Abstract
Objective
To assess the influence of pro-inflammatory IL-1 genotype status on the risk of CAD, defined as >50% diameter stenosis, and cardiovascular events mediated by OxPL and Lp(a).
Background
Oxidized phospholipids (OxPL) are pro-inflammatory, circulate on lipoprotein (a) [Lp(a)] and mediate coronary artery disease (CAD). Genetic variations in the interleukin-1 (IL-1) region are associated with increased inflammatory mediators.
Methods
IL-1 genotypes, OxPL on apolipoprotein B-100 (OxPL/apoB) and Lp(a) levels were measured in 499 patients undergoing coronary angiography. The composite genotype termed IL-1(+) was defined by three single nucleotide polymorphisms (SNPs) in the IL-1 gene cluster associated with higher levels of pro-inflammatory cytokines. All other IL-1 genotypes were termed IL-1(−).
Results
Among IL-1(+) patients, the highest quartile of OxPL/apoB was significantly associated with a higher risk of CAD compared to the lowest quartile (OR 2.84, P=0.001). This effect was accentuated in patients ≤60 years old (OR 7.03, P<0.001). In IL-1(−) patients, OxPL/apoB levels showed no association with CAD. The interaction was significant for OxPL/apoB (OR 1.99, P=0.004) and Lp(a) (OR 1.96, P<0.001) in IL-1(+) versus IL-1(−) groups for patients ≤60 years old but not for patients >60 years old. In IL-1(+) patients ≤60 years old, after adjusting for established risk factors, high sensitivity C-reactive protein and Lp(a), OxPL/apoB remained an independent predictor of CAD. IL-1(+) patients above the median OxPL/apoB presented to the cardiac catheterization laboratory a mean of 3.9 years earlier (P=0.002) and had worse 4-year event-free survival (death, MI, stroke, and revascularization) compared to other groups (P=0.006).
Conclusion
Our study suggests that IL-1 genotype status can stratify population risk for CAD and cardiovascular events mediated by OxPL. These data suggest a clinically-relevant biological link between pro-inflammatory IL-1 genotypes, oxidation of phospholipids, Lp(a) and genetic predisposition to CAD and cardiovascular events.
Keywords: lipoproteins, oxidation, atherosclerosis, lipoprotein (a), oxidized phospholipids, IL-1, polymorphism, haplotype, inflammation, genetic risk stratification
INTRODUCTION
The presence of chronic arterial inflammation in response to atherogenic stimuli provides a framework in understanding the development and destabilization of atherosclerotic plaques. Oxidized lipids play a central role in mediating a variety of immune, pro-inflammatory and plaque destabilizing processes that further amplify inflammatory responses(1). Underlying this inflammatory cascade is the production and secretion of cytokines, growth factors and metalloproteinases, such as interleukin-1 (IL-1), tumor necrosis factor α and C-reactive protein (CRP)(2). Genetic variations in the IL-1 gene family (chromosome 2q13 region), which include pro-inflammatory cytokines IL-1α, IL-1β and the anti-inflammatory IL-1 receptor antagonist (IL-1Ra)(3–5) are commonly found in the human population, affect pro-inflammatory gene regulation(6) and have been associated with elevated levels of pro-inflammatory mediators(7–10). The interplay of various single nucleotide polymorphisms within this IL-1 family determines the overall net effect on pro- or anti-inflammatory responses.
The majority of published studies have shown an association of IL-1 and cardiovascular disease, including early myocardial infarction/acute coronary syndromes (8,11–16) coronary artery disease (CAD)(17–20), acute ischemic stroke(21–23), restenosis following coronary stenting(24) and venous thrombosis(25). The Canakinumab Anti-inflammatory Thrombosis Outcomes Study (CANTOS) will test the hypothesis that treating patients with persistent elevation of CRP post myocardial infarction with a human monoclonal antibody that neutralizes IL-1β antibody will reduce cardiovascular events(26).
