Abstract
Objective
Short sleep duration induces hormonal perturbations contributing to hyperphagia, insulin resistance, and obesity. The majority of these studies are conducted in young adults. This analysis in a large (n= 769) sample of postmenopausal women (median age 63 y) sought to 1) confirm that sleep duration and sleep quality are negatively correlated with circulating leptin concentrations and 2) to examine the relationship between self-reported sleep, dietary energy intake, and diet quality, as well as, investigate the role of leptin in these associations.
Design and Methods
Sleep duration/quality, insomnia, and dietary intake were determined via self-report. Blood samples were collected following an overnight fast to assess serum leptin concentration. All analyses were adjusted for total body fat mass.
Results
Women reporting ≤6h sleep/night had lower serum leptin concentrations than those reporting ≥8h sleep (P= 0.04). Furthermore, those with ≤6h sleep/night reported higher dietary energy intake (p=0.01) and lower diet quality (P= 0.04) than the reference group (7h sleep/night). Women sleeping ≥8h also reported lower diet quality than the reference group (P= 0.02). Importantly, serum leptin did not confound these associations.
Conclusions
These results provide evidence that sleep duration is inversely associated with serum leptin and dietary energy intake in postmenopausal women.
Keywords: obesity, sleep duration, sleep quality, leptin, energy intake, diet quality
Introduction
Overweight and obesity have reached epidemic proportions (1) and the prevalence of chronic sleep loss has corresponded with this rise in obesity (2). Evidence suggests that short sleep duration may be a risk factor for weight gain and obesity in adults and children (3–6) (3, 4).
Sleep deprivation experiments demonstrate that the energetic response to inadequate sleep is similar to the human metabolic adaptation to negative energy balance, resulting in increased phagic drive and weight gain. Animal (7–10) and human (11–14) studies support causal pathways linking short sleep duration with weight gain, obesity, and the development of diabetes. An increase in hunger, driven by a decrease in circulating leptin as shown in observational (11) and experimental (12–14) studies in humans supports this mechanism. These hormonal changes reduce the anorexigenic drive from leptin, which normally contributes to feelings of satiety and increases energy expenditure.
In light of the rising prevalence of obesity, the identification of interventions for the treatment and prevention of weight gain is a priority. Evidence shows that low quantity and quality of sleep may hinder the success of dietary interventions targeting obesity (15) and that women with better quality and habitual sleep of > 7h/night have greater long term weight loss (16). Moreover, sleep loss decreases resting energy expenditure and physical activity associated energy expenditure, as well as daily physical activity (15, 17, 18). Thus, further investigation of the metabolic and hormonal perturbations induced with sleep loss, as well as the resultant impact on dietary intake, is imperative.
Short sleep duration, sleep disturbance, and insomnia are highly prevalent in older women (19). Although the impact of sleep duration and quality on hormonal regulation of energy homeostasis is increasingly being investigated, the majority of these studies in humans, both epidemiological (5, 11) and experimental (12–14, 20, 21), have been conducted in young or middle aged individuals. Postmenopausal women have a high risk of weight gain and resultant metabolic pathophysiologies (22). Thus, understanding the relationship between sleep and dietary energy intake/diet quality is of particular importance in understanding such age-related weight gain and metabolic disease among postmenopausal women. The purpose of this study was to 1) evaluate the association between sleep duration/quality and circulating leptin concentrations in older women and 2) examine the role of leptin in the relationship between sleep, energy intake and diet quality.
Methods
Study Design and Participants
Participants from the Women’s Health Initiative prospective Observational Study (WHI-OS) were recruited between 1994–1998 at 40 sites nationally. This analysis is restricted to women enrolled at the WHI Dual-energy X-ray Absorptiometry (DXA) centers (University of Arizona, University of Pittsburgh, and University of Alabama at Birmingham). The WHI-OS was designed to investigate risk factors for women’s health, including osteoporosis, cancer and cardiovascular diseases and has been previously described (23). Leptin, sleep, diet, DEXA, and anthropometric data were collected at baseline (n= 1001; 878 with complete dietary data) and studied cross-sectionally for these analyses. Because both sleep disturbance and disordered eating is frequent in individuals diagnosed with depression (24), 109 women were excluded for depression based on the Center for Epidemiologic Studies Depression Scale (CES-D short form cutoff of >0.06) (25), leaving a final sample of 769 women. This study was reviewed and approved by the Human Subjects Review Committee at each participating institution.
Covariates
Self-administered or interviewer-administered questionnaires for eligibility screening and baseline characteristics (such as demographic, reproductive, and health status data) were completed by each of the WHI-OS participants at baseline. At time of enrollment, physical examinations, including weight and height measurements, were conducted by trained WHI staff.
