Abstract
Fluorine (19F) MRI of perfluorocarbon labeled cells has become a powerful technique to track the migration and accumulation of cells in living organisms. It is common to label cells for 19F MRI with nanoemulsions of perfluoropolyethers that contain a large number of chemically equivalent fluorine atoms. Understanding the mechanisms of 19F nuclear relaxation, and in particular the spin-lattice relaxation of these molecules, is critical to improving experimental sensitivity. To date, the temperature and magnetic field strength dependence of spin-lattice relaxation rate constant (R1) for perfluoropolyethers has not been described in detail. In this study, we evaluated R1 of linear perfluoropolyether (PFPE) and cyclic perfluoro-15-crown-5 ether (PCE) at three magnetic field strengths (7.0, 9.4, and 14.1 T) and at temperatures ranging from 256-323K. Our results show that R1 of perfluoropolyethers is dominated by dipole-dipole interactions and chemical shift anisotropy. R1 increased with magnetic field strength for both PCE and PFPE. In the temperature range studied, PCE was in the fast motion regime (ωτc < 1) at all field strengths, but for PFPE, R1 passed through a maximum, from which the rotational correlation time was estimated. The importance of these measurements for the rational design of new 19F MRI agents and methods is discussed.
1. Introduction
Cyclic perfluoro-15-crown-5 ether (PCE) and linear perfluoropolyether (PFPE) molecules with repeating -CF2CF2O- units are increasingly being used for cellular and molecular MRI [1-4]. The use of 19F MRI has the advantage that there is no background signal in tissue, thus the imaging probe has high specificity. Moreover, quantification of the number of targeted probe molecules is feasible in vivo [5-6] leading to new cell tracking methods, such as in vivo cytometry [6]. For cell labeling, these molecules are formulated as an oil-in-water emulsion to enable use in biological applications [7]. PCE has desirable properties for imaging because each molecule has 20 chemically equivalent fluorine atoms giving rise to a single resonance peak. With these molecules, on the order of 1012 – 1013 fluorine atoms can be loaded into a cell of interest, providing a detection limit of order 104 – 105 labeled cells per voxel [8]. One advantage of 19F MRI cell tracking is that, with a known labeling efficiency, the cell number can be estimated from 19F spin-density weighted images [5], for which the acquisition time is limited by R1. Interestingly, unlike 1H MRI of tissue water [9], spin-lattice relaxation rate constant (R1) of PCE increases with increasing magnetic field strength [10], thereby allowing accelerated data acquisition at higher field strengths. PFPE is essentially a linear version of the cyclic PCE, which has a significantly long R1 compared with PCE, making it an attractive label for in vivo cell tracking by MRI where enhanced imaging speed and sensitivity is desirable.
Understanding the molecular mechanisms of spin-lattice-relaxation in these two closely related molecules could aid the development of novel agents that are optimized for 19F cellular MRI [7]. In this study, we measured the temperature dependence of 19F R1 for the linear PFPE and cyclic PCE between 256 K to 323 K at three different magnetic field strengths: 7.0 T (282 MHz), 9.4 T (376 MHz), and 14.1 T (564 MHz). These measurements were used to provide insight into the mechanisms 19F nuclear spin-lattice relaxation in these molecules and to estimate the apparent rotational correlation times.
2. Experimental
Sample preparation
PCE and PFPE with 98% purity were obtained from Exfluor LP (Round Rock, TX) and used without further modification. The molecular weights of PCE and PFPE were 580 and 1000 Da, respectively. The viscosity of PCE was 4.8 Pa s and PFPE was 14.74 Pa s. 200 μl of neat PCE and PFPE oil were transferred to a 2.5 mm NMR tube and purged with 100% nitrogen for 15 minutes to remove oxygen. Tubes were sealed gas-tight with epoxy resin. PCE and PFPE emulsions were obtained from Celsense (Pittsburgh, PA) at a concentration of 120 mg/ml. Emulsion samples for NMR measurements were prepared in the same way as described above.
