motility were assessed with computer-aided semen analysis (Hamilton-Thorne Version 10HTM-IVOS). The percentage of motile sperm was classified according to World Health Organization guidelines as progressive and total (progressive + non-progressive) (18). Total sperm count was calculated as sperm concentration x ejaculate volume. Similarly, total motile count was calculated as sperm concentration x ejaculate volume x total motility.
Dietary assessment
Participants completed a previously validated 131-item food frequency questionnaire (FFQ) at home (19). They were asked to report how often, on average, they consumed specific foods during the previous year. The FFQ had nine categories for intake frequency options that ranged from never to six or more times per day. 15 questions in the FFQ addressed dairy intake. The nutrient content of each food and the specific portion size was calculated by the nutrient database from the US Department of Agriculture (20) with additional information from manufacturers when necessary. Assessment of dairy food intake using this questionnaire has been validated against prospectively collected diet records representing 1 year of a diet (21). The de-attenuated correlation of dairy food intakes assessed with the FFQ and the 1 year average of prospectively collected diet records ranged from 0.52 for cottage cheese to 0.88 for skim milk (21). Low-fat milk was defined as the sum of skim milk and low-fat (1 and 2%) milk. Full-fat dairy intake was defined as the sum of whole milk, cream, ice cream, and cheese. Low-fat dairy was defined as the sum of low-fat milk, yogurt, and cottage cheese. Total dairy food intake was defined as the sum of full-fat and low-fat dairy. We used two data-derived dietary patterns to describe general patterns of food consumption (22): the “Prudent Pattern”, characterized by intakes of fish, low-fat dairy, fruits, vegetables, whole grains; and the “Western Pattern”, characterized by processed and red meats, fried fish and seafood, butter, margarine, full-fat dairy, French fries, refined grains, pizza, snacks, high energy drinks, mayonnaise, and sweets. We then calculated a summary score, ranging from −1.7 to 3.7 for the prudent pattern and −2.1 to 5.0 for the western pattern, reflecting how closely each man followed each of these dietary patterns (where higher scores reflect closer adherence).
Statistical analysis
We first summarized participant characteristics and compared them across quantiles of dairy food intake. We used the Kruskal-Wallis test to compare differences in continuous measures across categories of dairy intake and an extended Fisher’s Exact test for categorical variables. Linear mixed models with random intercepts were used to examine the relation between dairy food intake and semen parameters while adjusting for potential confounders and accounting for the correlation between multiple semen samples provided by the same man. Specifically, in these regression models, we compared semen quality parameters (total sperm count, sperm concentration, progressive motility, morphology, and semen volume) for men in increasing quantiles of dairy food intake in relation to those of men in the lowest quantile (reference). Robust estimators of the variance (23) were used in the computation of 95% confidence intervals. Population marginal means (24) were utilized to present marginal population averages adjusted for the covariates in the model. Total sperm count and sperm concentration were log-transformed to more closely approximate a normal distribution. Results for these parameters were back-transformed to allow presentation of results in the original scale. Tests for linear trend were performed using the median values of dairy intake in each category as a continuous variable and semen parameters as the response variable.
Potential confounders were baseline characteristics that have been associated with dairy intake and semen analysis, in our own analysis or in prior studies. Based on these criteria, all models were adjusted for age, body mass index (BMI), smoking status, race, and caloric intake. Abstinence time was associated with semen parameters but not with dairy intake and, therefore, was not a confounder. Following convention in semen quality studies, however, we included abstinence time in our multivariate adjusted models. History of previous infertility exam was not a confounder either but was included in multivariate models as a proxy for knowledge of one’s fertility in order to aid in identifying and accounting for reverse causation. Exclusion of abstinence time and history of infertility exam did not change the results. We further adjusted for principal components-derived dietary patterns, which have been previously related to semen parameters (22), to determine whether any observed association was specific to a particular dairy food or whether the overall food selection patterns explained the association. Since saturated fat intake has been previously related to lower semen quality (14, 25), and full-fat dairy is an important source of saturated fat, we further adjusted models for fat intake to explore whether any observed association was accounted for by fat intake. Similarly, we examined whether intake of breakfast cereals accounted for the association of milk and semen quality because intake of breakfast cereals is associated with milk intake and breakfast cereals contain large amounts of added vitamins and minerals which have been related to higher semen quality.
We performed a series of sensitivity analyses to evaluate the robustness of our findings. Specifically, we conducted analyses a) restricted to the first post-FFQ sample of each man, b) restricted to samples collected within 90 days after completion of the FFQ, c) restricted to men who gave only 1 sample, d) restricted to men with 2 samples only, and e) restricted to men with 3 or more samples. Last, we assessed effect modification of dietary associations with semen parameters by BMI (<25 kg/m2 and ≥25 kg/m2) and smoking status (current and never/former smokers) using cross-product terms. We analyzed the data using SAS (version 9.2; SAS Institute Inc., Cary, NC, USA), and two-sided p-values ≤ 0.05 were considered statistically significant.