Dietary energy intake and diet quality
Dietary energy intake was assessed at enrollment via the self-administered WHI Food Frequency Questionnaire, which reports consumption frequency and portion size over the previous 3 months. Because it has been previously described that self-reported energy intake was underreported in the WHI participant population (26), we utilized a mathematical calibration model to quantify daily dietary energy intake formulated from doubly-labeled water studies in a sub-sample of WHI participants (27). Diet Quality was estimated using the Alternate Healthy Eating Index (AHEI-2005) score (28). The AHEI was designed to assess diet quality based on foods and nutrients predictive of chronic disease risk and was created by summing its 9 component scores (fruit, vegetable, ratio of white to red meat, trans fat, ratio of polyunsaturated to saturated fat, total fiber, nuts and soy, alcohol consumption, and long-term multivitamin use) with each of the nine components having a potential score of 0–10 and a total AHEI score of 0–90, with a higher score corresponding to higher diet quality.
Subjective measures of sleep duration and quality
At enrollment, participants were asked the following sleep-related questions estimating behavior over the prior 4 weeks: 1) Did you have trouble falling asleep? 2) Did you wake up several times at night? 3) Did you wake up earlier than you planned to? 4) Did you have trouble getting back to sleep after you woke up too early? 5) Did you snore? 6) Overall, how was your typical night’s sleep during the past 4 weeks? 7) About how many hours of sleep did you get on a typical night during the past 4 weeks? Questions 6 and 7 were used to asses sleep quality and quantity, respectively. Sleep duration variables included the following categories: (1) 5h or less, (2) 6h, (3) 7h, (4) 8h, (5) 9h, and (6) 10h or more. For data analysis, categories 1 and 2 were collapsed to create ≤6h and categories 4–6 were collapsed to create ≥8h. Questions 1–5, comprising the 5-item Women’s Health Initiative validated Insomnia Rating Scale (WHIIRS) (29), assessed sleep latency, sleep maintenance insomnia, early morning awakening, and sleep quality. For each question, the score ranged from 0–4; summary scores range from 0–20, with a higher score indicating insomnia.
Body Composition
Total body fat and lean mass was determined via DXA as previously described (30).
Plasma Leptin Concentrations
Blood specimens were collected from each participant after a 12-h overnight fast at baseline and within the four weeks that the sleep-related questions referred to. Blood was processed within 1h of collection and aliquoted plasma was stored at −80°C. Total serum leptin concentrations were assayed using a bead-based, suspension multiplex assay on a Luminex 100 analyzer and the data were interpreted using software developed at Rules-Based Medicine (Human Multi-Analyte Profile (MAP) version 1.6, Rules Based Medicine, Austin, TX, USA). For each multiplex, both calibrators and controls were included on each microtiter plate. Testing results were determined first for the high, medium and low controls for each multiplex to ensure proper assay performance.
Statistical Analysis
Medians (interquartile ranges) and numbers (%) were used to describe baseline characteristics of subjects. Dietary energy and leptin values were natural log transformed prior to analysis of covariance (ANCOVA) and regression analysis. Physical activity (total MET hours per week) was square-root transformed. A comorbidity variable was created from women who answered positively to having hypertension, rheumatoid arthritis, or diabetes at baseline. The association of untransformed leptin values by sleep duration and sleep quality categories was explored using Kruskal Wallis tests. Comparison of leptin values by sleep duration and sleep quality categories were performed using ANCOVA, adjusting for age, race/ethnicity, smoking status, and total body fat mass. Multiple linear regression analysis was used to determine the association between dietary energy, diet quality, and percent kilocalories (kcal) from carbohydrate, protein, and fat with sleep predictors. Previous studies have reported an association with chronic disease risk with a cut-point of 7h/sleep per night, therefore a reference group of 7h sleep/night was used here in the multiple linear regression analyses. Models were built using a phased approach, including predictors which were identified ‘a priori’, and were significantly associated with dietary energy and/or diet quality in the sample population. Final models were adjusted for age (y), race/ethnicity (non-Hispanic white, Hispanic/Latino), total body fat mass (kg), physical activity (MET h/week), income (< $20,000, $20,000–34,999, $35,000–49,999, ≥$50,000), education (< College or College or Above), smoking status (never user, past user, current user), alcohol intake (non-drinker, past drinker, <1 drink/month, < 1 drink/week, 1 to < 7 drinks/week, 7+drinks/week), comorbidity (yes/no) and leptin (ng/ml). P-values were 2-tailed and statistical significance was set at <0.05. Statistical analyses were performed using STATA 12 (STATA, College Station, TX) and SAS 9.3 (SAS Institute, Cary, NC).
Results
Participant Characteristics
The characteristics of participants are summarized in Table 1. Most participants were non-Hispanic whites; with a median age of 63 years; 41% had a normal BMI. Median daily calibrated dietary energy intake was 2021 kcal, while the median AHEI value was 41.2. 33.7% of participants reported sleeping ≤6h/night. Forty nine percent reported having very sound or restful/sound or restful sleep.
Table 1.
