Abstract
Polymeric nanoparticles (NPs) are promising carriers of biological agents to lung due to advantages including biocompatibility, ease of surface modification, localized action and reduced systemic toxicity. However, there have been no studies extensively characterizing and comparing the behavior of polymeric NPs for pulmonary protein/DNA delivery both in vitro and in vivo. We screened six polymeric NPs: gelatin, chitosan, alginate, poly lactic-co-glycolic acid (PLGA), PLGA-chitosan, and PLGA-polyethylene glycol (PEG), for inhalational protein/ DNA delivery. All NPs except PLGA-PEG and alginate were <300 nm in size with bi-phasic core compound release profile. Gelatin, PLGA NPs and PLGA-PEG NPs remained stable in deionized water, serum, saline and simulated lung fluid (Gamble’s solution) over 5 days. PLGA-based NPs and natural polymer NPs exhibited highest cytocompatibility and dose-dependent in vitro uptake respectively by human alveolar type-1 epithelial cells. Based on these profiles, gelatin and PLGA NPs were used to encapsulate a) plasmid DNA encoding yellow fluorescent protein (YFP) or b) rhodamine-conjugated erythropoietin (EPO) for inhalational delivery to rats. Following a single inhalation, widespread pulmonary EPO distribution persisted for up to 10 days while increasing YFP expression was observed for at least 7 days for both NPs. The overall results support both PLGA and gelatin NPs as promising carriers for pulmonary protein/DNA delivery.
Keywords: Pulmonary, nanoparticles, protein, DNA, nebulization
1. Introduction
Nanomedicine in the area of pulmonary protein/DNA delivery has emerged as a cutting edge technology combining nanotechnology and pharmacotherapeutics for drug delivery and tissue remodeling. Conventional methods of delivering proteins and DNA are limited by low bioavailability, denaturing/instability of the product and variation between doses [1]. Delivery of nanoparticles (NPs) loaded with therapeutic agents via inhalation takes advantage of the ease and non-invasive nature of administration, the large alveolar surface area for rapid uptake, prolonged local action, and a lower effective dose resulting in lesser risk of toxicity compared to systemic drug delivery [2]. For instance, Terzano et al.[3] recently developed non-phospholipid vesicles encapsulating beclomethasone dipropionate for treatment of chronic obstructive pulmonary disease (COPD) that can enhance penetration through the mucus layer and provide localized therapy. In addition, NPs under ~200 nm could theoretically escape detection by alveolar macrophages [4], leading to more effective uptake and action.
Different types of nanocarriers such as liposomes, lipid or polymer-based micelles, dendrimers and polymeric NPs have been used for encapsulation and delivery of therapeutic agents to the lung [5]. Polymeric NPs are of growing interest, as the polymers can be co-polymerized, surface-modified or bioconjugated for better targeting capability and delivery of the encapsulated agents. The commonly used nanocarriers in pulmonary drug delivery include natural polymers such as gelatin, chitosan, alginate and synthetic polymers like poloxamer, poly lactic-co-glycolic acid (PLGA) and Poly-(ethylene glycol) (PEG) [6]. Gelatin is a biocompatible, biodegradable protein that covalently binds the active compound [7] resulting in greater loading efficiency. Chitosan, a polysaccharide, is a mucoadhesive and permeation enhancer that facilitates NP retention in the lung following administration [6]. Alginate is another highly biocompatible natural polymer with a hydrophilic matrix for efficient protein loading [8]. PLGA has been established as an FDA-approved biocompatible and biodegradable synthetic polymer that allows sustained drug release over a period of weeks to months depending on the ratio of monomers used [9]. Further, there is growing literature to support the use of PLGA-based nano/micro particles for pulmonary delivery due to their biocompatibility and the option of tailoring their rate of drug release and biodegradation based on the intended applications [10, 11] without causing tissue damage in the lung [12]. For example, in vitro studies by Tahara et al.[13] demonstrated that PLGA nanoparticles with and without chitosan coating are cytocompatible with A549 lung epithelial cells up to a high concentration of 5 mg/ml. Recent studies on PLGA nanoparticles prepared with poly vinyl alcohol (PVA) surfactant also demonstrated minimal inflammatory reaction and good cytocompatibility at <1 mg/ml concentration with A549 cells[14]. Further, histological examination of lung tissue sections following PLGA nanoparticle administration by intratracheal instillation have shown that these particles do not cause lung tissue damage [15]. PEG is known to improve the hydrophilicity, aerodynamic characteristics and retention time of NPs [16, 17].
Due to the small size of NPs, they tend to remain suspended in air, making direct delivery to and deposition in the deep lung difficult. Therefore, the mode of pulmonary delivery also plays a crucial role in facilitating NP deposition and distribution in distal lung tissue. Use of a metered dose inhaler or a dry powder inhaler could result in significant oropharyngeal NP deposition and variation in dosage when the device is not shaken correctly [18]. Use of a nebulizer on the other hand could maintain a relatively constant size of aerosol droplets in the range (4–6µm diameter) that easily allow the suspended NPs to reach the distal lung. For example, the celecoxib-loaded lipid nanocarriers developed by Patolla et al. [19] (~217 nm size) were shown to deposit in the alveolar region of murine lungs following nebulization. A recent study demonstrated that aerosol droplets containing 5(6)-carboxyfluorescein-loaded nanoparticles (195 nm) generated by an Aeroneb™ nebulizer possessed aerodynamic properties suitable for alveolar deposition [20].
A survey of literature indicates that although several polymeric NPs have been characterized for pulmonary delivery of different compounds, there have been no studies to the authors’ knowledge that corroborated the in vitro cellular uptake and retention time of NPs with their behavior in vivo. Further, the optimum formulation that facilitates prolonged core compound delivery and release as well as comparatively longer retention in the lung is unknown. Therefore, it is essential that polymeric NPs are thoroughly evaluated in terms of physical and chemical properties and release efficacy of therapeutic agent to choose the optimum nanocarrier for the specific type of compounds being delivered. Studies have been conducted previously to compare the properties of selected polymeric (e.g. PLGA, chitosan, gelatin) nano/micro particles for pulmonary delivery of therapeutic agents like tobramycin and rifampicin [12, 21]. Recently, chitosan and PLGA-based NPs have also been developed for pulmonary delivery of proteins/peptides such as insulin and calcitonin [22, 23]. However, it is essential to determine the most promising nanoparticle formulation that can efficiently deliver these core compounds to the alveoli for treatment of pulmonary ailments. Therefore, the goal of this project was to compare selected naturally and synthetically-derived biocompatible polymer-based NPs encapsulating model proteins (bovine serum albumin [BSA] and rhodamine conjugated to recombinant human erythropoietin [EPO]) or plasmid cDNA (encoding yellow fluorescent protein, YFP) in terms of their physical-chemical properties, in vitro cell uptake and compatibility with human alveolar epithelial cells, and in vivo pulmonary uptake following inhalation in rats. Our goal was to determine the most promising formulation(s) for further development as carriers for pulmonary delivery of biological agents.
2. Materials and Methods
2.1. Synthesis of natural polymer-based NPs
Gelatin NPs were prepared by two step desolvation method described by Shutava et al.[24] Briefly, 0.05% (w/v) gelatin solution was prepared in DI water and 25 ml of acetone was rapidly added to it. The gel-like precipitate obtained was re-dissolved in water and 75 ml of acetone was added dropwise at 40°C to obtain a milky-white solution. 0.2ml of 25% glutaraldehyde as a crosslinker was then added and stirred overnight, following which the solution was dialyzed and lyophilized to obtain gelatin NPs.
Chitosan NPs were prepared by ionic gelation using sodium tripolyphosphate (TPP) [25]. Chitosan (Polyscience Inc., Warrington, PA) solution in 1% (w/v) acetic acid was adjusted to a pH of 5.5 following which TPP was added dropwise to allow formation of particles. After 1 hr stirring, the particles were dialyzed and freeze-dried.
Alginate NPs were prepared by cation induced controlled gelification of alginate described by Rajaonarivony et al.[26] with slight modifications [27]. Briefly, 18 mM of calcium chloride was added dropwise to sodium alginate solution (0.06% w/v). Chitosan solution of concentration 0.05% w/v was then added followed by stirring overnight. The NPs were recovered by centrifugation at 19,000 rpm for 30 mins, followed by lyophilization to obtain the NPs.