Results
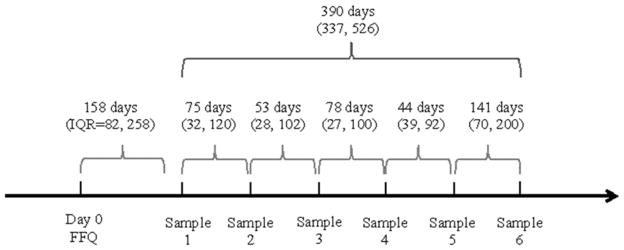
Participants were primarily Caucasian (83%), had never smoked (63%), and had a mean (SD) age of 36.5 (4.9) years. The majority (71%) were overweight or obese (BMI ≥25 kg/m2). Forty six percent of men had one or more semen analysis parameters below the 2010 WHO reference values (18) in their first semen analysis: 12% had <15 million sperm/mL, 42% had <40% motile sperm, and 39% had <4% morphologically normal sperm, 17% had <1.5mL ejaculate volume and 12% had a total sperm count <39 million. The median (interquartile range (IQR)) time between FFQ return and 1st semen sample was 158 days (IQR=82 to 258); and the time between FFQ return and last semen sample was 266 days (IQR =160 to 408) (Supplementary Figure 1). The number of semen samples produced by each man was not related to dairy intake, infertility diagnosis, or semen quality parameters. Cheese (34%) and low-fat milk (28%) accounted for more than half of all dairy food intake.
Men with a high intake of dairy foods were more likely to be Caucasian and never smokers. They also had lower intakes of poly-unsaturated fats and higher intakes of total calories, cold breakfast cereal, dairy protein, saturated and mono-unsaturated fats, and ranked higher in the Prudent Pattern score (Table 1).
Table 1.
Participants’ characteristics according to quartiles of dairy food intake.
| Dairy food intake (quartiles) | |||||
|---|---|---|---|---|---|
|
| |||||
| Q1 (lowest) | Q2 | Q3 | Q4 (highest) | p a | |
| N | 39 | 39 | 38 | 39 | |
| Range, servings/day | 0.02 to 1.32 | 1.39 to 1.86 | 1.90 to 2.69 | 2.71 to 5.77 | |
| Median (IQR) or N (%) | |||||
| Demographics | |||||
| Age, years | 37 (33, 40.2) | 36.4 (32.8, 39) | 36.2 (33.8, 38.4) | 35.8 (32.9, 41) | 0.93 |
| Race/ethnicity, N (%) | 0.43 | ||||
| White, not Hispanic | 28 (71.8) | 36 (92.3) | 30 (79.0) | 35 (89.7) | |
| Black | 2 (5.1) | 0 (0.00) | 1 (2.6) | 1 (2.6) | |
| Asian | 5 (12.8) | 1 (2.6) | 3 (7.9) | 1 (2.6) | |
| Hispanic or Latino | 4 (10.3) | 2 (5.1) | 4 (10.5) | 2 (5.1) | |
| Body Mass Index, kg/m2 | 26.2 (22.7, 29.9) | 27.8 (26.5, 29.2) | 27.1 (24.8, 29.4) | 26.7 (23.7, 28.6) | 0.15 |
| Smoker, N (%) | 0.04 | ||||
| Never smoker | 25 (64.1) | 20 (51.3) | 23 (60.5) | 30 (76.9) | |
| Past smoker | 12 (30.8) | 19 (48.7) | 13 (34.2) | 6 (15.4) | |
| Current smoker | 2 (5.1) | 0 (0.00) | 2 (5.3) | 3 (7.7) | |
| Abstinence time <2 days, N (%)b | 10 (25.6) | 9 (23.1) | 8 (21.1) | 8 (20.5) | 0.97 |
| Diet | |||||
| Total energy intake, kcal/day | 1461.5 (1178.3, 2241.3) | 1996.9 (1635.4, 2258.3) | 2184.2 (1829.4, 2520.1) | 2321.6 (1935.6, 2808) | <.0001 |
| Caffeine intake, mg/day | 113.5 (37.9, 283.5) | 218.4 (71.4, 281.4) | 181.6 (86.8, 253.5) | 170.2 (80.8, 286.1) | 0.58 |
| Alcohol intake, g/day | 7 (2.7, 16.6) | 13.3 (7.5, 22.6) | 8.3 (3.6, 19) | 10.6 (2, 19.2) | 0.39 |
| Cold breakfast cereal, 1 or more servings/day, N (%) | 9 (23.1) | 18 (46.2) | 27 (71.1) | 23 (59.0) | <.0001 |
| Cooked oatmeal or oat bran, 1 or more servings/day, N (%) | 3 (7.7) | 7 (18.0) | 7 (18.4) | 7 (18.0) | 0.46 |
| Saturated fat, % energy | 9.6 (8, 11.2) | 10.6 (8.8, 11.6) | 10.4 (9, 11.9) | 11.1 (8.8, 12.8) | 0.15 |
| Monounsaturated fat, % energy | 12.3 (10.8, 15.2) | 12.7 (11.8, 14.9) | 11.9 (10.3, 13.6) | 11.4 (10, 13.3) | 0.06 |
| Polyunsaturated fat, % energy | 6.1 (5.1, 7.4) | 6.5 (5.1, 7.6) | 5.6 (4.7, 6) | 5.4 (4.2, 6.1) | 0.001 |
| Trans fat, % energy | 1.1 (0.8, 1.3) | 1.0 (0.8, 1.2) | 0.9 (0.8, 1.1) | 1.0 (0.8, 1.2) | 0.55 |
| Protein intake, % energy | 15.7 (13.8, 17.3) | 16.4 (14.7, 18.8) | 16.1 (14.8, 17.5) | 15.9 (14.5, 18) | 0.72 |
| Dairy protein intake, % energy | 1.9 (1.4, 3) | 3 (2.3, 3.6) | 3.4 (2.8, 4) | 4.6 (3.8, 5.8) | <.0001 |
| Prudent pattern score | −0.5 (−1.1, 0.2) | −0.1 (−0.8, 0.6) | 0.1 (−0.4, 0.6) | 0.1 (−0.4, 0.7) | 0.01 |
| Western pattern score | −0.5 (−0.9, 0.1) | 0 (−0.6, 0.5) | −0.1 (−0.4, 0.6) | 0 (−0.9, 0.9) | 0.07 |
| Reproductive history | |||||
| Male factor infertility diagnosis, N (%) | 16 (41.0) | 14 (35.9) | 11 (29.0) | 15 (38.5) | 0.73 |
| Previous infertility exam, N (%) | 28 (71.8) | 29 (74.4) | 29 (76.3) | 32 (82.1) | 0.76 |
| Undescended testes, N (%) | 1 (2.6) | 0 (0.00) | 2 (5.3) | 3 (7.7) | 0.34 |
| Varicocele, N (%) | 3 (7.7) | 6 (15.4) | 2 (5.3) | 4 (10.3) | 0.54 |
| Any reproductive surgery, N (%) c | 2 (5.1) | 4 (10.3) | 4 (10.5) | 6 (15.4) | 0.55 |
From Kruskal-Wallis test for continuous variables, Fisher’s exact test for categorical variables.