Characteristics of Study Participants
| Variable | Median | IQR1 |
|---|---|---|
| Leptin (ng/ml) | 16 | (10,27) |
| Calibrated energy intake (kcal/d) | 2021.0 | (1908,2134.3) |
| Alternate Healthy Eating Index (AHEI) at baseline | 41.2 | (33.3,48.9) |
| WHI Insomnia Rating Scale | 5.0 | (3,9) |
| Age at screening (years) | 63.0 | (57,69) |
| Total body fat mass (kg) | 28.0 | (22,35.2) |
| Physical activity (Total METS/week) | 9.8 | (3.7,19.9) |
| Weight (kg) | 67.2 | (59.4,76.5) |
| Body Mass Index (kg/m2) | 25.9 | (23.1,29.4) |
| No | % | |
|
|
||
| Sleep Duration | ||
| <=6 hours | 256 | 33.7 |
| 7 hours | 299 | 39.3 |
| >=8 hours | 205 | 27.0 |
| Sleep Quality | ||
| Very sound or restful/Sound or restful | 374 | 49.1 |
| Average quality | 297 | 39.0 |
| Very restless/restless | 91 | 11.9 |
| Ethnicity | ||
| White non-Hispanic | 561 | 73.0 |
| Hispanic/Latino | 208 | 27.0 |
| Smoking Status | ||
| Never smoked | 425 | 56.1 |
| Past smoker | 282 | 37.2 |
| Current smoker | 51 | 6.7 |
| Alcohol Intake | ||
| Non-drinker | 91 | 11.9 |
| Past drinker | 134 | 17.5 |
| < 1 drink per month | 95 | 12.4 |
| < 1 drink per week | 170 | 22.2 |
| 1 to < 7 drinks per week | 202 | 26.3 |
| 7+ drinks per week | 75 | 9.8 |
| HRT Usage Status | ||
| Never used | 352 | 45.8 |
| Past user | 102 | 13.3 |
| Current user | 315 | 41.0 |
| Hypertension Ever (Baseline) | ||
| No | 553 | 72.8 |
| Yes | 207 | 27.2 |
| Diabetes at Screening | ||
| No | 726 | 94.4 |
| Yes | 43 | 5.6 |
| Arthritis Ever (Baseline) | ||
| No | 386 | 51.1 |
| Yes | 369 | 48.9 |
| Rheumatoid Arthritis | ||
| No arthritis | 386 | 51.1 |
| Rheumatoid arthritis | 37 | 4.9 |
| Other/Missing | 332 | 44.0 |
| Comorbidity Status | ||
| No diabetes/hypertension/rheumatoid arthritis | 522 | 67.9 |
| Hypertension/diabetes/rheumatoid arthritis | 247 | 32.1 |
| Income | ||
| < $20,000 | 209 | 27.2 |
| $20,000–34,999 | 209 | 27.2 |
| $35,000–49,999 | 141 | 18.3 |
| >= $50,000 | 210 | 27.3 |
| Education | ||
| < College | 508 | 66.5 |
| College or Above | 256 | 33.5 |
| Body Mass Index Category | ||
| Underweight (<18.5 kg/m2) | 11 | 1.4 |
| Normal weight (18.5–24.9 kg/m2) | 318 | 41.4 |
| Overweight (25–29.9 kg/m2) | 271 | 35.2 |
| Obese (>= 30 kg/m2) | 169 | 22.0 |
IQR = Interquartile Range (25th, 75th percentiles)
Leptin and Sleep Quality and Sleep Duration
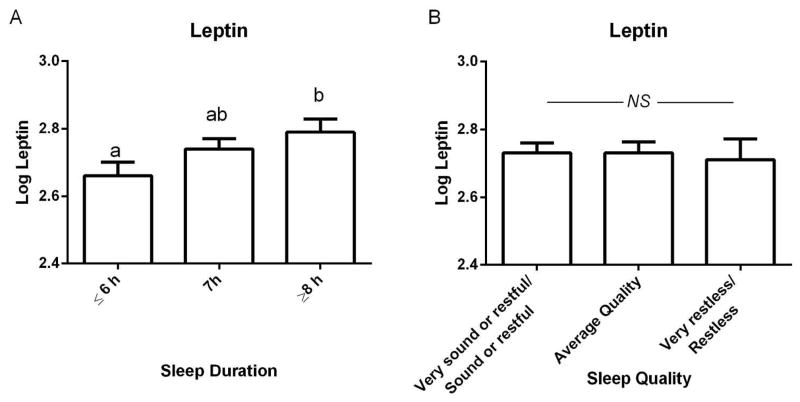
It is well established that serum leptin concentrations are highly correlated with fat mass (31, 32) as was shown here (R2= 0.494, P<0.0001). Thus, we utilized total body fat mass as a covariate when comparing leptin concentrations, dietary energy, and diet quality by sleep duration and sleep quality categories. ANCOVA analyses of categorized sleep duration and sleep quality adjusted for age, race, total body fat mass, and smoking status revealed that log leptin values differed significantly over sleep duration categories. Specifically, participants reporting ≤6h sleep demonstrated lower circulating leptin concentrations than those reporting ≥8h sleep (P= 0.04) (Figure 2A). Leptin concentrations were not different between sleep quality categories (Figure 2B).