2.2. Fabrication of synthetic polymer-based NPs
Emulsion - solvent evaporation method was used to prepare PLGA NPs. For this procedure, 3% w/v PLGA (Lakeshore Biomaterials, Birmingham, AL) solution was prepared in chloroform to form a primary emulsion. This emulsion was then added to an aqueous solution of 5% w/v PVA to create double emulsion, and sonicated. This particle suspension was stirred overnight at room temperature allowing the solvent to evaporate. NPs were recovered by ultracentrifugation at 25, 000 rpm for 30 mins at 10°C. For BSA loaded NPs, 3% BSA solution (30mg in 300µl of DI water) was emulsified in PLGA solution, while for cDNA loaded NPs, 0.1% of the cDNA was dispersed in DI water and used for emulsification.
For the preparation of PLGA-CS NPs, carboxymethyl chitosan (CMC) was mixed with PVA solution and allowed to be adsorbed onto the surface of the PLGA NPs. The NP preparation procedure is similar to PLGA NPs except for the addition of 0.5% (w/v) CMC in 12 ml of 4.5% (w/v) PVA.
The copolymer of PLGA-PEG was synthesized by conjugation of COOH-PEG-NH2 (Laysan Bio Inc., Arab, AL) to the free COOH groups on PLGA using carbodiimide chemistry. PLGA-NHS was obtained by addition of excess N-hydroxysuccinimide (NHS) and 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide (EDC) to PLGA solution in dichloromethane. The resultant polymer was precipitated by cold methanol and vacuum dried. 1g of PLGA-NHS was dissolved in 4 ml of chloroform and then 250mg of COOH-PEG-NH2 and 28 mg N,N-diisopropylethylamine was added and stirred for 12 hours. The copolymer was precipitated with cold methanol and washed three times to remove unreacted PEG. This polymer was dried under vacuum and used further for NP preparation [28]. Bovine serum albumin (BSA) was used as protein model while YFP plasmid cDNA was used as cDNA model for encapsulation within all six NPs. All NPs were lyophilized and stored in powder form at −20°C when not being used. For all of our in vitro and in vivo studies, the particles were freshly constituted either in DI water, media, or saline.
2.3. Characterization of NPs
The NPs were characterized for their particle size, polydispersity and zeta potential using DLS (ZetaPALS dynamic light scattering (DLS) detector (Brookhaven Instruments, Holtsville, NY). A fixed amount of nanoparticle solution was added to a transparent cuvette and placed in the instrument. The nanoparticle properties were detected by laser light scattering due to Brownian motion of the particles. The morphology of the particles was also analyzed using Transmission electron microscopy (TEM, FEI Tecnai G2 Spirit BioTWIN, Hillsboro, OR). A drop of particle solution was placed on a Formvar-coated 200-mesh copper grid (Electron Microscopy Sciences, Hartfield, PA) at room temperature and allowed to air-dry. The sample was then inserted into the TEM instrument for observation.
2.4. Stability Study
To determine in vitro stability, the NPs were suspended in DI water, saline (0.9% sodium chloride solution), 10% Fetal Bovine Serum (FBS, Atlanta Biological, Lawerenceville, GA), or simulated lung fluid (Gamble’s solution, prepared as described by Marques et al.[29]) and incubated at 37°C for 5 days. Particle size was measured after every 6 hours for up to 12 hours and then every 24 hours for 5 days. DLS was used to measure particle size at each interval.
2.5. Loading and release studies
The amount of encapsulated agent entrapped in NPs was calculated by quantifying the amount of un-entrapped reagent collected in the supernatant after centrifugation. The protein/cDNA encapsulation efficiency was calculated as the percentage of protein/cDNA used initially for loading into the NPs (Equation 1).
| (1) |
For in vitro protein release studies, stock solutions of BSA-loaded NPs were prepared in DI water and 1 ml was added to dialysis bags with a molecular weight cut-off of 100 kDa (Spectrum Laboratories Inc., Rancho Dominguez, CA) and dialyzed against DI water at 37°C for 21 days. Four replicates were used for analysis. At predetermined time points, 1 ml of dialysate was collected from each sample and replaced with 1 ml of fresh DI water. This dialysate was stored at −20°C for further analysis. The amount of BSA released was analyzed using Pierce BCA protein assays (Fisher Scientific, Hampton, NH), according to the manufacturer’s instructions. The amount of protein released was analyzed against BSA standards to determine cumulative percentage protein release.
2.6. In Vitro cell studies
Cytocompatibility
Human alveolar type 1 epithelial cells (Applied Biological Materials Inc., Richmond, BC, Canada) were seeded in tissue culture well plates at a density of 16000 cells/cm2 and incubated at 37°C and 5% CO2 for 24h to promote cell attachment. Following incubation, the media was aspirated and replaced with increasing concentration of NP suspensions (in media) for 24 h. The samples were washed twice with 1× Phosphate buffered saline (PBS) and incubated with MTS reagent (CellTiter 96®AQueous One Solution Cell Proliferation Assay, Promega, Madison, WI). Absorbance readings were obtained using a UV–vis spectrometer (Infinite M200 plate reader, Tecan, Durham, NC) at a wavelength of 490 nm to determine cell viability. In order to confirm the results obtained using MTS assay, a Picogreen dsDNA assay was also performed to determine the total DNA content per treatment group. To perform this assay, the cells incubated with the nanoparticles were first washed thrice with PBS and then lysed using 1% Triton X-100. Picogreen dsDNA assay was then performed on the cell lysates per manufacturer’s instructions.
Cellular uptake
Human alveolar type 1 epithelial cells were seeded at a density of 16000 cells/cm2 in tissue culture plates and incubated at 37°C for 24 h. The cells were then incubated with increasing concentrations of Indocyanine green (ICG)-loaded NPs in media for 2 h. At the end of the study, the cells were washed well using PBS and lysed using 1% Triton X-100. Fluorescence intensity measurement of each well was carried out using a spectrophotometer to determine the ICG present within the particles taken up the cells. These measurements were analyzed against a nanoparticle standard. These fluorescence intensity values were then normalized with the total DNA content per sample using Picogreen dsDNA assays (Invitrogen, Grand Island, NY). The cell lysate sample is usually quantified for the total cell protein or DNA, which presents the cell number per sample, using protein or DNA assays. However, EPO- and cDNA-loaded NPs will interfere with the readings for both these assays. Therefore we used ICG-loaded particles since the fluorescence readings will not interfere with our quantification of the cell lysate samples.
2.7. In vivo animal studies
The Institutional Animal Care and Use Committee of the University of Texas Southwestern Medical Center (UT Southwestern) approved all animal procedures. Sprague-Dawley rats (300–400 grams body weight, Charles River Laboratories, Wilmington, MA) were anesthetized by an intraperitoneal injection of ketamine (50 mg/kg) and xylazine (5 mg/kg). The larynx was visualized using an otoscope and a 14 gauge cannula was inserted into the trachea using a guide wire so that the NPs can be delivered to the lung. Heart rate and transcutaneous oxygen saturation were monitored via a tail cuff (Kent Scientific, Torrington, CT). PLGA or gelatin NPs (4.5 mg) encapsulating a) plasmid DNA vector encoding for yellow fluorescent protein (pEYFP-N1, kindly provided by Dr. Makoto Kuro-o, Dept. of Pathology, UT Southwestern, 40.28% loading efficiency) or b) recombinant human EPO (Cell Sciences, Canton, MA, 100 IU/kg body weight) conjugated to rhodamine, were suspended in 0.5 ml of sterile saline, sonicated (Model 300VT ultrasonic homogenizer, Biologics Inc., Manassas, VA) and aerosolized (4–6µm droplets) via the tracheal cannula over 3 min using a pediatric mesh nebulizer (Aeroneb™, Aerogen, Galway, Ireland). Each animal received one aerosolized NP preparation. Control rats received the corresponding NP encapsulating empty vector (DNA control) or empty NP (protein control) by the same method. The rats were observed following nebulization until complete recovery from anesthesia.