NMR measurements
19F NMR measurements were made at 282, 376, and 564 MHz at temperatures between 256 to 323K using three different Bruker Avance III NMR spectrometers (Bruker Biospin, Billerica, MA). A 5mm Bruker QNP probe was used at 282 MHz and a BBFO-Plus probe was used at 376 and 564 MHz. On both these probes the inner radio frequency coil was used for 19F observation. The probe temperature was calibrated using ethylene glycol by measuring the chemical shift difference between the OH and CH2 resonance [11] before every measurement. The longitudinal relaxation time constant, T1 (1/R1) measurements were made using an inversion recovery sequence with phase cycling for a total of 8 averages per sample. A non-selective 90° pulse length of 15.5 μs was used and the recycle delay was 20s. 14 recovery points were used for each relaxation measurement. T1 was determined with a two-parameter monoexponential fit using Topspin software (Bruker). The 19F COSY spectrum was measured using 2048 and 128 TD points in the direct and indirect dimensions respectively with a recycle delay of 4 s and 8 scans were average per TD point in the F1 dimension. The data were processed with SI of 2048 × 1024. Spectral width of 100 ppm was used for both inversion recovery and COSY experiments. Data analysis was performed using Origin software (Originlab, Northampton, MA). The R1 versus temperature data for PFPE was fit to a third degree polynomial. To calculate the maximum point, the first derivative of the fit was solved for f′(T)=0.
3. Results
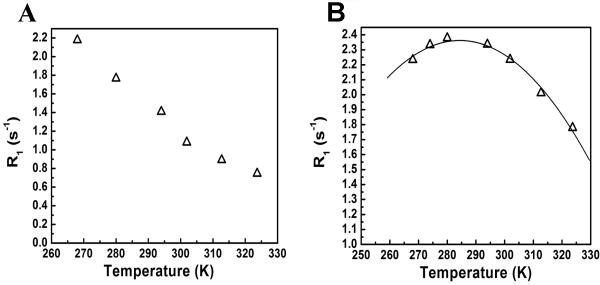
The structures of PCE and PFPE are shown in Figures 1A and 1C, respectively. PCE has 20 fluorine atoms with equivalent chemical shifts at −92.8 ppm (Figure 1B) (relative to CFCl3 (trichloro-fluoro-methane) at 0.00 ppm). PFPE is provided as a mixture of polymers with 28-36 fluorine atoms per molecule and major resonance peaks between −90.7 and −90.9 ppm that arise from the perfluoropolyether chain and minor peaks at −58 and −93 ppm that arises from the terminal perfluorocarbon moieties (Figure 1D). Theoretically, the fluorine atoms of perfluoropolyether backbone should only exhibit a single resonance peak; however, the large size and conformation of the molecule within the local environment can give rise to different chemical shifts. The range of chemical shifts could also result from a difference in the polymer chain lengths present in PFPE. The separation between two major resonance peaks (90.7 and 90.9 ppm) increases linearly with magnetic field ranging from 14 Hz (0.49 ppm) at 282 MHz, 19 Hz (0.50 ppm) at 376 MHz and 29 Hz (0.51 ppm) at 564 MHz, consistent with chemical shift separation and not spin-spin coupling, as this would display field independence. 19F-COSY experiments further confirmed that there is no spin-spin coupling between these two major resonance peaks (Figure 1E). A broad-band COSY experiment showed that there is a weak correlation between the major peaks and minor peak supporting the assignment of the minor peak to the end groups (Figure 1F). T1 was measured at three different magnetic field strengths (7.0, 9.4, and 14.1T) and at temperatures ranging from 256 K to 323 K. T1 was estimated using a two-parameter monoexponential recovery curve (Figure 2A and C). For PCE, R1 decreased with increasing temperature for this range for all magnetic field strengths (Figure 2B). For PFPE, R1 was calculated using the area integrated over the two major resonance peaks. For all magnetic field strengths, R1 increased with temperature from 256 K to reach a maximum before decreasing (Figure 2D). To estimate the maximum point, the data was fit to a third order polynomial, followed by solving f′(T) = 0. The maximum R1 was observed at 281.6 K for 14.1T, 270.6 K for 9.4T and 264 K at 7T. No maximum was observed for PCE at these temperatures and magnetic field strengths. The T1 of PCE and PFPE was then measured at 14.1 T in aqueous nanoemulsions for the same temperature range. The temperature dependence of R1 was similar to that found for the neat preparations. For PCE, the R1 decreased with increasing temperatures (Figure 3A), and R1 values of the nanoemulsions and neat preparations were similar. For PFPE, R1 increased from 256 K to reach a maximum at 279.4 K and then decreased (Figure 3B). This temperature at which f′(T) = 0 is in good agreement with the neat measurement; however, for PFPE, the R1 values were reduced by about 20% in the nanoemulsion.
Figure 1.