Association of Calibrated Dietary Energy Intake with Sleep
To investigate the relationship between sleep and dietary energy intake/quality and to determine if leptin served as a mediator of these associations, we performed multiple linear regression analyses using models including, and not including leptin. Results of these analyses are summarized in Table 2. Daily dietary energy intake was associated with sleep duration when controlling for the covariates listed in model 2, independent of leptin. Women who reported sleeping ≤6h/night demonstrated a 1% increase in mean dietary energy intake relative to women who reported sleeping 7h/night (reference group) (P=0.01) (Table 2). Women reporting “very restless/restless” sleep had higher daily energy intake intercepts (P= 0.03). However, this association was not significant after controlling for covariates listed in model 2 and leptin concentrations (Table 2).
Table 2.
The association between sleep duration, sleep quality and WHI Insomnia Scale with dietary energy intake and diet quality score.
| ln* Dietary Energy Intake (kcal/day) | Diet Quality (AHEI) | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Coeff | SE | p | Coeff | SE | p | |
|
Sleep Duration
| ||||||
| Crude | ||||||
| <= 6 hours | 0.008 | 0.008 | 0.338 | −2.755 | 0.943 | 0.004 |
| 7 hours | Ref | - | - | Ref | - | - |
| >= 8 hours | 0.007 | 0.008 | 0.384 | −2.375 | 1.031 | 0.022 |
| Adjusted Model 1a | ||||||
| <= 6 hours | 0.007 | 0.004 | 0.129 | −2.246 | 0.905 | 0.013 |
| 7 hours | Ref | - | - | Ref | - | - |
| >= 8 hours | 0.0001 | 0.004 | 0.975 | −2.341 | 0.987 | 0.018 |
| Adjusted Model 2b | ||||||
| <= 6 hours | 0.009 | 0.004 | 0.015 | −1.511 | 0.784 | 0.054 |
| 7 hours | Ref | - | - | Ref | - | - |
| >= 8 hours | 0.001 | 0.004 | 0.808 | −2.168 | 0.873 | 0.013 |
| Adjusted Model 3c | ||||||
| <= 6 hours | 0.009 | 0.004 | 0.014 | −1.634 | 0.779 | 0.036 |
| 7 hours | Ref | - | - | Ref | - | - |
| >= 8 hours | 0.001 | 0.004 | 0.814 | −2.077 | 0.872 | 0.017 |
|
| ||||||
|
Sleep Quality
| ||||||
| Crude | ||||||
| Very Sound or Restful/Sound or Restful | Ref | - | - | Ref | - | - |
| Average Quality | 0.005 | 0.007 | 0.510 | −1.148 | 0.883 | 0.194 |
| Very restless/Restless | 0.025 | 0.012 | 0.033 | −4.346 | 1.180 | 0.000 |
| Adjusted Model 1a | ||||||
| Very Sound or Restful/Sound or Restful | Ref | - | - | Ref | - | - |
| Average Quality | 0.002 | 0.004 | 0.641 | −1.117 | 0.853 | 0.191 |
| Very restless/Restless | 0.005 | 0.006 | 0.376 | −3.384 | 1.154 | 0.004 |
| Adjusted Model 2b | ||||||
| Very Sound or Restful/Sound or Restful | Ref | - | - | Ref | - | - |
| Average Quality | 0.004 | 0.004 | 0.259 | −0.969 | 0.750 | 0.197 |
| Very restless/Restless | 0.003 | 0.005 | 0.503 | −2.204 | 1.080 | 0.042 |
| Adjusted Model 3c | ||||||
| Very Sound or Restful/Sound or Restful | Ref | - | - | Ref | - | - |
| Average Quality | 0.004 | 0.004 | 0.259 | −0.979 | 0.748 | 0.191 |
| Very restless/Restless | 0.003 | 0.005 | 0.504 | −2.241 | 1.069 | 0.036 |
|
| ||||||
|
WHI Insomnia Scale
| ||||||
| Crude | −0.0001 | 0.0009 | 0.893 | −0.1361 | 0.0903 | 0.132 |
| Adjusted Model 1a | −0.0001 | 0.0004 | 0.715 | −0.1020 | 0.0902 | 0.258 |
| Adjusted Model 2b | <−0.0001 | 0.0004 | 0.933 | −0.0530 | 0.0808 | 0.512 |
| Adjusted Model 3c | <−0.0001 | 0.0004 | 0.933 | −0.0534 | 0.0806 | 0.508 |
ln, natural log
Adjusted for age, race/ethnicity and total body fat mass
Adjusted for the variables in Model 1 plus sqrt(physical activity), income, education, smoking status, alcohol intake, antidepressant use and comorbidity status (diabetes/hypertension/rheumatoid arthritis)
Adjusted for the variables in Model 2 plus circulating ln leptin concentrations
Association of Diet Quality with Sleep Predictors
Diet quality was significantly associated with sleep duration and this association remained after addition of leptin to the model (Table 2). Women who reported sleeping ≥8h (P= 0.02) had significantly lower diet quality intercepts than women who slept for 7h. This inverse relationship was also significant among women who reported sleeping ≤6h (P=0.04). Women reporting the lowest category of sleep quality (“very restless or restless”) had lower diet quality intercepts than women reporting very sound or restful/sound or restful sleep (P= 0.04), independent of leptin concentrations (Table 2). To further explore the relationship between diet quality and sleep, we investigated the association of sleep predictors with macronutrient intake. While sleep duration was not significantly associated with the composition of macronutrient intake, we found that women who reported average quality sleep consumed a lower percent of kcal from carbohydrate than women who reported sound/very sound sleep in both the crude (P=0.011) and age, ethnicity, and total body fat mass adjusted models (P=0.015). Women who reported restless/very restless sleep consumed a higher percentage of kcal from fat than women who reported sound/very sound sleep in crude models (P=0.016). However, these associations were no longer statistically significant on adjustment for covariates in models 2 and 3. The WHI insomnia scale was not significantly associated with diet quality, dietary energy intake, or the macronutrient composition of dietary energy intake.