At selected days post-treatment, rats were killed by an intraperitoneal injection of Euthasol™ overdose (pentobarbital 86 mg/kg and phenytoin 11 mg/kg) to stop the heart. The lungs were inflation fixed by tracheal instillation of 4% paraformaldehyde at 25 cmH2O of airway pressure and removed intact. The fixed lobes were serially sliced (3 mm intervals) and the slice faces were imaged by a biofluorescence imager (CRI Maestro 2, Cambridge Research & Instrumentation Inc, Waltham, MA; excitation/emission wavelengths 529nm/580nm for rhodamine, 520nm/532nm for YFP). Fixed tissue blocks were embedded in paraffin, and histological sections (4 µm thickness) were examined under a confocal fluorescence microscope (Zeiss LSM510, excitation/emission wavelengths 543nm/560nm for rhodamine, 514nm/535nm for YFP).
3. Results
3.1. Characterization of NPs
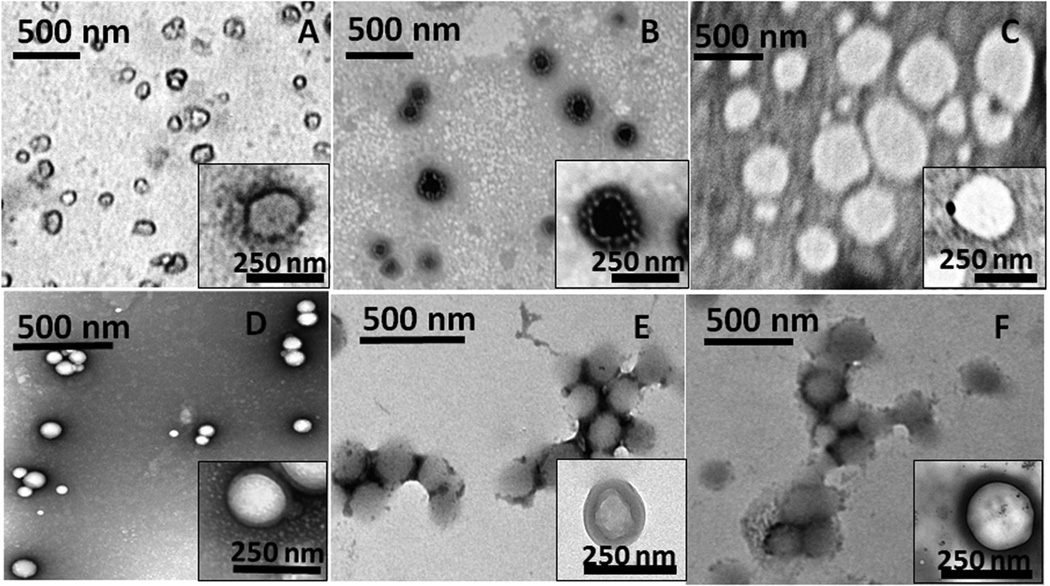
The NPs were characterized for their size, polydispersity and surface charge (Table 1). Among natural polymers, alginate NPs are large (hydrodynamic diameter 556±56 nm) while gelatin and chitosan were comparatively smaller (191 and 253 nm, respectively). The positive zeta potential value of chitosan NPs indicates the presence of cationic NH2 groups on the surface of the particle. Among the synthetic polymers, PLGA-PEG NPs were larger with an average hydrodynamic diameter of 335 nm while PLGA and PLGA-Chitosan (PLGA-CS) NPs were smaller (164 and 191 nm respectively). The smaller polydispersity values of PLGA and PLGA-CS NPs (0.14 and 0.07, respectively) indicate that they are relatively uniformly dispersed. In contrast, chitosan and alginate NPs demonstrated larger polydispersity values (0.28 and 0.29, respectively), indicating more variation in their particle size distribution. Transmission Electron Microscopy (TEM) images indicate that the particles are spherical in morphology and are uniformly dispersed except for alginate NPs (Figure 1). In addition, the particle size range determined from TEM images agreed with that observed using the DLS instrument. After NP formation and purification, the residual solvent present have been previously analyzed using high-performance liquid chromatography (HPLC) [30] and gas chromatography [31]. Parameters such as method of solvent evaporation, number of washes and surfactant concentration can influence the residual solvent content [30, 31]. The particles we had prepared for this project using organic solvents were extensively evaporated overnight, dialyzed and/or washed thoroughly to remove most of the residual organic solvent. The purified nanoparticles were then collected via centrifugation and freeze-drying. Due to these extensive washing and drying steps we do not expect any adverse effects to occur due to the use of organic solvents during preparation.
Table 1.
Size, charge and polydispersity of NP formulations
| Polymer | Particle Diameter (nm) | Polydispersity | Zeta Potential (mV) |
|---|---|---|---|
| Gelatin | 187 ± 83 | 0.22 ± 0.007 | −18.2 ± 2.61 |
| Chitosan | 253 ± 110 | 0.28 ± 0.017 | 4.8 ± 1.08 |
| Alginate | 556 ± 56 | 0.29 ± 0.014 | −28.7 ± 0.89 |
| PLGA | 160 ± 63 | 0.14 ± 0.017 | −20.2 ± 1.16 |
| PLGA – CS | 191 ± 60 | 0.07 ± 0.006 | −17.2 ± 1.34 |
| PLGA – PEG | 335 ± 131 | 0.22 ± 0.033 | −25.4 ± 1.01 |
Figure 1.
TEM images of nanoparticles prepared using (A) gelatin, (B) chitosan, (C) alginate, (D) PLGA, (E) PLGA-CS and (F) PLGA-PEG. The insets represent the morphology of a single nanoparticle of each type.
The stability of NPs was assessed by suspension in de-ionized (DI) water, 10% fetal bovine serum, 0.9% saline and simulated lung fluid (Gamble’s solution) and taking DLS particle size measurements at fixed time intervals. The PLGA and gelatin NPs were stable in all 4 solutions over 5 days with no significant aggregation or change in size. However, chitosan and alginate NPs showed fluctuations in size, indicating nanoparticle aggregation or polymer degradation. PLGA-CS NPs also tended to show aggregation in saline although it remained relatively stable in DI water, serum and Gamble’s solution (Figure 2).
Figure 2.
Stability of all NPs was tested by measuring particle size in (A) DI water, (B) 10% FBS (C) saline solution and (D) simulated lung fluid at 37°C. The PLGA-based and gelatin NPs remained stable for up to 5 days while alginate NPs tended to show aggregation by the 4th day. Chitosan NPs showed fluctuations in size indicating comparatively less stability.
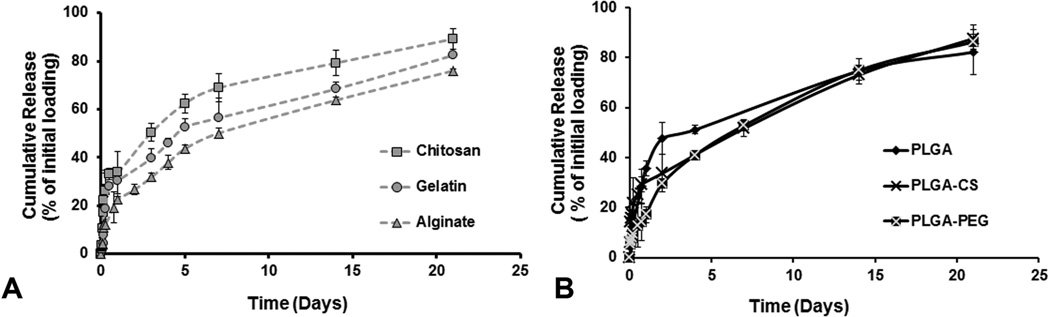
All NPs demonstrated a biphasic release profile of the incorporated compound (shown for BSA). PLGA NPs showed the greatest burst release (about 48%) of the loaded compound within 2 days while PLGA-PEG and PLGA-CS showed burst releases of 30% and 34%, respectively. Among the natural polymer-based NPs, gelatin showed a burst release of about 32% of the drug in 2 days while chitosan and alginate NPs showed burst release of 43% and 27% of the drug, respectively, within 2 days. For all NPs, 80% or more of the encapsulated compound was released within 21 days (Figure 3).
Figure 3.