Calculated for the first sample for each man
Report of any of the following: orchidopexy, varicocelectomy, hydrocelectomy, hernia repair, urethral repair, hypospadias repair, sympathectomy, bladder neck surgery, or other reproductive surgery
Full-fat dairy food intake was not associated with semen parameters but cheese intake was associated with lower sperm concentration (p-trend=0.03) (Table 2). Adjustment for dairy fat intake did not change the relation between cheese intake and sperm concentration. There was also a suggestion of inverse associations of cheese intake with total sperm count (p-trend=0.06) and progressive motility (p-trend=0.07) (Table 2). Compared to men in the lowest tertile of cheese intake (<0.43 servings/day), men in the highest tertile (0.82–2.43 servings/day) had 31.9% (95% CI −82.9, 4.8) lower total sperm count, 38.5 (95% CI −98.3, 3.2) lower sperm concentration, and 5.4% units (95% CI −0.7, 11.6) lower progressive motility.
Table 2.
Adjusted a semen quality parameters (mean (95% confidence interval)) according to intake of full-fat dairy foods
| Quantiles of dairy intake [range, servings/d] | N | Total sperm count (million) | Sperm concentration (million/mL) | Progressive motility (% motile) | Sperm morphology (% normal) | Ejaculate volume (mL) |
|---|---|---|---|---|---|---|
| Full-fat dairy food b | ||||||
| Quartile 1 [0.00–0.57] | 37 | 137 (105–177) | 57.7 (43.3–76.9) | 28.4 (24.0–32.8) | 6.8 (5.7–7.8) | 2.7 (2.3–3.2) |
| Quartile 2 [0.59–0.94] | 40 | 110 (85–143) | 46.3 (35.5–60.4) | 27.6 (23.0–32.2) | 5.9 (4.9–7.0) | 2.7 (2.3–3.0) |
| Quartile 3 [0.96–1.67] | 39 | 126 (98–161) | 52.5 (39.6–69.5) | 26.5 (21.0–32.1) | 6.4 (5.5–7.4) | 2.7 (2.3–3.0) |
| Quartile 4 [1.68–5.45] | 39 | 114 (87–149) | 43.6 (33.0–57.6) | 22.9 (18.1–27.7) | 5.8 (4.6–7.0) | 2.9 (2.5–3.4) |
| P-trend | 0.65 | 0.35 | 0.08 | 0.47 | 0.41 | |
| Cheese c | ||||||
| Tertile 1 [0.00–0.43] | 43 | 134 (107–167) | 54.3 (42.6–69.2) | 28.9 (24.9–32.8) | 6.8 (5.9–7.7) | 2.9 (2.4–3.3) |
| Tertile 2 [0.45–0.80] | 56 | 133 (109–163) | 58.2 (47.2–71.8) | 27.3 (23.9–30.8) | 6.4 (5.6–7.2) | 2.6 (2.3–2.9) |
| Tertile 3 [0.82–2.43] | 56 | 101 (81–127) | 39.2 (30.8–49.9) | 23.4 (19.0–27.9) | 5.6 (4.6–6.6) | 2.8 (2.5–3.1) |
| P-trend | 0.06 | 0.03 | 0.07 | 0.11 | 0.95 | |
| Cream | ||||||
| Tertile 1 [0.00] | 39 | 99 (77–128) | 40.8 (31.9–52.2) | 23.4 (19.5–27.3) | 5.5 (4.6–6.4) | 2.7 (2.3–3.1) |
| Tertile 2 [0.02–0.08] | 65 | 125 (105–150) | 53.1 (43.6–64.6) | 28.2 (24.8–31.5) | 6.6 (5.8–7.4) | 2.7 (2.4–2.9) |
| Tertile 3 [0.14–2.0] | 51 | 135 (107–169) | 52.9 (41.4–67.6) | 26.4 (21.7–31.1) | 6.4 (5.5–7.2) | 2.9 (2.5–3.2) |
| P-trend | 0.27 | 0.51 | 0.97 | 0.65 | 0.38 | |
| Ice cream | ||||||
| Tertile 1 [0.00–0.02] | 51 | 128 (105–156) | 48.9 (40.1–59.8) | 24.8 (21.5–28.1) | 6.4 (5.5–7.2) | 2.9 (2.6–3.2) |
| Tertile 2 [0.08] | 55 | 106 (85–133) | 44.0 (34.6–55.8) | 26.7 (21.9–31.5) | 6.1 (5.2–7.0) | 2.7 (2.4–3.0) |
| Tertile 3 [0.14–2.00] | 49 | 132 (107–164) | 57.8 (46.4–72.1) | 27.9 (24.2–31.6) | 6.3 (5.4–7.1) | 2.7 (2.3–3.0) |
| P-trend | 0.41 | 0.12 | 0.34 | 0.96 | 0.41 | |
| Whole milk | ||||||
| None | 96 | 114 (98–134) | 48.8 (41.3–57.7) | 26.7 (23.4–30.0) | 6.0 (5.4–6.6) | 2.7 (2.4–2.9) |
| Any [0.02–1.00] | 59 | 135 (110–165) | 51.3 (41.3–63.7) | 25.9 (22.6–29.3) | 6.6 (5.8–7.5) | 2.9 (2.6–3.2) |
| P-value (comparing two groups) | 0.23 | 0.73 | 0.75 | 0.25 | 0.31 | |
Adjusted for age, total energy intake, body mass index, smoking status, abstinence time, previous infertility diagnosis, race, and dietary patterns
Includes cheese, cream, ice cream, and whole milk
Includes cream cheese and other cheese
P<0.05 compared to men in the lowest category of intake