Discussion
To our knowledge, this study is the first to examine the association of sleep duration and quality with leptin in the context of dietary energy intake and diet quality among post-menopausal women. Our study shows significantly higher energy intake among women sleeping ≤6h/night. Although apparently modest, based on the average energy intake of the participants in this study, this 1% increase in daily energy intake in the form of fat could result in excess of an estimated 7373 kcal/year, equivalent to 0.8 kg of extra body lipid. Our finding that short sleep duration is associated with decreased serum leptin, is consistent with results from some, but not all, epidemiological studies of sleep duration and likely supports the role of many other physiological mediators of leptin. Some of these studies have been conducted in obese populations, in which, leptin insensitivity separates serum leptin from leptin signaling. Circadian rhythm can also affect serum leptin. Finally, when examining the effect of leptin one must segregate effects of adiposity from those of leptin. The altered leptin signaling and regulation in the obese, the circadian changes in leptin, and the statistical correction for adiposity may explain why some studies have not shown an association between sleep and leptin, dietary energy, and diet quality.
It has been reported that in hyperleptinemic obese people short sleep duration is not associated with a decrease in leptin (33, 34). Knutson et al. (34) found that leptin controlled for fat mass was not associated with objectively measured (actigraphy) sleep duration or quality in a cohort of 80 obese men and premenopausal obese women (mean BMI of 37.5 and 38.4 in men and women, respectively) aged 18–50 years (34). Similarly, an intervention study conducted by Littman et al. in 173 postmenopausal, inactive women (aged 50–75 y, mean BMI 30.5) reported that neither subjectively reported sleep duration or sleep quality was associated with serum leptin at baseline (35). In contrast, Taheri et al. reported that in a study of men and women with a mean age of 52.7 and median BMI of 29.7 (26.2, 34.7 IQR), objectively (polysomnography) and subjectively (sleep log) measured short sleep duration was, in fact, associated with reduced leptin, independent of BMI and sex (11). The participants in the study of Knutson et al. have BMI values close to morbid obesity (BMI ≥ 40), while the participants in the studies of Littman et al. and Taheri et al. have BMI values representing the lower end of obesity. Thus, the degree of obesity may impact leptin values in relation to lifestyle factors such as sleep.
Differences in the measure of sleep, methods of sleep quality assessment, markers of adiposity and statistical analyses may also contribute to the differences in reported results. In our study, sleep duration and quality was measured by subjective sleep logs and questionnaires versus objective measurements of polysomnography and actigraphy as measured by Hayes et al. (33) and Knutson et al. (34), respectively. We utilized total body fat mass as a covariate when analyzing the association of sleep variables with leptin while Knutson et al. calculated a relative leptin level by dividing leptin by body fat percentage. However, the studies of Littman et al. and Taheri et al. utilized BMI as a covariate rather than fat mass. Leptin is secreted from adipocytes in proportion to body fat stores and is, thus, tightly correlated with fat mass (31, 32). Because physiological factors associated with increased adiposity, such as respiratory disorders (i.e. sleep apnea) can impact sleep duration and quality, it is imperative to control for fat mass when examining the association between sleep and leptin.
Circulating leptin concentrations have a discrete circadian rhythm with levels rising to maximum at night and dropping to minimum during the day (36, 37). To limit changes associated with the diurnal cycle, our study and the aforementioned studies (11, 35) (34) collected blood samples at one time point after an overnight fast. Variations in bed-time and waking time may, however, confound the relationship between sleep duration and leptin. Previous studies have investigated the impact of sleep curtailment on the diurnal rhythm of circulating leptin (13, 21) and these studies provide detail of the disruptive impact of sleep duration on the diurnal rhythm of leptin release, causing a reduction in the amplitude of the diurnal variation of circulating leptin concentrations. Because we do not have data on bed time or waking time on the day of blood sampling in our study population, we cannot confirm that blood sampling took place within a specific window of this circadian rhythm for all subjects. Another potential limitation of this study is the time frame described in the FFQ compared to that of the sleep-related questions. The FFQ queried about food type and quantity over the previous 3 months and the sleep-related questions were in the context of the previous 4 weeks. However, the participants completed these 2 questionnaires at the same time and did so within 4 weeks of blood collection.