Drug release studies done on all NPs using BSA as a protein model for a period of 21 days. All NPs showed a bi-phasic release consisting of a burst release for the first 2 days followed by sustained release for 3 weeks. Gelatin, Chitosan and PLGA NPs showed an initial burst release of more than 40% loaded protein within 4 days.
3.2. In Vitro Studies of NPs
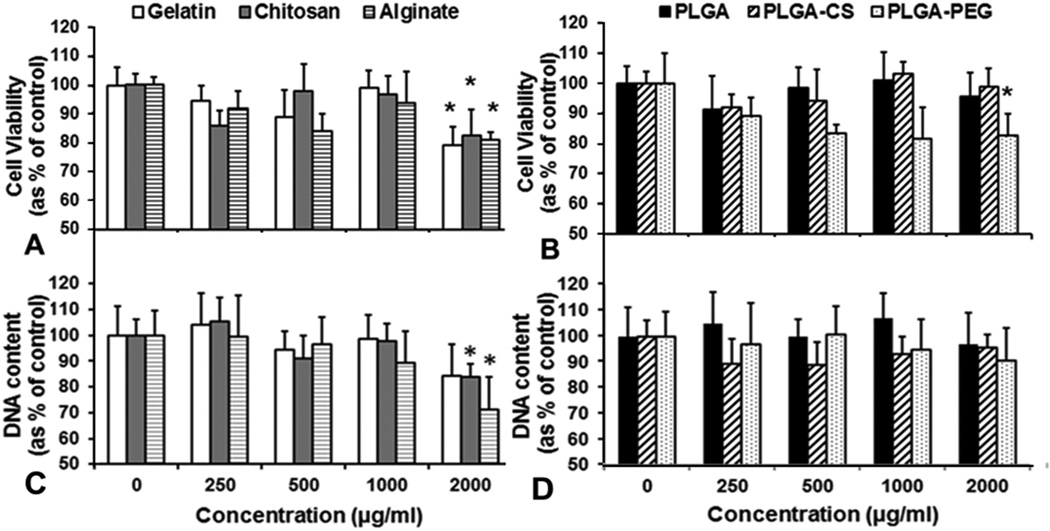
Human alveolar type 1 epithelial cells incubated with gelatin, chitosan or alginate NPs showed greater than 90% cell viability up to an NP concentration of 1000 µg/ml compared to control cells (not incubated with NPs), when tested using MTS assay. The natural polymer-based NPs showed about 80% cell viability at 2000 µg/ml, indicating that they are cytocompatible even at high concentrations. In comparison, human alveolar epithelial cells incubated with PLGA or PLGA-CS NPs showed greater than 90% viability at all concentrations while PLGA-PEG NPs demonstrated a slight decline in cell viability to 83% of control at 2000 µg/ml (Figure 4 A, B). To confirm these results, a Picogreen dsDNA assay was also performed. We observed that more than 90% of the cell DNA was present following incubation with PLGA, PLGA-CS and PLGA-PEG NPs at all concentrations. In addition, about 80% of the DNA content was retained following treatment with gelatin and chitosan NPs up to 2 mg/ml concentration. Treatment with alginate NPs resulted in a decrease in DNA content to 76% at 2mg/ml concentration. It should be noted, however, that more than 80% of the DNA content was present in all samples following incubation with all particles up to 1 mg/ml concentration indicating that these particles are cytocompatible up to 1mg/ml.
Figure 4.
Type 1 alveolar epithelial cell viability studies using (A,B) MTS assay and (C,D) Picogreen ds DNA assay indicated that gelatin, chitosan, alginate and PLGA-PEG NPs maintained were cytocompatible up to a concentration of 1000 µg/ml. All NPs except alginate showed greater than 80% DNA content at 2000 µg/ml concentration. (n=3, *p<0.05 w.r.t control).
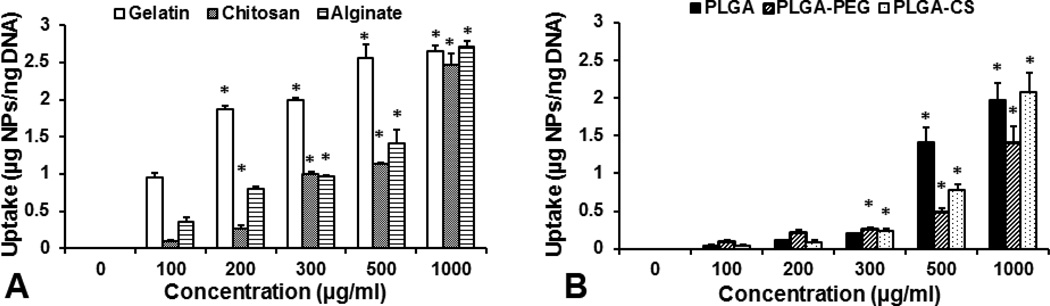
The uptake of NPs by human alveolar Type 1 epithelial cells was studied by incubating with increasing concentrations of NPs over 2 h. All NP formulations showed significant dose-dependent cellular uptake up to a concentration of 1000 µg/ml with the exception that cellular uptake of gelatin NPs became saturated at 500 µg/ml concentration (Figure 5). At a given concentration, cellular uptake of natural polymeric NPs was higher than that of synthetic polymeric NPs, possibly due to different rates of uptake of different NPs by the cells over time.
Figure 5.
Cellular uptake of all six NPs by Type 1 alveolar epithelial cells was studied using Picogreen dsDNA assay and fluorescence readings following 2h incubation with NPs of increasing concentration. Results showed dose-dependence in uptake with increasing NP concentration (n=3, *p<0.05 w.r.t to cellular uptake at 100 µg/ml).
3.3. In Vivo Studies of NPs
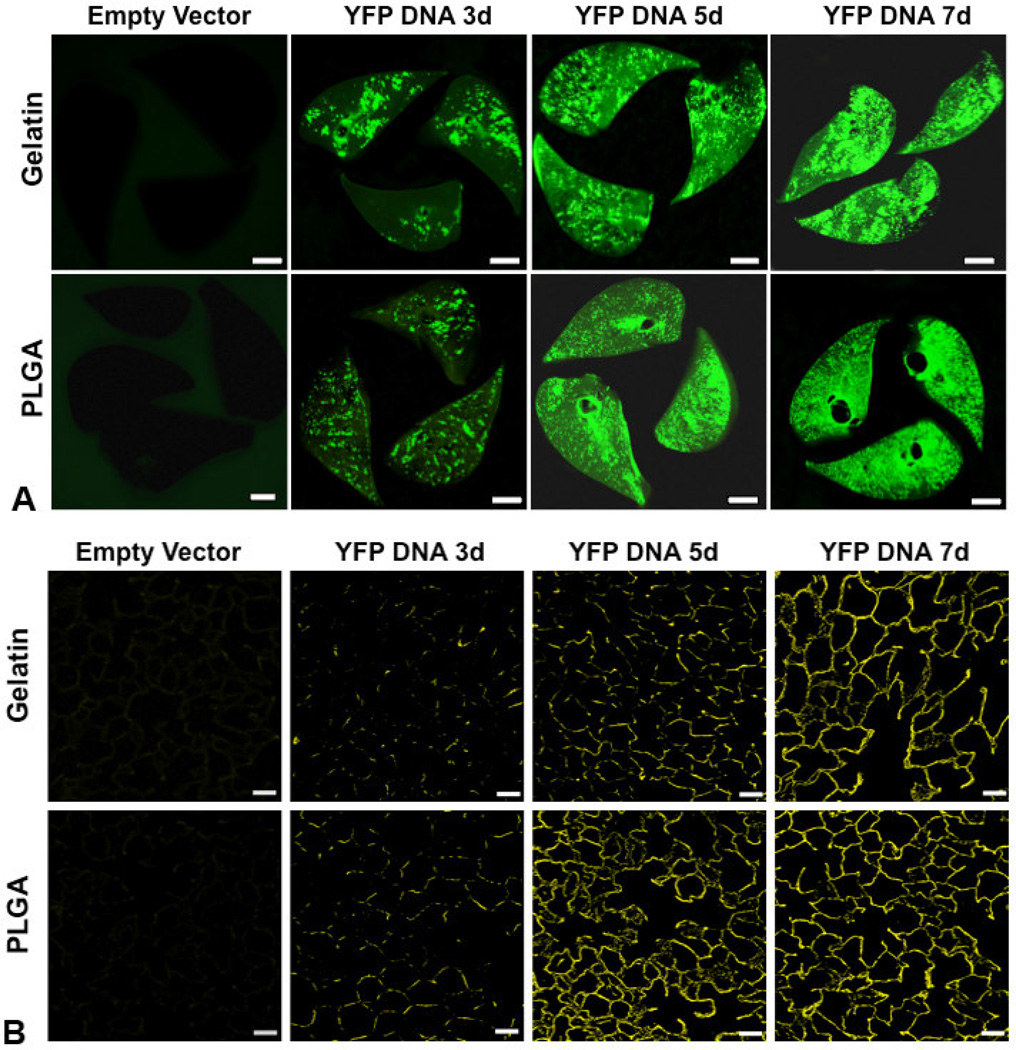
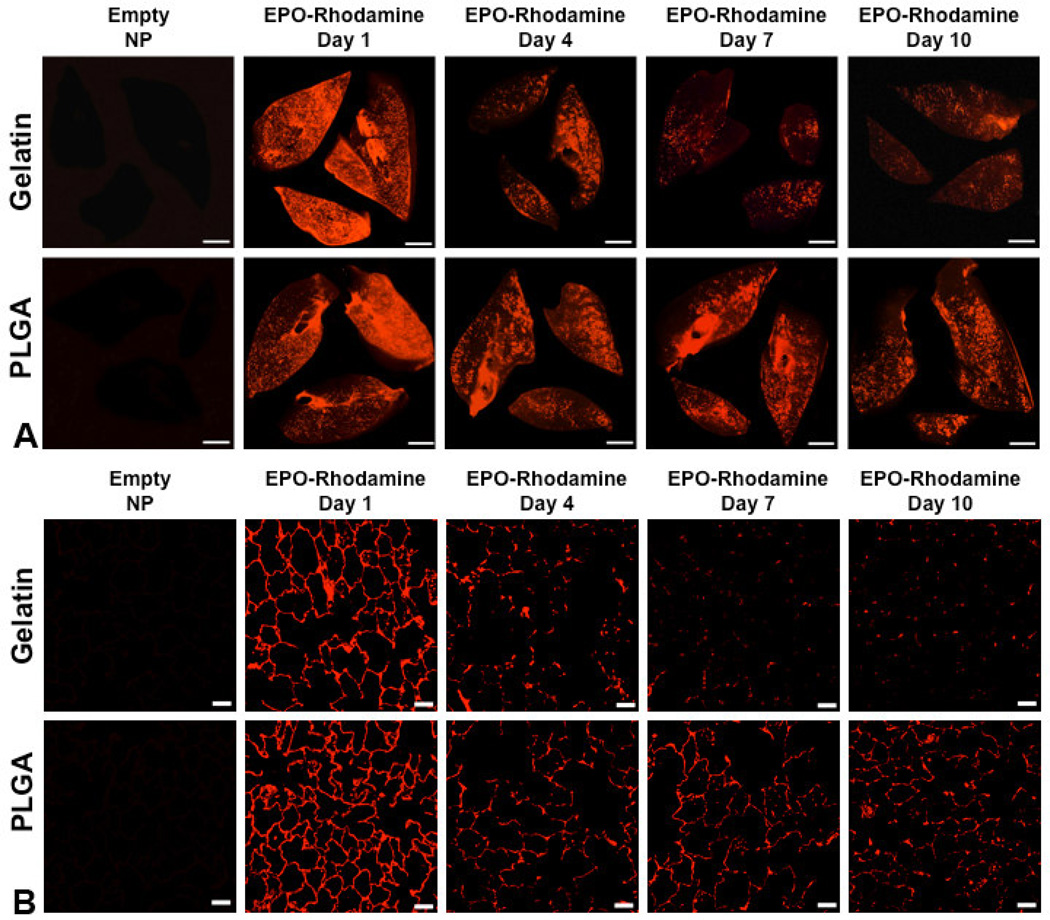
Based on the composite in vitro characteristics (Table 2), gelatin and PLGA NPs exhibit the most favorable profiles for effective delivery to lung tissue (small size, low aggregation, high stability, and biocompatibility); therefore, these two formulations were selected for further testing in vivo. A single dose of gelatin or PLGA NPs incorporating plasmid DNA encoding YFP and delivered by nebulization to the lungs of anesthetized, intubated rats resulted in widespread and increasing fluorescence throughout lung tissue. The pattern of distribution