Structure and 19F NMR spectra of PCE and PFPE. (A) Structure of PCE and (C) PFPE (R=CF2CF3 or CF3). (B) PCE has 20 equivalent 19F atoms showing a single peak at −92.8 ppm. (D) PFPE has about 28-36 19F atoms with two major chemical shifts at −90.7 and −90.9 ppm. The inset shows the magnified view of the major peaks. (E) 19F COSY shows that there is no spin-spin coupling between the peaks. (F) 19F COSY spectrum of PFPE at broad bandwidth shows a weak correlation between major and minor resonances supporting the assignment of minor peak to the endgroup of the molecule
Figure 2.
R1 versus temperature at 7.0, 9.4, and 14.1T. (A) A representative mono-exponential R1 inversion recovery curve of PCE and (C) PFPE. (B) R1 decreased with temperature and increased with magnetic field strength for PCE. (D) For PFPE, R1 increased from 256 K until a maximum and then decreased. The data were fit to a third degree polynomial, and the maximum point was determined by solving f′(T)=0. The R1 maximum was observed at 281.63 K (14.1T), 270.61 K (9.4T), and 264 K (7.0T). The rotational correlation time for PFPE was estimated to be 1.77×10−9 s at 281.63 K, 2.66×10−9 s at 270.61 K, and 3.55×10−9 s at 264 K. At all temperatures and magnetic field strengths, PCE has a slower R1 compared to PFPE. (▲) = 14.1 T, (●) = 9.4 T, and (■) = 7.0 T.
Figure 3.
R1 versus temperature of PCE and PFPE emulsions at 14.1T. (A) R1 decreased with temperature for PCE emulsions. B) For PFPE, R1 increased from 268 K until a maximum and then reduced. The data were fit to a third degree polynomial, and the maximum point was determined by solving f′(T)=0. The R1 maximum was observed at 279. 4 K.
We calculated the rotational correlation time (τc) for PFPE directly from the apparent maximum of R1 versus temperature (Figure 2D). Assuming ω2 τc2 =1 at the R1 maximum, the calculated rotational correlation times for the neat PFPE were 1.77×10−9 s at 281.6 K, 2.66×10−9 s at 270.6 K, and 3.55×10−9 s at 264 K. For the PFPE emulsion, a rotational correlation time of 1.77×10−9 s was calculated at 279.4 K. As expected, an inverse linear relationship between rotational correlation time and temperature was observed. The rotational correlation time for PCE could not be calculated from the range of temperatures and magnetic field studied. Measurements at higher magnetic fields and/or lower temperatures would be needed to find the maximum for R1.
4. Discussion and Conclusions
The temperature and field strength dependence of R1 for perfluoropolyethers is consistent with contributions from dipole-dipole and CSA interactions [12-13].
| Eq: 105, pg. 300, ref. 13 |
| Eq: 115, pg. 302, ref. 13 |
| Eq: 141, pg. 316, ref.13 |
where J(ω) is the spectral density function given by
| Eq: 91, pg.297, ref.13 |
τc is the rotational correlation time and R1 is a maximum at τc=1/ω
Our measurements show that R1 increases with increasing magnetic field strengths due to CSA. The contribution of CSA increases with increasing magnetic field with a dependence of B02. For tissue water in the fast motion regime (ωτc < 1), dipole-dipole interactions dominate nuclear relaxation and R1 decreases with increasing magnetic field strength. The observed trend of R1 versus temperature for the perfluorocarbon molecules supports contributions from both dipole-dipole and CSA interactions. However, we did not attempt to quantify the relative contributions of these two relaxation mechanisms. We calculated the τc for PFPE using the assumption that ω2 τc2 =1 at R1 maximum. From the equation for R1rot, it follows that at R1 maximum, 4 ω2 τc2=1 can also be assumed. The range of temperatures did not allow us to separate the contributions ofJ(ω) or J(2ω), but exploring a larger temperature range may allow separation of the rotation (J(ω) + 4J(2ω)) from CSA contributions (J(ω)). The decrease in R1 from 293 K to 323 K for PFPE and 256 K to 323 K for PCE suggest that, at these temperatures, the molecules experience fast to intermediate tumbling motion within the NMR time scale (ωτc < 1). R1 versus temperature trends observed in neat liquids at 14.1T were similar to that of the respective emulsions. For PFPE, the temperature at which the R1 maximum was observed in emulsion was in good agreement with the R1 maximum observed in neat liquid. However, unlike PCE, the PFPE R1 was found to be about 20% lower in the emulsion compared to the neat liquid. The presence of residual O2 in the neat liquid could contribute to relaxation, although all the samples were prepared by bubbling N2 thought the samples. One could speculate that translational diffusion could be limited in the emulsion and translational intermolecular dipolar relaxation could make a significant contribution in the neat liquid.