Low-fat dairy foods, on the other hand, were positively related to sperm concentration and progressive motility (Table 3). Compared to men in the lowest quartile of low-fat dairy intake, men in the highest quartile had 33.3% (95% CI 0.6, 55.2) higher sperm concentration and 9.3% units (95% CI 1.4, 17.2) higher progressive motility. This association was driven by intake of low-fat milk (Table 3). Men in the highest tertile of low-fat milk intake had 29.9% (95% CI 0.6, 50.5) higher sperm concentration and 8.7% (95%CI 3.0, 14.4) higher progressive motility than men in the lowest tertile. Adjustment for dairy protein intake attenuated the association of low-fat milk intake with sperm concentration. In a model including an additional term for dairy protein intake, the adjusted means (95% CI) in increasing categories of low-fat milk intake were 41.0 (32.1, 52.5), 52.4 (42.2, 65.2), 56.1 (42.2, 74.6) million sperm/mL (p-trend=0.14) Adjustment for dairy protein intake had no impact on the relation between low-fat milk intake and sperm motility.
Table 3.
Adjusted a semen quality parameters (mean (95% confidence interval)) according to intake of low-fat dairy foods
| Quantiles of dairy intake [range, servings/d] | N | Total sperm count (million) | Sperm concentration (million/mL) | Progressive motility (% motile) | Sperm morphology (% normal) | Ejaculate volume (mL) |
|---|---|---|---|---|---|---|
| Low-fat dairy food b | ||||||
| Quartile 1 [0.00–0.28] | 39 | 111 (86–144) | 40.9 (31.8–52.5) | 21.6 (17.7–25.5) | 6.4 (5.3–7.5) | 3.0 (2.6–3.4) |
| Quartile 2 [0.30–0.75] | 39 | 114 (87–151) | 48.9 (36.7–65.2) | 26.5 (22.1–30.9) | 5.5 (4.7–6.4) | 2.6 (2.2–3.0) |
| Quartile 3 [0.77–1.20] | 39 | 129 (100–166) | 51.5 (38.6–68.5) | 27.5 (23.2–31.8)* | 7.1 (6.0–8.1) | 2.8 (2.5–3.2) |
| Quartile 4 [1.22–3.54] | 38 | 135 (103–177) | 61.2 (46.1–81.3)* | 30.9 (24.4–37.4)* | 6.0 (4.8–7.2) | 2.5 (2.0–3.0) |
| P-trend | 0.26 | 0.06 | 0.03 | 0.72 | 0.44 | |
| Low-fat milk c | ||||||
| Tertile 1 [0.00–0.10] | 47 | 101 (81–127) | 40.3 (31.9–50.9) | 20.8 (17.6–24.1) | 5.9 (5.1–6.8) | 2.7 (2.4–3.1) |
| Tertile 2 [0.14–0.57] | 57 | 129 (105–159) | 52.6 (42.3–65.3) | 28.8 (25.4–32.2)* | 6.3 (5.4–7.1) | 2.8 (2.4–3.1) |
| Tertile 3 [0.80–2.64] | 51 | 135 (109–168) | 57.5 (44.8–73.7)* | 29.5 (24.8–34.3)* | 6.5 (5.6–7.5) | 2.7 (2.3–3.1) |
| P-trend | 0.08 | 0.05 | 0.003 | 0.36 | 1.00 | |
| Yogurt d | ||||||
| Tertile 1 [0.00–0.08] | 55 | 109 (88–136) | 45.0 (36.5–55.4) | 27.7 (23.8–31.7) | 6.4 (5.6–7.2) | 2.8 (2.4–3.1) |
| Tertile 2 [0.10–0.30] | 49 | 127 (102–159) | 47.3 (37.2–60.2) | 23.6 (19.9–27.3) | 5.9 (4.9–6.9) | 2.9 (2.7–3.2) |
| Tertile 3 [0.36–1.16] | 51 | 130 (106–159) | 58.7 (46.8–73.5) | 27.9 (23.4–32.3) | 6.4 (5.6–7.3) | 2.5 (2.2–2.9) |
| P-trend | 0.35 | 0.08 | 0.65 | 0.72 | 0.31 | |
Adjusted for age, total energy intake, body mass index, smoking status, abstinence time, previous infertility diagnosis, race, and dietary patterns
Includes low fat milk, yogurt, and cottage cheese
Includes skim milk and 1 and 2% milk
Includes frozen yogurt, plain yogurt and flavored yogurt
P<0.05 compared to men in the lowest category of intake
Although the observed relations appeared to be independent of overall food choices, as captured by data-derived dietary patterns, we further examined the possibility of residual confounding by breakfast cereal intake. The adjusted sperm concentrations (95% CI) in increasing tertiles of low-fat milk intake were 39.1 (30.7, 49.8), 52.2 (42.0, 64.8), and 59.6 (45.8, 77.5) million sperm per mL in models further adjusted for cereal breakfast intake (p-trend=0.03). The corresponding values for progressive motility were 20.4% (16.9, 23.8), 28.7% (25.2, 32.1), and 30.2% (24.9, 35.4) progressively motile sperm (p-trend=0.004). Low-fat milk intake was also associated with higher total motile count (Figure 1).