observed in lung slices and histological sections increased from punctate (day 3) to diffuse (days 5 and 7) in all lobes, consistent with persistent gene expression and YFP production by lung cells (Figure 6A and 6B). Similarly, a single inhalational dose of gelatin or PLGA NPs incorporating EPO-rhodamine resulted in widespread fluorescence in lung tissue that persisted for at least 10 days (Figure 7A and 7B). At the same delivered dose of cDNA or protein, tissue expression was uniform following inhalation of both loaded PLGA and gelatin NPs. However, uniform protein expression was observed for a longer time following inhalation of PLGA NPs than that following gelatin NP inhalation.
Table 2.
Comparison of physical-chemical, in vitro and in vivo characteristics of polymeric nanoparticles for pulmonary delivery
| Nanoparticle | Natural polymer based | Synthetic polymer based | ||||
|---|---|---|---|---|---|---|
| Gelatin | Chitosan | Alginate | PLGA | PLGA- CS |
PLGA- PEG |
|
| Size <200 nm | Yes | No | No | Yes | Yes | No |
| Stability at 5 d | Yes | No | No | Yes | Yes | Yes |
| Burst core release (2 d) | <40% | >40% | <40% | >40% | <40% | <40% |
| Sustained core release (3 wk) | >80% | >80% | <80% | >80% | >80% | >80% |
| Time and concentration dependent cell uptake | Yes | Yes | Yes | Yes | Yes | Yes |
| Cytocompatibility | Up to 1 mg/ml | Up to 1 mg/ml | Up to 1 mg/ml | Up to 2 mg/ml | Up to 2 mg/ml | Up to 1 mg/ml |
| Pulmonary distribution of delivered or expressed protein following nebulization | Less uniform or sustained | More uniform and sustained | ||||
Figure 6.
Panel A: Biofluorescence of rat lung slices fixed at 3, 5 and 7 d following nebulization of gelatin or PLGA based NPs loaded with YFP cDNA, compared to control lungs following nebulization of the corresponding NPs loaded with empty vector (bar=0.5 cm). The panels show increasing YFP expression up to 7 d following nebulization; expression was greater and more uniform using PLGA than gelatin NPs. Panel B: Confocal fluorescence microscopy of histological sections taken from the corresponding lungs shows increasing and widespread YFP expression up to 7 d post-inhalation compared to the respective controls (bar=50 µm).
Figure 7.
Panel A: Biofluorescence of rat lung slices fixed at 1, 4, 7 and 10 d following nebulization of gelatin or PLGA-based NPs loaded with rhodamine-conjugated recombinant human erythropoietin (EPO-Rhodamine) compared to control lungs following nebulization of the corresponding empty NPs (bar=0.5 cm). Panel B: Confocal fluorescence microscopy of histological sections taken from the corresponding lungs. These panels show more sustained fluorescence up to 10 days post-inhalation using PLGA rather than gelatin NPs (bar=50 µm).
4. Discussion
In this study we screened six common NP preparations consisting of natural and synthetic polymers to determine the most promising formulations for pulmonary delivery and uptake by distal lung cells. Our characterization showed that most NPs except PLGA-PEG and alginate NPs maintained a size under 300 nm. The larger size of alginate NPs (556±56 nm) concurs with results of Yang et al.[32] who prepared alginate NPs having sizes ranging from 536 nm to 1.8 µm for gene therapy, based on the ionic gelation method However, NPs in the size range of 0.5 to 3 µm tend to be phagocytosed rapidly by macrophages [33] while particles having a diameter between 50 to 200 nm show greater alveolar deposition and are more advantageous for pulmonary drug delivery [4]. Thus, most of our NP formulations except alginate and PLGA-PEG were in the desirable size range as carriers for pulmonary delivery.