In addition to temperature and magnetic field strengths, the spin-lattice relaxation of perfluorocarbons including perfluoropolyethers are influenced by the oxygen content. The paramagnetic properties of molecular oxygen increase the R1 which has been used to measure tissue oxygenation in vivo [14, 15]. In vitro calibration studies at different magnetic field strengths have shown that R1 exhibits a linear increase with pO2 [10, 14, 15].
CSA is a predominant mechanism for NMR relaxation in many solids, however, in liquids, CSA components average out due to rapid tumbling motion. The motions that produce this average value can cause fluctuations in the local magnetic field, and these time-varying fields lead to relaxation [16]. Although PCE and PFPE are liquids in the temperature range studied, our data show that CSA makes a significant contribution to R1. Perfluorocarbons are particularly interesting because of their low intermolecular dipole-dipole interactions and high vapor pressures compared to corresponding hydrocarbons of equal chain length. Low inter-molecular interactions are due to the large electronegativity of the F in C-F bonds, which effectively repel any other similar molecules in their vicinity [17].
Understanding the molecular dynamics underlying NMR relaxation, such as rotational correlation times can aid in the development of optimized perfluoropolyethers for 19F MRI. The rotational correlation time for PFPE was calculated from the maximum R1 versus temperature, but the rotational correlation time of PCE could not be determined since the molecule remained in the fast motion regime over the temperature and field strength used in our study. However, our relaxation data support a shorter effective rotational correlation time for PCE than PFPE at a given temperature, likely a result of the lower molecular weight and viscosity [18]. In addition, the shape of the molecules may also influence the rotational tumbling motion [19]. PFPE has a long chain, and the strong electronegativity of C-F bonds tends to align the chains in a rigid linear structure compared to PCE, where the crown-ether structure gives rise to a compact alignment.
The increase in R1 with increasing magnetic field strength observed in perfluoropolyethers is desirable for imaging at high magnetic field strengths because signal-to-noise ratio per unit time can be increased with an optimized T1-weighted sequence. However, at high field strengths, increased CSA and magnetic field inhomogenieties can lead to undesirable line-broadening. For cell tracking, however, 19F MRI is typically acquired at 2-4 times lower resolution than 1H MRI, therefore, the effect of line broadening on signal localization can be minimal. PFPE has a long spin-spin relaxation time constant (T2), which is also attractive for imaging applications. There are other perfluorocarbon molecules used for cellular imaging such as perfluorooctyl bromide [1, 2]; however, these molecules have multiple major resonance peaks over a broad chemical shift range resulting in a reduction in the sensitivity and confounding MRI results. Therefore, these molecules were not included in the study.
An ideal 19F contrast agent for molecular MRI should have long R1 and short R2. Based on our measurements, for PFPE and PCE, R1 can be increased by increasing magnetic field strengths. For PFPE, R1 maximum was observed in the R1 versus temperature plots, from which we estimated the rotational correlation times. For in vivo imaging, it is desirable to have a R1 maximum at about 310 K (37°C). From our data, a right shift in R1 versus temperature plots could achieve it. A right shift in R1 maximum towards 310 K was observed with increasing magnetic field strengths suggesting that PFPE is an optimized agent for high field MRI. For imaging at clinical field strengths of 3T and below, PFPE can be optimized by increasing the chain length. For PCE, enlarging the crown structure could result in more signals per molecule and more desirable R1 characteristics. The emulsion formulation can be optimized by increasing the size of the emulsion droplets and viscosity of the emulsions into an acceptable range without affecting the stability of the emulsion. For ex vivo imaging of tissues, the sensitivity can be increased by reducing the temperature of the samples. Understanding the fundamental principles of 19F NMR relaxation of these molecules will aid in the rational design of 19F contrast agents.
Highlights.