Figure 1.
a Adjusted for age, total energy intake, body mass index, smoking status, abstinence time, previous infertility diagnosis, race, and dietary patterns
* P-value <0.05 compared to men in the lowest tertile of intake
Results of our different sensitivity analyses were in the same direction regardless of how many samples were used or time-related exclusions (Supplementary table 1). There was no evidence of effect modification by BMI or smoking for the observed associations with low-fat dairy or low-fat milk. The association of cheese intake and sperm concentration, on the other hand, was modified by smoking (p, heterogeneity = 0.01). The adjusted sperm concentration in increasing tertiles of cheese intake was 63.8 (39, 104.2), 63.3 (47.9, 83.7), and 29.9 (20.1, 44.3) million per mL (p-trend=0.009) among ever smokers (past and current), and 49.9 (39, 63.8), 53.1 (39.9, 70.6), and 46.4 (34.9, 61.6) million per mL (p-trend=0.62) among never smokers.
Discussion
We prospectively investigated the association of dairy foods intake and semen quality parameters in a cohort of men attending a fertility clinic and found that low-fat dairy food intake was associated with higher sperm concentration and motility. This association was driven by intake of low-fat milk and was independent of overall food choices as captured by data-derived dietary patterns. Further, the association between low-fat dairy and sperm concentration appeared to be explained in part by dairy protein intake. We also observed an inverse relation between cheese intake and sperm concentration that appeared to be restricted to ever smokers. These associations were independent of overall food choices as captured by data-derived dietary patterns.
The inverse relation between cheese intake and sperm concentration among smokers is not entirely consistent with our initial hypothesis that full-fat dairy products would be associated with lower semen quality regardless of smoking status. Favoring this hypothesis, decreased sperm production, manifested in lower concentration, could be the result from of estrogens from dairy contributing to a negative feedback loop on LH and FSH. Some of our findings, however, argue against this hypothesis. For example, because sex steroids are lipid soluble, we expected that adjustment for dairy fat intake would attenuate associations between full fat dairy foods and semen quality but this was not the case. Also, while we had previously related cheese intake with lower semen quality among healthy young men (12), the previously observed relation was with sperm morphology rather than with concentration. Previous studies suggesting that sex steroids from dairy may have limited biological activity also argue against this hypothesis (26–28). Furthermore, the association of cheese intake and sperm concentration was restricted to ever smokers raising the possibility that this relation may be reflective of unhealthy behaviors not adequately captured in this study. Equally plausible alternative hypotheses include the possibility that this relation is reflective of environmental contaminants present in full-fat dairy products (29) that have been related to lower sperm parameters (30) or that this association is a chance finding. Further examination of this relation is warranted.
We found strong positive relations of low-fat dairy foods intake, particularly of low-fat milk, with sperm concentration and motility. While not part of our original hypothesis, this finding may reflect known effects of low-fat dairy intake on circulating insulin growth factor-1 (IGF-1) and insulin levels. Low-fat milk intake is associated with higher circulating levels of IGF-1 in free living populations (31, 32) and increases IGF-1 levels in feeding trials (33, 34). Intake of protein from animal sources, which appeared to account for the association between low-fat dairy and sperm concentration, increases post-prandial insulinemia in animal and human feeding experiments (35, 36). Moreover, experimental models show that insulin rescues spermatogenesis in type I diabetic mice (37) and increases total sperm count and sperm motility in type I diabetic rats (38) while IGF-1 protects equine Leydig cells from undergoing apoptosis in vitro (39). Given that spermatogenesis is a process of active cell division requiring insulin and that IGF-1 can bind and activate Leydig cell insulin receptors (40), it is possible that the observed relations of low-fat dairy with higher sperm concentration and motility represent a biological effect in humans. Although insulin and IGF-1 cannot cross the blood-testis barrier in humans (37), the observed association could represent their effects on Leydig cells (which are outside of the blood testis barrier), Sertoli cells (which create the blood testis barrier) or spermatogonia (also outside of the blood testis barrier). Given the limited data available, further work is needed to clarify whether the observed associations represent true biological effects and whether these are mediated by the mechanisms described above.