We further conducted studies to determine the stability of our NPs in DI water, serum, saline and simulated lung fluid. Our results for gelatin NPs are in agreement with previous studies on these particles prepared by the layer-by-layer method, which demonstrated stability up to 4 weeks after preparation [24]. The observed aggregation of our chitosan NPs in media and serum concurs with the results by Gan et al.[34], which showed chitosan particle aggregation at different pH conditions and particle concentrations due to thermodynamic instability of the system. Other studies have found that alginate-chitosan NPs tend to break apart at a pH of 7.0 indicating that these particles are unstable when the pH is close to physiological pH [35]. On the other hand, our PLGA-based NPs maintained stability without aggregation in all solutions for up to 5 days, which is in agreement with previous studies [9, 36]. PLGA-CS NPs showed some aggregation in saline, which may also have occurred due to chitosan’s thermodynamic instability with pH changes. Based on this study, chitosan and alginate NPs are less favorable for pulmonary administration as their instability may lead to inflammation and reduced therapeutic efficacy due to faster clearance from the lung.
Next we studied the drug release kinetics of all our NP formulations containing BSA as a protein model, over a period of 21 days. All of our NPs exhibited a biphasic release profile of the core compound. Among natural polymers, the highest burst release of 43% of encapsulated BSA from chitosan NPs within 2 days was similar to the ~40% BSA release within 48 h observed by Gan et al.[34] using NPs prepared with medium molecular weight chitosan (that is similar to the polymer used by us). Our PLGA-based NPs showed a high burst release followed by a characteristic sustained release up to 21 days, which was similar to the drug release profile observed in the literature [9, 37]. Although we observed higher burst release from PLGA than from PLGA-PEG NPs, Li et al.[38] detected greater initial BSA release from the latter. This may have occurred due to the different PEG compounds used in their experiments and the availability of different functional groups on the NPs that may differentially interact with different encapsulated compound. On the other hand, Parveen et al.[39] observed slightly reduced paclitaxel release from PLGA-CS and PLGA-PEG NPs than from PLGA NPs, which is consistent with the results obtained by us.
Following physical and chemical characterization of the nanoparticles, in vitro studies were conducted to assess the compatibility of our nanoparticle formulations with mammalian cells. The cytocompatibility with human alveolar type 1 epithelial cells observed by us for gelatin NPs (~80% viability) is similar to the value reported by Tseng et al.[40] using human fetal lung fibroblasts (HFL1). Our chitosan NPs maintained greater than 80% cytocompatibility with alveolar type 1 epithelial cells up to a concentration of 1 mg/ml. This observation agrees with results by Grenha et al.[41], which showed up to 80% viability with human bronchial Calu-3 cells and A549 alveolar epithelial cells up to NP concentration of 1 mg/ml. Similarly, our alginate NPs showed a comparable cell viability (92% cell viability up to 250 µg/ml) as reported in previous studies (~90% viability at 50 µg/ml NP concentration, 24h incubation) using T47D breast cancer cells [42]. The higher cytocompatibility observed for PLGA-based NPs in our studies agrees with results by Mura et al.[43], which showed more than 80% viability of Calu-3 cells incubated with PLGA and PLGA-CS NPs up to a concentration of 5 mg/ml for 24 h. These results indicate that all of our NP formulations are compatible with lung cells up to a high concentration of 1 mg/ml.
Further studies were conducted to determine the optimum in vitro NP concentration for uptake by epithelial cells in the lung. All of our NP formulations exhibited concentration-dependent uptake by human alveolar type 1 epithelial cells. These results are in keeping with previous reports by other groups that tested various NP formulations on different cell lines. For example, Tseng et al.[40] used complexes of gelatin NPs and biotinylated epithelial growth factor (EGF) conjugated with NeutrAvidinFITC to demonstrate increasing NP uptake by A549 cells up to a concentration of 200 µg/ml. Nam et al.[44] observed a similar dose-dependent uptake of hydrophobically modified glycol chitosan NPs by HeLa cervical cancer cells up to a concentration of 200 µg/ml. The dioctylsodium sulfosuccinate (AOT)-sodium alginate NPs formulated by Chavanpatil’s group[45] also showed dose-dependent uptake when incubated with MCF7 breast cancer cells and MCF7-ADR cells (a multidrug resistant sub-line of MCF7). Chen et al.[46] demonstrated concentration-dependent uptake of PLGA-PEG NPs by MCF7 cells. Further, the dose-dependent uptake of PLGA and chitosan-modified PLGA NPs by A549 cells observed by Tahara et al.[13] is similar to our results using PLGA and PLGA-CS NPs. However, contrary to our results, Tahara’s group observed greater uptake of PLGA-CS than PLGA NPs. This difference may have been due to differences in the concentrations of chitosan used during preparation as well as variations in formulation techniques.
We observed a higher in vitro NP uptake of natural polymer-based NPs than synthetic-polymer based NPs by lung cells grown in culture. This disparity may be related to differential uptake rates of different polymeric NPs by the cells over time. We previously demonstrated differential uptake rates of PLGA-based NPs by different cells in a concentration and incubation time-dependent manner [9, 47]. Interactions between the cell membrane and polymers could affect the uptake of NPs by human alveolar Type 1 cells [48]. For example, the negatively-charged cell membranes would favor the positively-charged chitosan NPs, resulting in higher cellular uptake of these particles [49]. Taken together, our results indicate that gelatin and PLGA NPs possess the most promising properties as nanocarriers for pulmonary delivery of biological agents. The sizes of both carriers were within the range appropriate for deposition at the alveolar surface without being cleared by alveolar macrophages. Further, they showed excellent stability in addition to good cytocompatibility and dose-dependent uptake by Type-1 alveolar epithelial cells.
Due to their overall promising characteristics, both PLGA and gelatin NPs were chosen for our preliminary in vivo studies. Although alveolar Type 1 cells show higher uptake of gelatin NPs than PLGA nanoparticles in vitro, the in vivo lung tissue distribution profile was relatively similar for both PLGA and gelatin NPs loaded with YFP cDNA and Rhodamine-tagged EPO. On the other hand, greater fluorescence was observed with time in lung slices of animals administered Rhodamine-tagged EPO encapsulated PLGA NPs than gelatin NPs containing the same protein. Studies have shown that cellular uptake decreases with increasing size and hydrophilicity of the polymeric NPs [50]. Therefore, the observed variation between in vitro and in vivo results could potentially be explained by the slightly larger size of gelatin NPs following drug encapsulation (~260 nm), which might attract more rapid clearance by alveolar macrophages. The inherent hydrophobicity of PLGA may have contributed to greater PLGA NP uptake in vivo compared to the hydrophilic gelatin NPs. Additional factors in intact lung, e.g., the amount and physical properties of alveolar lining fluid as well as various extracellular and intracellular clearance mechanisms, could also differentially influence the distribution, penetration and retention of nebulized NPs in tissues. These data illustrate the importance of corroborating in vitro test results with those obtained in vivo. Our results suggest that the potential use of PLGA and gelatin NPs for inhalational delivery of proteins and DNA are about equal, but PLGA NPs are more effectively retained in the distal lung under physiological conditions.
5. Conclusions
We formulated six different NPs encapsulating protein or DNA, and characterized their physical and chemical properties, in vitro cytocompatibility and particle uptake as well as in vivo deposition and action of the core compound. Among these formulations, gelatin and PLGA NPs possess the smallest sizes (187 and 160 nm respectively), within the optimal range for deposition in the alveolar region by nebulization while avoiding phagocytosis by alveolar macrophages. The PLGA-based and gelatin NPs maintained consistent particle sizes in water, serum, saline and simulated lung fluid indicating high stability. All NPs showed a bi-phasic drug release profile, although PLGA NPs had the highest burst release within 2 days. All NPs formulations were cytocompatible and showed dose-dependent uptake by type 1 alveolar epithelial cells; however, PLGA-based NPs showed the highest cytocompatibility while natural polymeric NPs showed the highest uptake at a given concentration. Of the respective natural and synthetic polymers, gelatin and PLGA NPs exhibit the most favorable in vitro profiles. Following nebulization and inhalational delivery into rat lungs, PLGA NPs yielded more uniform and sustained tissue distributions of the payload compound than gelatin NPs. Our major contribution to the field of drug delivery is that the in vitro observations of NP properties such as cellular uptake and cytotoxicity may not completely reflect their behavior in vivo. Therefore, it is important to validate in vitro characterization results of NPs encapsulating biological reagents with in vivo results in order to choose the most favorable nanocarrier for the desired application. Considering the aggregate in vitro and in vivo results, PLGA NPs demonstrate the most favorable set of characteristics as carriers for pulmonary protein/DNA delivery while gelatin NPs are an acceptable alternative choice. Future work will determine their respective optimal therapeutic dose and frequency of administration as well as the local and systemic effects of specific encapsulated agents following delivery.