While the association between sleep duration, energy intake, energy expenditure, and hormones that regulate intake and expenditure has been described (7–10), there are sparse data describing the association between sleep and diet quality. This study demonstrates that diet quality is significantly associated with sleep duration and that this relationship is independent of serum leptin concentration. It is interesting to note the “U” shaped relationship between sleep duration and diet quality among the participants of our study. That is, women sleeping ≥8h had significantly lower diet quality than women who slept for 7h, while women sleeping ≤6 hours also demonstrated lower diet quality than the 7h reference group. Given the previously discussed hormonal changes that stimulate hyperphagic drive, some studies have investigated the relationship between short sleep duration and eating habits, most of which conclude that short sleep duration is associated with increased intake of calories from snacks and carbohydrates (38, 39), eating behaviors that could reduce the overall diet quality score. Our findings that percent kcal from carbohydrate and fat were significantly associated with self-reported sleep quality suggests that sleep quality may also be associated with macronutrient diet composition supporting lower overall diet quality. These associations, however, were attenuated on adjustment for covariates known to modulate macronutrient intake. What is less clear is why sleeping >8h per night is associated with decreased diet quality in this study. Future studies examining the impact of sleep duration on diet quality in postmenopausal women would be worthwhile to elucidate these findings.
Given the previously discussed hormonal changes that stimulate hyperphagic drive, some studies have investigated the relationship between short sleep duration and eating habits. It has been recently reported that neuronal activity among healthy men and women aged 35 to 45 y in response to food stimuli is enhanced after experimentally restricted sleep compared to habitual sleep, providing evidence that sleep curtailment influences brain centers that affect food choices (40). Similarly, a study in young females (18–28 y) described an inverse relationship between sleep duration and diet quality (38). To our knowledge, our study is the first to examine this relationship in a large population of older, postmenopausal women. Our observation that post-menopausal women with better sleep quality had higher values of diet quality suggests that both sleep duration and sleep quality may have an impact on food cravings and food choices. Although causal relationships cannot be determined by this cross-sectional analysis, it is possible that differences in food intake among our study population are impacting sleep habits. However, data from previously discussed experimental studies of induced sleep restriction suggest that sleep affects dietary energy intake and diet quality under controlled conditions.
We have demonstrated that sleep duration, but not sleep quality is associated with fat mass-adjusted circulating leptin in a large sample of post-menopausal women. Additionally, we have described an inverse relationship between sleep duration and dietary energy intake, as well as sleep duration/quality and diet quality. These findings suggest that sleep may be a relevant factor in weight management. While suggestive that 7h sleep per night and improved sleep quality could help augment other approaches to improve dietary choices in postmenopausal women, a clinical trial would be needed to test such hypotheses. Given the increased risk of metabolic disorders and obesity with menopause, our findings provide evidence that improving sleep duration and quality may be a novel target for a lifestyle intervention aimed at modulating dietary energy intake and diet quality in post-menopausal women.
Figure 1.
Circulating leptin concentrations partitioned by A) sleep duration and B) sleep quality. Values presented as least square mean ± SEM, adjusted for age, race, fat mass, and smoking status; superscripts that differ indicate differences between sleep duration categories, ANCOVA bonferroni corrected P<0.05.
What is already known about this subject?
Observational and experimental studies in humans have shown that short sleep duration induces a decrease in circulating leptin, an increase in hunger ratings, and an increase in adiposity.
Evidence also shows that low quantity and quality of sleep may hinder the success of dietary interventions targeting obesity.
The majority of these studies have been conducted in young adults.
What does this study add?
This analysis demonstrates that postmenopausal women reporting ≤6h sleep/night had lower fat-mass-adjusted serum leptin concentrations than those reporting ≥8h sleep, while women reporting 7h sleep/night did not differ from those sleeping ≤6h or ≥8h sleep/night.
Linear regression analyses using 7h sleep per night as a reference group shows that postmenopausal women reporting ≤6h sleep/night reported significantly higher dietary energy intake and lower diet quality than those with 7h/night. These associations were independent of circulating leptin concentrations.
Acknowledgments
Funding
JHS is funded by NIH 2R25 CA078447-1. The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, US Department of Health, and Human Services through contracts HHSN268201100046C, HHSN268201100001C, HHSN268201100002C, HHSN268201100003C, HHSN268201100004C and HHSN271201100004C.
Footnotes
Conflicts of Interest
The authors declare that they have no competing interests.
Author Contributions
Conceived hypotheses and manuscript concept: JHS. Analyzed and interpreted data: ASG, JHS, and ZC. Wrote the paper: JHS, ASG, CAT, and ZC. Conceived and designed ancillary study: ZC. Women’s Health Initiative study design, implementation, and data collection: LT. Edited and provided feedback on the manuscript: LT, LH, KMB, and NFW.