Our findings are in partial agreement with existing literature among subfertile men. Mendiola et al. found that oligoasthenoteratospermic men had lower intakes of skimmed milk than controls (11). In addition, in a case-control study of asthenospermic men in Iran, the odds of asthenospermia were significantly lower with increasing intake of skim milk (10). These two studies also found that risk of oligoasthenoteratospermia increased with intake of full-fat dairy products (11) and that risk of asthenospermia was marginally elevated with higher intake of total dairy products (p-trend=0.06) (10). Nevertheless, a third cross-sectional study among fertility patients in the Netherlands, found that dairy intake was unrelated to semen quality (13). In addition, we have previously reported no association between low-fat dairy and semen quality and inverse relations of cheese intake with sperm morphology among healthy young men (12).
Although this study contributes to the scarce literature on this topic, it does have limitations. First, because all participants were male partners in subfertile couples presenting for evaluation at a fertility center, and many (76%) had previously undergone fertility evaluations, it is possible that they would have changed specific aspects of their diet in response to having difficulties conceiving or specific knowledge of their semen quality. However, dairy food intake is generally not regarded as a risk factor for low semen quality or male factor infertility so it is unlikely men would have instituted this specific dietary change. Moreover, we tried to minimize the possibility of reverse causation by limiting the analysis to semen analyses performed after completion of the dietary assessment in order to maintain a strictly prospective analysis of dairy food intake in relation to semen parameters. An additional limitation of an infertility clinic population is that results may not be generalizable to men without known fertility problems. However, around half (47%) of the men in this population had no detectable problems in their semen analysis, ruling out male factor infertility. On the other hand, men in this study are comparable to men in fertility clinics nationwide and therefore results could be informative to men facing fertility problems. Strengths of this study include the use of a previously validated diet assessment questionnaire (19), and the assessment and adjustment for a variety of other lifestyle factors that could be potential confounding variables. Another major strength is the use of multiple samples on most men given that semen parameters are known to be highly variable within-person (18).
In summary, we prospectively investigated dairy foods intake in relation to semen quality among men attending a fertility clinic in an academic medical center and found that low-fat dairy foods, especially low-fat milk, were positively associated with sperm concentration and progressive motility resulting in higher total motile sperm counts. We also found that cheese intake was associated with lower sperm concentration among ever smokers. Although the observed relations are biologically plausible, data on the relation of diet in general and dairy foods in particular with semen quality or male factor infertility remains limited. Therefore, additional prospective studies of this relation are needed including studies exploring the biological mechanisms explaining these associations.
Supplementary Material
A total of 155 men with 338 semen samples were included in the analysis; 98 men provided only 1 sample, 51 men provided 2 samples, and 47 men provided 3 or more samples.
Acknowledgments
We thank the patients from the MGH fertility center who participated in the study. We also thank the HSPH research staff, especially the research nurses, Jennifer Ford and Myra Keller.
Abbreviations
- BMI
body mass index
- FFQ
food frequency questionnaire
- IGF-1
insulin growth factor-1
- IQR
interquartile range
- MGH
Massachusetts General Hospital
- WHO
World Health Organization
Footnotes
Study funding/competing interest(s): This work was supported by NIH grants R01-ES009718 from NIEHS, P30 DK046200 from NIDDK. MCA and AJG were supported by a Ruth L. Kirschstein National Research Service Award T32 DK 007703-16 from NIDDK.
Financial disclosures: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Louis JF, Thoma ME, Sørensen DN, McLain AC, King RB, Sundaram R, et al. The prevalence of couple infertility in the United States from a male perspective: evidence from a nationally representative sample. Andrology. 2013;1:741–8. doi: 10.1111/j.2047-2927.2013.00110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thoma ME, McLain AC, Louis JF, King RB, Trumble AC, Sundaram R, et al. Prevalence of infertility in the United States as estimated by the current duration approach and a traditional constructed approach. Fertility and Sterility. 2013;99:1324–31. e1. doi: 10.1016/j.fertnstert.2012.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thonneau P, Marchand S, Tallec A, Ferial M-L, Ducot B, Lansac J, et al. Incidence and main causes of infertility in a resident population (1 850 000) of three French regions (1988–1989) Hum Reprod. 1991;6:811–6. doi: 10.1093/oxfordjournals.humrep.a137433. [DOI] [PubMed] [Google Scholar]