Acknowledgements
We acknowledge the assistance of UT Southwestern imaging and microscopy core facilities for the use of their TEM. This work was supported by the National Heart Blood and Lung Institute grants NHLBI UO1 HL-111146, NHLBI RO1 HL40070, and NHLBI R01 HL118498. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Andrade F, Videira M, Ferreira D, Sarmento B. Nanocarriers for pulmonary administration of peptides and therapeutic proteins. Nanomedicine. 2011;6:123–141. doi: 10.2217/nnm.10.143. [DOI] [PubMed] [Google Scholar]
- 2.Beck-Broichsitter M, Merkel OM, Kissel T. Controlled pulmonary drug and gene delivery using polymeric nano-carriers. J Control Release. 2012;161:214–224. doi: 10.1016/j.jconrel.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 3.Terzano C, Allegra L, Alhaique F, Marianecci C, Carafa M. Non-phospholipid vesicles for pulmonary glucocorticoid delivery. European Journal of Pharmaceutics and Biopharmaceutics. 2005;59:57–62. doi: 10.1016/j.ejpb.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 4.Dandekar P, Venkataraman C, Mehra A. Pulmonary targeting of nanoparticle drug matrices. J Aerosol Med Pulm Drug Deliv. 2010;23:343–353. doi: 10.1089/jamp.2009.0784. [DOI] [PubMed] [Google Scholar]
- 5.Smola M, Vandamme T, Sokolowski A. Nanocarriers as pulmonary drug delivery systems to treat and to diagnose respiratory and non respiratory diseases. Int J Nanomedicine. 2008;3:1–19. [PMC free article] [PubMed] [Google Scholar]
- 6.Sung JC, Pulliam BL, Edwards DA. Nanoparticles for drug delivery to the lungs. Trends in Biotechnology. 2007;25:563–570. doi: 10.1016/j.tibtech.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 7.Sham JOH, Zhang Y, Finlay WH, Roa WH, Lobenberg R. Formulation and characterization of spray-dried powders containing nanoparticles for aerosol delivery to the lung. International Journal of Pharmaceutics. 2004;269:457–467. doi: 10.1016/j.ijpharm.2003.09.041. [DOI] [PubMed] [Google Scholar]
- 8.Nesamony J, Singh PR, Nada SE, Shah ZA, Kolling WM. Calcium alginate nanoparticles synthesized through a novel interfacial cross-linking method as a potential protein drug delivery system. J Pharm Sci. 2012;101:2177–2184. doi: 10.1002/jps.23104. [DOI] [PubMed] [Google Scholar]
- 9.Menon JU, Kona S, Wadajkar AS, Desai F, Vadla A, Nguyen KT. Effects of surfactants on the properties of PLGA nanoparticles. J Biomed Mater Res A. 2012;100:1998–2005. doi: 10.1002/jbm.a.34040. [DOI] [PubMed] [Google Scholar]
- 10.Bivas-Benita M, Romeijn S, Junginger HE, Borchard G. PLGA-PEI nanoparticles for gene delivery to pulmonary epithelium. Eur J Pharm Biopharm. 2004;58:1–6. doi: 10.1016/j.ejpb.2004.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ungaro F, d'Angelo I, Miro A, La Rotonda MI, Quaglia F. Engineered PLGA nano- and micro-carriers for pulmonary delivery: challenges and promises. J Pharm Pharmacol. 2012;64:1217–1235. doi: 10.1111/j.2042-7158.2012.01486.x. [DOI] [PubMed] [Google Scholar]
- 12.Ungaro F, d'Angelo I, Coletta C, d'Emmanuele di Villa Bianca R, Sorrentino R, Perfetto B, et al. Dry powders based on PLGA nanoparticles for pulmonary delivery of antibiotics: modulation of encapsulation efficiency, release rate and lung deposition pattern by hydrophilic polymers. J Control Release. 2012;157:149–159. doi: 10.1016/j.jconrel.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 13.Tahara K, Sakai T, Yamamoto H, Takeuchi H, Hirashima N, Kawashima Y. Improved cellular uptake of chitosan-modified PLGA nanospheres by A549 cells. International Journal of Pharmaceutics. 2009;382:198–204. doi: 10.1016/j.ijpharm.2009.07.023. [DOI] [PubMed] [Google Scholar]
- 14.Grabowski N, Hillaireau H, Vergnaud J, Santiago LA, Kerdine-Romer S, Pallardy M, et al. Toxicity of surface-modified PLGA nanoparticles toward lung alveolar epithelial cells. International Journal of Pharmaceutics. 2013;454:686–694. doi: 10.1016/j.ijpharm.2013.05.025. [DOI] [PubMed] [Google Scholar]
- 15.Hara K, Tsujimoto H, Tsukada Y, Huang CC, Kawashima Y, Tsutsumi M. Histological examination of PLGA nanospheres for intratracheal drug administration. International Journal of Pharmaceutics. 2008;356:267–273. doi: 10.1016/j.ijpharm.2007.12.041. [DOI] [PubMed] [Google Scholar]
- 16.El-Sherbiny IM, McGill S, Smyth HDC. Swellable microparticles as carriers for sustained pulmonary drug delivery. J Pharm Sci. 2010;99:2343–2356. doi: 10.1002/jps.22003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ely L, Roa W, Finlay WH, Lobenberg R. Effervescent dry powder for respiratory drug delivery. Eur J Pharm Biopharm. 2007;65:346–353. doi: 10.1016/j.ejpb.2006.10.021. [DOI] [PubMed] [Google Scholar]
- 18.Fernandez Tena A, Casan Clara P. Deposition of Inhaled Particles in the Lungs. Archivos de Bronconeumol. 2012;48:240–246. doi: 10.1016/j.arbres.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 19.Patlolla RR, Chougule M, Patel AR, Jackson T, Tata PNV, Singh M. Formulation, characterization and pulmonary deposition of nebulized celecoxib encapsulated nanostructured lipid carriers. Journal of Controlled Release. 2010;144:233–241. doi: 10.1016/j.jconrel.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beck-Broichsitter M, Gauss J, Packhaeuser CB, Lahnstein K, Schmehl T, Seeger W, et al. Pulmonary drug delivery with aerosolizable nanoparticles in an ex vivo lung model. International Journal of Pharmaceutics. 2009;367:169–178. doi: 10.1016/j.ijpharm.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 21.Manca ML, Mourtas S, Dracopoulos V, Fadda AM, Antimisiaris SG. PLGA, chitosan or chitosan-coated PLGA microparticles for alveolar delivery? A comparative study of particle stability during nebulization. Colloids Surf B Biointerfaces. 2008;62:220–231. doi: 10.1016/j.colsurfb.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 22.Yang M, Yamamoto H, Kurashima H, Takeuchi H, Yokoyama T, Tsujimoto H, et al. Design and evaluation of inhalable chitosan-modified poly (dl-lactic-co-glycolic acid) nanocomposite particles. Eur J Pharm Sci. 2012;47:235–243. doi: 10.1016/j.ejps.2012.05.016. [DOI] [PubMed] [Google Scholar]
- 23.Al-Qadi S, Grenha A, Carrion-Recio D, Seijo B, Remunan-Lopez C. Microencapsulated chitosan nanoparticles for pulmonary protein delivery: In vivo evaluation of insulin-loaded formulations. J Control Release. 2012;157:383–390. doi: 10.1016/j.jconrel.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 24.Shutava TG, Balkundi SS, Vangala P, Steffan JJ, Bigelow RL, Cardelli JA, et al. Layer-by-Layer-Coated Gelatin Nanoparticles as a Vehicle for Delivery of Natural Polyphenols. ACS Nano. 2009;3:1877–1885. doi: 10.1021/nn900451a. [DOI] [PubMed] [Google Scholar]