References
- 1.Finucane MM, Stevens GA, Cowan MJ, et al. National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9. 1 million participants. Lancet. 2011;377:557–67. doi: 10.1016/S0140-6736(10)62037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.2009 Sleep in America Poll, Summary of Findings. National Sleep Foundation; Washington: 2009. [Google Scholar]
- 3.Appelhans BM, Janssen I, Cursio JF, et al. Sleep Duration and Weight Change in Midlife Women: The SWAN Sleep Study. Obesity. 2012 doi: 10.1002/oby.20251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carter PJ, Taylor BJ, Williams SM, Taylor RW. Longitudinal analysis of sleep in relation to BMI and body fat in children: the FLAME study. BMJ. 2011;342:d2712. doi: 10.1136/bmj.d2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jennings JR, Muldoon MF, Hall M, Buysse DJ, Manuck SB. Self-reported sleep quality is associated with the metabolic syndrome. Sleep. 2007;30:219–23. doi: 10.1093/sleep/30.2.219. [DOI] [PubMed] [Google Scholar]
- 6.Mozaffarian D, Hao T, Rimm EB, Willett WC, Hu FB. Changes in diet and lifestyle and long-term weight gain in women and men. The New England journal of medicine. 2011;364:2392–404. doi: 10.1056/NEJMoa1014296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barf RP, Desprez T, Meerlo P, Scheurink AJ. Increased food intake and changes in metabolic hormones in response to chronic sleep restriction alternated with short periods of sleep allowance. American journal of physiology Regulatory, integrative and comparative physiology. 2012;302:R112–7. doi: 10.1152/ajpregu.00326.2011. [DOI] [PubMed] [Google Scholar]
- 8.Koban M, Le WW, Hoffman GE. Changes in hypothalamic corticotropin-releasing hormone, neuropeptide Y, and proopiomelanocortin gene expression during chronic rapid eye movement sleep deprivation of rats. Endocrinology. 2006;147:421–31. doi: 10.1210/en.2005-0695. [DOI] [PubMed] [Google Scholar]
- 9.Koban M, Sita LV, Le WW, Hoffman GE. Sleep deprivation of rats: the hyperphagic response is real. Sleep. 2008;31:927–33. [PMC free article] [PubMed] [Google Scholar]
- 10.Martins PJ, Marques MS, Tufik S, D’Almeida V. Orexin activation precedes increased NPY expression, hyperphagia, and metabolic changes in response to sleep deprivation. American journal of physiology Endocrinology and metabolism. 2010;298:E726–34. doi: 10.1152/ajpendo.00660.2009. [DOI] [PubMed] [Google Scholar]
- 11.Taheri S, Lin L, Austin D, Young T, Mignot E. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS medicine. 2004;1:e62. doi: 10.1371/journal.pmed.0010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gonnissen HK, Hursel R, Rutters F, Martens EA, Westerterp-Plantenga MS. Effects of sleep fragmentation on appetite and related hormone concentrations over 24 h in healthy men. The British journal of nutrition. 2012:1–9. doi: 10.1017/S0007114512001894. [DOI] [PubMed] [Google Scholar]
- 13.Spiegel K, Leproult R, L’Hermite-Baleriaux M, Copinschi G, Penev PD, Van Cauter E. Leptin levels are dependent on sleep duration: relationships with sympathovagal balance, carbohydrate regulation, cortisol, and thyrotropin. The Journal of clinical endocrinology and metabolism. 2004;89:5762–71. doi: 10.1210/jc.2004-1003. [DOI] [PubMed] [Google Scholar]
- 14.Spiegel K, Tasali E, Penev P, Van Cauter E. Brief communication: Sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Annals of internal medicine. 2004;141:846–50. doi: 10.7326/0003-4819-141-11-200412070-00008. [DOI] [PubMed] [Google Scholar]
- 15.Nedeltcheva AV, Kilkus JM, Imperial J, Schoeller DA, Penev PD. Insufficient sleep undermines dietary efforts to reduce adiposity. Annals of internal medicine. 2010;153:435–41. doi: 10.1059/0003-4819-153-7-201010050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thomson CA, Morrow KL, Flatt SW, et al. Relationship Between Sleep Quality and Quantity and Weight Loss in Women Participating in a Weight-Loss Intervention Trial. Obesity. doi: 10.1038/oby.2012.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Booth JN, Bromley LE, Darukhanavala AP, Whitmore HR, Imperial JG, Penev PD. Reduced physical activity in adults at risk for type 2 diabetes who curtail their sleep. Obesity. 2012;20:278–84. doi: 10.1038/oby.2011.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benedict C, Hallschmid M, Lassen A, et al. Acute sleep deprivation reduces energy expenditure in healthy men. The American journal of clinical nutrition. 2011;93:1229–36. doi: 10.3945/ajcn.110.006460. [DOI] [PubMed] [Google Scholar]
- 19.Dzaja A, Arber S, Hislop J, et al. Women’s sleep in health and disease. Journal of psychiatric research. 2005;39:55–76. doi: 10.1016/j.jpsychires.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 20.Broussard JL, Ehrmann DA, Van Cauter E, Tasali E, Brady MJ. Impaired insulin signaling in human adipocytes after experimental sleep restriction: a randomized, crossover study. Annals of internal medicine. 2012;157:549–57. doi: 10.7326/0003-4819-157-8-201210160-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mullington JM, Chan JL, Van Dongen HP, et al. Sleep loss reduces diurnal rhythm amplitude of leptin in healthy men. Journal of neuroendocrinology. 2003;15:851–4. doi: 10.1046/j.1365-2826.2003.01069.x. [DOI] [PubMed] [Google Scholar]