- 4.Sharpe RM. The ‘oestrogen hypothesis’– where do we stand now? International Journal of Andrology. 2003;26:2–15. doi: 10.1046/j.1365-2605.2003.00367.x. [DOI] [PubMed] [Google Scholar]
- 5.Davaasambuu G, Wang PY, Qin LQ, Hoshi K, Sato A. Is milk responsible for male reproductive disorders? Medical Hypotheses. 2001;57:510–4. doi: 10.1054/mehy.2001.1380. [DOI] [PubMed] [Google Scholar]
- 6.Daxenberger A, Ibarreta D, Meyer HHD. Possible health impact of animal oestrogens in food. Human Reproduction Update. 2001;7:340–55. doi: 10.1093/humupd/7.3.340. [DOI] [PubMed] [Google Scholar]
- 7.García-Peláez B, Ferrer-Lorente R, Gómez-Ollés S, Fernández-López JA, Remesar X, Alemany M. Technical Note: Measurement of Total Estrone Content in Foods. Application to Dairy Products Journal of Dairy Science. 2004;87:2331–6. doi: 10.3168/jds.S0022-0302(04)73354-8. [DOI] [PubMed] [Google Scholar]
- 8.Pape-Zambito DA, Roberts RF, Kensinger RS. Estrone and 17β-estradiol concentrations in pasteurized-homogenized milk and commercial dairy products. Journal of Dairy Science. 2010;93:2533–40. doi: 10.3168/jds.2009-2947. [DOI] [PubMed] [Google Scholar]
- 9.Hartmann S, Lacorn M, Steinhart H. Natural occurrence of steroid hormones in food. Food Chemistry. 1998;62:7–20. [Google Scholar]
- 10.Eslamian G, Amirjannati N, Rashidkhani B, Sadeghi M-R, Hekmatdoost A. Intake of food groups and idiopathic asthenozoospermia: a case-control study. Hum Reprod. 2012;27:3328–36. doi: 10.1093/humrep/des311. [DOI] [PubMed] [Google Scholar]
- 11.Mendiola J, Torres-Cantero AM, Moreno-Grau JM, Ten J, Roca M, Moreno-Grau S, et al. Food intake and its relationship with semen quality: a case-control study. Fertility and Sterility. 2009;91:812–8. doi: 10.1016/j.fertnstert.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 12.Afeiche M, Williams PL, Mendiola J, Gaskins A, Jørgensen N, Swan S, et al. Dairy Food Intake in Relation to Semen Quality and Reproductive Hormone Levels among Physically Active Young Men. Human Reproduction. 2013;28:2265–75. doi: 10.1093/humrep/det133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vujkovic M, de Vries JH, Dohle GR, Bonsel GJ, Lindemans J, Macklon NS, et al. Associations between dietary patterns and semen quality in men undergoing IVF/ICSI treatment. Hum Reprod. 2009;24:1304–12. doi: 10.1093/humrep/dep024. [DOI] [PubMed] [Google Scholar]
- 14.Attaman JA, Toth TL, Furtado J, Campos H, Hauser R, Chavarro JE. Dietary fat and semen quality among men attending a fertility clinic. Hum Reprod. 2012;27:1466–74. doi: 10.1093/humrep/des065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jensen TK, Heitmann BL, Jensen MB, Halldorsson TI, Andersson AM, Skakkebaek NE, et al. High dietary intake of saturated fat is associated with reduced semen quality among 701 young Danish men from the general population. Am J Clin Nutr. 2013;97:411–8. doi: 10.3945/ajcn.112.042432. [DOI] [PubMed] [Google Scholar]
- 16.Hauser R, Meeker JD, Duty S, Silva MJ, Calafat AM. Altered semen quality in relation to urinary concentrations of phthalate monoester and oxidative metabolites. Epidemiology. 2006;17:682–91. doi: 10.1097/01.ede.0000235996.89953.d7. [DOI] [PubMed] [Google Scholar]
- 17.Kruger TF, Acosta AA, Simmons KF, Swanson RJ, Matta JF, Oehninger S. Predictive value of abnormal sperm morphology in in vitro fertilization. Fertil Steril. 1988;49:112–7. doi: 10.1016/s0015-0282(16)59660-5. [DOI] [PubMed] [Google Scholar]
- 18.WHO. WHO Laboratory Manual for the Examination and Processing of Human Semen. 5. Geneva: World Health Organization; 2010. [Google Scholar]
- 19.Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and Validity of an Expanded Self-Administered Semiquantitative Food Frequency Questionnaire among Male Health Professionals. American Journal of Epidemiology. 1992;135:1114–26. doi: 10.1093/oxfordjournals.aje.a116211. [DOI] [PubMed] [Google Scholar]
- 20.Gebhardt SE, Lemar LE, Haytowitz DB, Pehrsson PR, Nickle MS, Showell BA, et al. USDA national nutrient database for standard reference, release 21. 2008. [Google Scholar]
- 21.Feskanich D, Rimm EB, Giovannucci EL, Colditz GA, Stampfer MJ, Litin LB, et al. Reproducibility and validity of food intake measurements from a semiquantitative food frequency questionnaire. Journal of the American Dietetic Association. 1993;93:790–6. doi: 10.1016/0002-8223(93)91754-e. [DOI] [PubMed] [Google Scholar]
- 22.Gaskins AJ, Colaci DS, Mendiola J, Swan SH, Chavarro JE. Dietary patterns and semen quality in young men. Hum Reprod. 2012;27:2899–907. doi: 10.1093/humrep/des298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.White H. A heteroskedasticity-consistent covariance matrix estimator and a direct test for heteroskedasticity. Econometrica. 1980;48:817–30. [Google Scholar]