T1 of perfluoropolyethers can be explained by dipole-dipole interactions and CSA
Molecular motion determines the differences in R1 between PCE and PFPE
The rotational correlation time of PFPE was estimated from R1 vs. temp plots
Acknowledgements
We are grateful to Dr. Paul A. Bottomley for his helpful discussion and insight into relaxation mechanisms. This work was supported in part by the National Institutes of Health (R01 CA134633, R01 EB17271 and P41 EB 001977), the Maryland Stem Cell Research Foundation (MSCRFII-0161), and the California Institute for Regenerative Medicine (LA1-C12-06919).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Chen J, Lanza GM, Wickline SA. Quantitative magnetic resonance fluorine imaging: today and tomorrow. WIRES Nanomed. Nanobi. 2010;2:431–440. doi: 10.1002/wnan.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Ruiz-Cabello J, Barnett BP, Bottomley PA, Bulte JWM. Fluorine (19F) MRS and MRI in biomedicine. NMR Biomed. 2011;24:114–129. doi: 10.1002/nbm.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ahrens ET, Flores R, Xu H, Morel PA. In vivo imaging platform for tracking immunotherapeutic cells. Nature Biotechnol. 2005;23:983–987. doi: 10.1038/nbt1121. [DOI] [PubMed] [Google Scholar]
- [4].Bulte JW. Hot spot MRI emerges from the background. Nature Biotechnol. 2005;23:945–946. doi: 10.1038/nbt0805-945. [DOI] [PubMed] [Google Scholar]
- [5].Srinivas M, Morel PA, Ernst LA, Laidlaw DH, Ahrens ET. Fluorine-19 MRI for visualization and quantification of cell migration in a diabetes model. Magn. Reson. Med. 2007;58:725–734. doi: 10.1002/mrm.21352. [DOI] [PubMed] [Google Scholar]
- [6].Srinivas M, Turner MS, Janjic JM, Morel PA, Laidlaw DH, Ahrens ET. In vivo cytometry of antigen-specific t cells using 19F MRI. Magn. Reson. Med. 2009;62:747–753. doi: 10.1002/mrm.22063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Janjic JM, Ahrens ET. Fluorine-containing nanoemulsions for MRI cell tracking. WIREs Nanomed. Nanobi. 2009;1:492–501. doi: 10.1002/wnan.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ahrens ET, Zhong J. In vivo MRI cell tracking using perfluorocarbon probes and fluorine-19 detection. NMR Biomed. 2013;26:860–71. doi: 10.1002/nbm.2948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Bottomley PA, Foster TH, Argersinger RE, Pfeifer LM. A review of normal tissue hydrogen NMR relaxation times and relaxation mechanisms from 1-100 MHz: dependence on tissue type, NMR frequency, temperature, species, excision, and age, Medical physics. 1984;11:425–448. doi: 10.1118/1.595535. [DOI] [PubMed] [Google Scholar]
- [10].Duong TQ, Iadecola C, Kim SG. Effect of hyperoxia, hypercapnia, and hypoxia on cerebral interstitial oxygen tension and cerebral blood flow, Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine/Society of Magnetic Resonance in Medicine. 2001;45:61–70. doi: 10.1002/1522-2594(200101)45:1<61::aid-mrm1010>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- [11].Raiford DS, Fisk CL, Becker ED. Calibration of methanol and ethylene glycol nuclear magnetic resonance thermometers. Anal. Chem. 1979;51:2050–2051. [Google Scholar]
- [12].Bloembergen N, Purcell EM, Pound RV. Relaxation Effects in Nuclear Magnetic Resonance Absorption. Phys. Rev. 1948;73:679–712. [Google Scholar]
- [13].Abragam A. The principles of nuclear magnetism. Clarendon Press; Oxford: 1961. [Google Scholar]
- [14].Dardzinski BJ, Sotak CH. Rapid tissue oxygen tension mapping using 19F inversion-recovery echo-planar imaging of perfluoro-15-crown-5-ether, Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine/Society of Magnetic Resonance in Medicine. 1994;32:88–97. doi: 10.1002/mrm.1910320112. [DOI] [PubMed] [Google Scholar]
- [15].Kadayakkara DK, Janjic JM, Pusateri LK, Young WB, Ahrens ET. In vivo observation of intracellular oximetry in perfluorocarbon-labeled glioma cells and chemotherapeutic response in the CNS using fluorine-19 MRI, Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine/Society of Magnetic Resonance in Medicine. 2010;64:1252–1259. doi: 10.1002/mrm.22506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Gerig J. Fluorine NMR. Monograph published online. 2001:1–35. Available from http://www.biophysics.org/portals/1/pdfs/education/gerig.pdf.
- [17].Neilson AH. Organofluorines. Springer; Berlin: 2002. Partitioning of Organofluorine Compounds in the Environment; pp. 68–69. [Google Scholar]
- [18].Debye PJW. Polar molecules. The Chemical Catalog Company, inc; New York: 1929. [Google Scholar]
- [19].Hu CM, Zwanzig R. Rotational Frictional Coefficients for Spheroids with Slipping Boundary-Condition. J. Chem. Phys. 1974;60:4354–4357. [Google Scholar]