- 22.Wing RR, Matthews KA, Kuller LH, Meilahn EN, Plantinga PL. Weight-Gain at the Time of Menopause. Arch Intern Med. 1991;151:97–102. [PubMed] [Google Scholar]
- 23.Langer RD, White E, Lewis CE, Kotchen JM, Hendrix SL, Trevisan M. The Women’s Health Initiative Observational Study: baseline characteristics of participants and reliability of baseline measures. Annals of epidemiology. 2003;13:S107–21. doi: 10.1016/s1047-2797(03)00047-4. [DOI] [PubMed] [Google Scholar]
- 24.American Psychiatric Association., American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-IV-TR. 4. American Psychiatric Association; Washington, DC: 2000. Task Force on DSM-IV. [Google Scholar]
- 25.Burnam MA, Wells KB, Leake B, Landsverk J. Development of a brief screening instrument for detecting depressive disorders. Medical care. 1988;26:775–89. doi: 10.1097/00005650-198808000-00004. [DOI] [PubMed] [Google Scholar]
- 26.Tinker LF, Sarto GE, Howard BV, et al. Biomarker-calibrated dietary energy and protein intake associations with diabetes risk among postmenopausal women from the Women’s Health Initiative. The American journal of clinical nutrition. 2011;94:1600–6. doi: 10.3945/ajcn.111.018648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neuhouser ML, Tinker L, Shaw PA, et al. Use of recovery biomarkers to calibrate nutrient consumption self-reports in the Women’s Health Initiative. American journal of epidemiology. 2008;167:1247–59. doi: 10.1093/aje/kwn026. [DOI] [PubMed] [Google Scholar]
- 28.McCullough ML, Willett WC. Evaluating adherence to recommended diets in adults: the Alternate Healthy Eating Index. Public health nutrition. 2006;9:152–7. doi: 10.1079/phn2005938. [DOI] [PubMed] [Google Scholar]
- 29.Levine DW, Kaplan RM, Kripke DF, Bowen DJ, Naughton MJ, Shumaker SA. Factor structure and measurement invariance of the Women’s Health Initiative Insomnia Rating Scale. Psychological assessment. 2003;15:123–36. doi: 10.1037/1040-3590.15.2.123. [DOI] [PubMed] [Google Scholar]
- 30.Bea JW, Zhao Q, Cauley JA, et al. Effect of hormone therapy on lean body mass, falls, and fractures: 6-year results from the Women’s Health Initiative hormone trials. Menopause. 2011;18:44–52. doi: 10.1097/gme.0b013e3181e3aab1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395:763–70. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- 32.Oswal A, Yeo G. Leptin and the control of body weight: a review of its diverse central targets, signaling mechanisms, and role in the pathogenesis of obesity. Obesity. 2010;18:221–9. doi: 10.1038/oby.2009.228. [DOI] [PubMed] [Google Scholar]
- 33.Hayes AL, Xu F, Babineau D, Patel SR. Sleep duration and circulating adipokine levels. Sleep. 2011;34:147–52. doi: 10.1093/sleep/34.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Knutson KL, Galli G, Zhao X, Mattingly M, Cizza G. No association between leptin levels and sleep duration or quality in obese adults. Obesity. 2011;19:2433–5. doi: 10.1038/oby.2011.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Littman AJ, Vitiello MV, Foster-Schubert K, et al. Sleep, ghrelin, leptin and changes in body weight during a 1-year moderate-intensity physical activity intervention. Int J Obes (Lond) 2007;31:466–75. doi: 10.1038/sj.ijo.0803438. [DOI] [PubMed] [Google Scholar]
- 36.Licinio J, Mantzoros C, Negrao AB, et al. Human leptin levels are pulsatile and inversely related to pituitary-adrenal function. Nature medicine. 1997;3:575–9. doi: 10.1038/nm0597-575. [DOI] [PubMed] [Google Scholar]
- 37.Sinha MK, Ohannesian JP, Heiman ML, et al. Nocturnal rise of leptin in lean, obese, and non-insulin-dependent diabetes mellitus subjects. The Journal of clinical investigation. 1996;97:1344–7. doi: 10.1172/JCI118551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Haghighatdoost F, Karimi G, Esmaillzadeh A, Azadbakht L. Sleep deprivation is associated with lower diet quality indices and higher rate of general and central obesity among young female students in Iran. Nutrition. 2012;28:1146–50. doi: 10.1016/j.nut.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 39.Nedeltcheva AV, Kilkus JM, Imperial J, Kasza K, Schoeller DA, Penev PD. Sleep curtailment is accompanied by increased intake of calories from snacks. The American journal of clinical nutrition. 2009;89:126–33. doi: 10.3945/ajcn.2008.26574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.St-Onge MP, McReynolds A, Trivedi ZB, Roberts AL, Sy M, Hirsch J. Sleep restriction leads to increased activation of brain regions sensitive to food stimuli. The American journal of clinical nutrition. 2012;95:818–24. doi: 10.3945/ajcn.111.027383. [DOI] [PMC free article] [PubMed] [Google Scholar]