- 24.Searle SR, Speed FM, Milliken GA. Population marginal means in the linear model: an alternative to least square means. Am Stat. 1980;34:216–21. [Google Scholar]
- 25.Jensen TK, Heitmann BL, Jensen MB, Halldorsson TI, Andersson A-M, Skakkebæk NE, et al. High dietary intake of saturated fat is associated with reduced semen quality among 701 young Danish men from the general population. The American Journal of Clinical Nutrition. 2013;97:411–8. doi: 10.3945/ajcn.112.042432. [DOI] [PubMed] [Google Scholar]
- 26.Ruoff WL, Dziuk PJ. Circulation of estrogens introduced into the rectum or duodenum in pigs. Domestic Animal Endocrinology. 1994;11:383–91. doi: 10.1016/0739-7240(94)90010-8. [DOI] [PubMed] [Google Scholar]
- 27.O’Connell MB. Pharmacokinetic and Pharmacologic Variation Between Different Estrogen Products. The Journal of Clinical Pharmacology. 1995;35:18S–24S. doi: 10.1002/j.1552-4604.1995.tb04143.x. [DOI] [PubMed] [Google Scholar]
- 28.Furnari C, Maroun D, Gyawali S, Snyder BW, Davis AM. Lack of biologically active estrogens in commercial cow milk. Journal of Dairy Science. 2012;95:9–14. doi: 10.3168/jds.2011-4365. [DOI] [PubMed] [Google Scholar]
- 29.Schaum J, Schuda L, Wu C, Sears R, Ferrario J, Andrews K. A national survey of persistent, bioaccumulative, and toxic (PBT) pollutants in the United States milk supply. Journal of Exposure Analysis & Environmental Epidemiology. 2003;13:177. doi: 10.1038/sj.jea.7500269. [DOI] [PubMed] [Google Scholar]
- 30.Rozati R, Reddy PP, Reddanna P, Mujtaba R. Role of environmental estrogens in the deterioration of male factor fertility. Fertility and Sterility. 2002;78:1187–94. doi: 10.1016/s0015-0282(02)04389-3. [DOI] [PubMed] [Google Scholar]
- 31.Giovannucci E, Pollak M, Liu Y, Platz EA, Majeed N, Rimm EB, et al. Nutritional Predictors of Insulin-like Growth Factor I and Their Relationships to Cancer in Men. Cancer Epidemiology Biomarkers & Prevention. 2003;12:84–9. [PubMed] [Google Scholar]
- 32.Tsilidis KK, Travis RC, Appleby PN, Allen NE, Lindström S, Albanes D, et al. Insulin-like growth factor pathway genes and blood concentrations, dietary protein and risk of prostate cancer in the NCI Breast and Prostate Cancer Cohort Consortium (BPC3) International Journal of Cancer. 2013:n/a–n/a. doi: 10.1002/ijc.28042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hoppe C, Molgaard C, Juul A, Michaelsen KF. High intakes of skimmed milk, but not meat, increase serum IGF-I and IGFBP-3 in eight-year-old boys. Eur J Clin Nutr. 2004;58:1211–6. doi: 10.1038/sj.ejcn.1601948. [DOI] [PubMed] [Google Scholar]
- 34.Bonjour J-P, Benoit V, Rousseau B, Souberbielle J-C. Consumption of Vitamin D-and Calcium-Fortified Soft White Cheese Lowers the Biochemical Marker of Bone Resorption TRAP 5b in Postmenopausal Women at Moderate Risk of Osteoporosis Fracture. The Journal of Nutrition. 2012;142:698–703. doi: 10.3945/jn.111.153692. [DOI] [PubMed] [Google Scholar]
- 35.Toden S, Belobrajdic DP, Bird AR, Topping DL, Conlon MA. Effects of Dietary Beef and Chicken With and Without High Amylose Maize Starch on Blood Malondialdehyde, Interleukins, IGF-I, Insulin, Leptin, MMP-2, and TIMP-2 Concentrations in Rats. Nutrition and Cancer. 2010;62:454–65. doi: 10.1080/01635580903532382. [DOI] [PubMed] [Google Scholar]
- 36.Larsson SC, Wolk K, Brismar K, Wolk A. Association of diet with serum insulin-like growth factor I in middle-aged and elderly men. The American Journal of Clinical Nutrition. 2005;81:1163–7. doi: 10.1093/ajcn/81.5.1163. [DOI] [PubMed] [Google Scholar]
- 37.Schoeller EL, Albanna G, Frolova AI, Moley KH. Insulin Rescues Impaired Spermatogenesis via the Hypothalamic-Pituitary-Gonadal Axis in Akita Diabetic Mice and Restores Male Fertility. Diabetes. 2012;61:1869–78. doi: 10.2337/db11-1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Singh S, Malini T, Rengarajan S, Balasubramanian K. Impact of experimental diabetes and insulin replacement on epididymal secretory products and sperm maturation in albino rats. Journal of Cellular Biochemistry. 2009;108:1094–101. doi: 10.1002/jcb.22337. [DOI] [PubMed] [Google Scholar]
- 39.Yoon MJ, Roser JF. Insulin-like growth factor-I (IGF-I) protects cultured equine Leydig cells from undergoing apoptosis. Animal Reproduction Science. 2010;122:353–8. doi: 10.1016/j.anireprosci.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 40.Holly JMP, Perks CM. Insulin-Like Growth Factor Physiology: What we have Learned from Human Studies. Endocrinology and Metabolism Clinics of North America. 2012;41:249–63. doi: 10.1016/j.ecl.2012.04.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A total of 155 men with 338 semen samples were included in the analysis; 98 men provided only 1 sample, 51 men provided 2 samples, and 47 men provided 3 or more samples.