- 25.Gan Q, Wang T, Cochrane C, McCarron P. Modulation of surface charge, particle size and morphological properties of chitosan-TPP nanoparticles intended for gene delivery. Colloids Surf B Biointerfaces. 2005;44:65–73. doi: 10.1016/j.colsurfb.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 26.Rajaonarivony M, Vauthier C, Couarraze G, Puisieux F, Couvreur P. Development of a new drug carrier made from alginate. J Pharm Sci. 1993;82:912–917. doi: 10.1002/jps.2600820909. [DOI] [PubMed] [Google Scholar]
- 27.Ahmad Z, Sharma S, Khuller GK. Inhalable alginate nanoparticles as antitubercular drug carriers against experimental tuberculosis. Int J Antimicrob Agents. 2005;26:298–303. doi: 10.1016/j.ijantimicag.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 28.Cheng J, Teply BA, Sherifi I, Sung J, Luther G, Gu FX, et al. Formulation of functionalized PLGA-PEG nanoparticles for in vivo targeted drug delivery. Biomaterials. 2007;28:869–876. doi: 10.1016/j.biomaterials.2006.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marques MR, Loebenberg R, Almukainzi M. Simulated biological fluids with possible application in dissolution testing. Dissolution Technol. 2011;18:15–28. [Google Scholar]
- 30.Mainardes RM, Evangelista RC. PLGA nanoparticles containing praziquantel: effect of formulation variables on size distribution. Int J Pharm. 2005;290:137–144. doi: 10.1016/j.ijpharm.2004.11.027. [DOI] [PubMed] [Google Scholar]
- 31.Han E-J, Chung A-H, Oh I-J. Analysis of residual solvents in poly(lactide-co-glycolide) nanoparticles. Journal of Pharmaceutical Investigation. 2012;42:251–256. [Google Scholar]
- 32.Yang S-J, Chang S-M, Tsai K-C, Chen W-S, Lin F-H, Shieh M-J. Effect of chitosan-alginate nanoparticles and ultrasound on the efficiency of gene transfection of human cancer cells. J Gene Med. 2010;12:168–179. doi: 10.1002/jgm.1418. [DOI] [PubMed] [Google Scholar]
- 33.Mansour HM, Rhee YS, Wu X. Nanomedicine in pulmonary delivery. Int J Nanomedicine. 2009;4:299–319. doi: 10.2147/ijn.s4937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gan Q, Wang T. Chitosan nanoparticle as protein delivery carrier - Systematic examination of fabrication conditions for efficient loading and release. Colloids and Surfaces B: Biointerfaces. 2007;59:24–34. doi: 10.1016/j.colsurfb.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 35.Chang C-H, Lin Y-H, Yeh C-L, Chen Y-C, Chiou S-F, Hsu Y-M, et al. Nanoparticles Incorporated in pH-Sensitive Hydrogels as Amoxicillin Delivery for Eradication of Helicobacter pylori. Biomacromolecules. 2009;11:133–142. doi: 10.1021/bm900985h. [DOI] [PubMed] [Google Scholar]
- 36.Chan JM, Zhang L, Yuet KP, Liao G, Rhee J-W, Langer R, et al. PLGA-lecithin-PEG core-shell nanoparticles for controlled drug delivery. Biomaterials. 2009;30:1627–1634. doi: 10.1016/j.biomaterials.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 37.Musumeci T, Ventura CA, Giannone I, Ruozi B, Montenegro L, Pignatello R, et al. PLA/PLGA nanoparticles for sustained release of docetaxel. Int J Pharm. 2006;325:172–179. doi: 10.1016/j.ijpharm.2006.06.023. [DOI] [PubMed] [Google Scholar]
- 38.Li Y-P, Pei Y-Y, Zhang X-Y, Gu Z-H, Zhou Z-H, Yuan W-F, et al. PEGylated PLGA nanoparticles as protein carriers: synthesis, preparation and biodistribution in rats. Journal of Controlled Release. 2001;71:203–211. doi: 10.1016/s0168-3659(01)00218-8. [DOI] [PubMed] [Google Scholar]
- 39.Parveen S, Sahoo SK. Long circulating chitosan/PEG blended PLGA nanoparticle for tumor drug delivery. Eur J Pharmacol. 2011;670:372–383. doi: 10.1016/j.ejphar.2011.09.023. [DOI] [PubMed] [Google Scholar]
- 40.Tseng C-L, Wang T-W, Dong G-C, Yueh-Hsiu Wu S, Young T-H, Shieh M-J, et al. Development of gelatin nanoparticles with biotinylated EGF conjugation for lung cancer targeting. Biomaterials. 2007;28:3996–4005. doi: 10.1016/j.biomaterials.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 41.Grenha A, Grainger CI, Dailey LA, Seijo Ba, Martin GP, Remuñán-López C, et al. Chitosan nanoparticles are compatible with respiratory epithelial cells in vitro. European Journal of Pharmaceutical Sciences. 2007;31:73–84. doi: 10.1016/j.ejps.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 42.Gazori T, Haririan I, Fouladdel S, Namazi A, Nomani A, Azizi E. Inhibition of EGFR expression with chitosan/alginate nanoparticles encapsulating antisense oligonucleotides in T47D cell line using RT-PCR and immunocytochemistry. Carbohydrate Polymers. 2010;80:1042–1047. [Google Scholar]
- 43.Mura S, Hillaireau H, Nicolas J, Le Droumaguet B, Gueutin C, Zanna S, et al. Influence of surface charge on the potential toxicity of PLGA nanoparticles towards Calu-3 cells. Int J Nanomedicine. 2011;6:2591–2605. doi: 10.2147/IJN.S24552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nam HY, Kwon SM, Chung H, Lee S-Y, Kwon S-H, Jeon H, et al. Cellular uptake mechanism and intracellular fate of hydrophobically modified glycol chitosan nanoparticles. Journal of Controlled Release. 2009;135:259–267. doi: 10.1016/j.jconrel.2009.01.018. [DOI] [PubMed] [Google Scholar]
- 45.Chavanpatil MD, Khdair A, Gerard B, Bachmeier C, Miller DW, Shekhar MPV, et al. Surfactant-Polymer Nanoparticles Overcome P-Glycoprotein-Mediated Drug Efflux. Mol Pharm. 2007;4:730–738. doi: 10.1021/mp070024d. [DOI] [PubMed] [Google Scholar]
- 46.Chen J, Li S, Shen Q. Folic acid and cell-penetrating peptide conjugated PLGA-PEG bifunctional nanoparticles for vincristine sulfate delivery. European Journal of Pharmaceutical Sciences. 2012;47:430–443. doi: 10.1016/j.ejps.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 47.Lin A, Sabnis A, Kona S, Nattama S, Patel H, Dong J-F, et al. Shear-regulated uptake of nanoparticles by endothelial cells and development of endothelial-targeting nanoparticles. J Biomed Mater Res Part A. 2010;93A:833–842. doi: 10.1002/jbm.a.32592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lesniak A, Salvati A, Santos-Martinez MJ, Radomski MW, Dawson KA, Aberg C. Nanoparticle adhesion to the cell membrane and its effect on nanoparticle uptake efficiency. J Am Chem Soc. 2013;135:1438–1444. doi: 10.1021/ja309812z. [DOI] [PubMed] [Google Scholar]
- 49.He C, Hu Y, Yin L, Tang C, Yin C. Effects of particle size and surface charge on cellular uptake and biodistribution of polymeric nanoparticles. Biomaterials. 2010;31:3657–3666. doi: 10.1016/j.biomaterials.2010.01.065. [DOI] [PubMed] [Google Scholar]
- 50.Sahoo SK, Panyam J, Prabha S, Labhasetwar V. Residual polyvinyl alcohol associated with poly (d,l-lactide-co-glycolide) nanoparticles affects their physical properties and cellular uptake. J Control Release. 2002;82:105–114. doi: 10.1016/s0168-3659(02)00127-x. [DOI] [PubMed] [Google Scholar]