Oxidized phospholipids (OxPL) are pro-inflammatory(27), mediate atherothrombosis and are abundant in pathologically-defined human vulnerable plaques(28). Plasma levels of specific OxPL on apolipoprotein B-100 (apoB) particles (OxPL/apoB) are elevated in patients with coronary, carotid and peripheral artery disease(29), as well as in acute coronary syndromes(30), and following percutaneous coronary intervention(31). Importantly, they predict the occurrence of cardiac death, myocardial infarction and stroke in unselected populations(32–34). Additionally, they reclassify up to one third of patients in intermediate Framingham risk categories into either higher or lower categories(33). In human plasma, OxPL are preferentially carried by Lp(a) lipoprotein (a) [Lp(a)], compared to other apoB-100 particles (reviewed in Taleb et al (35)). OxPL are also covalently bound by plasminogen, but early data suggest different pathophysiological implications when OxPL are on Lp(a) versus plasminogen (36).
Since OxPL mediate pro-inflammatory responses on endothelial cells and monocytes/macrophages(27), it is possible that the risk they confer on atherothrombosis is potentiated by genetic predisposition to inflammation. In the present study, we hypothesized that the risk of CAD conferred by OxPL/apoB and Lp(a) may be influenced by IL-1 genotypes known to be associated with enhanced inflammatory responses.
METHODS (A full description of the Methods is available in the Supplement)
Study design
The study was prospectively designed to test the association of CAD with specific IL-1 genotype groups known to be associated with higher inflammatory responses. The study design has been described previously in detail(37). Briefly, 504 eligible, consecutive patients undergoing clinically indicated coronary angiography were recruited. We focused our analyses on angiographically significant disease defined as diameter stenosis (DS) >50%. Two patients had incomplete OxPL/apoB data and 3 patients incomplete IL-1 data, therefore 499 patients were available for the present analysis. Four hundred sixty-six patients (92.5%) followed for up of 4.0 years (interquartile range, 3.9–4.2 years)]. The follow-up events consisted of 20 deaths (6 cardiac), 14 myocardial infarctions, 26 coronary revascularizations (15 percutaneous intervention only, 9 coronary artery bypass surgery only, and 2 with both), and 10 strokes.
Genetic analyses
Single nucleotide polymorphisms (SNPs) were genotyped at two loci in the gene for IL-1β, IL1B(-511; C>T; rs16944) and IL1B(+3954; C>T; rs1143634); and at one locus in the gene for IL-1α, IL1A (+4845; G>T; rs17561)(38).
IL-1 composite genotype patterns used for association with biochemical and clinical parameters
We designed the study to evaluate the relationship between CAD and IL-1 genotypes that are associated with differential expression of interleukin-1β (IL-1β). Four single-nucleotide polymorphisms (SNPs) in the promoter region of IL1B have been shown to be functional at the molecular level and operate in haplotype context to alter transcriptional activity of IL-1β(6). The functional IL1B SNPs define four predominant haplotypes that as pairs observed together account for significantly different clinical levels of IL-1β protein in tissue fluid samples(10). All possible composite genotype combinations of the 3 SNPs used in the study provided an efficient tagging of the composite genotypes resulting from combinations of the functional IL1B promoter haplotypes that define differential expression of IL-1β protein(10). Table 1 shows the composite genotypes in this study that defined the IL-1(+) group, which are associated with over-expression of IL-1β, and the IL-1(−) group, which are the composite genotypes that have not been associated with over-expression of IL-1β. The IL-1(+) and IL-1(−) groups were defined and published (Francis S.E., Crossman D.C., Duff G.W., Kornman K. S., Stephenson K. 2003. Diagnostics for cardiovascular disorders. United States Patent # 6,524,795; Filed November 1, 1999; granted February 25, 2003) prior to data analysis.
Table 1.
Composite Genotypes Used in Study
| Composite Genotypes Used in Study | |||
|---|---|---|---|
| Group Classification for Analysis | IL1A(+4845) | IL1B(+3954) | IL1B(−511) |
| rs17561 G>T | rs1143634 C>T | rs16944 C>T | |
| IL-1(+) | T* | T* | CC |
| GG | T* | CC | |
| ** | CC | CC | |
| T* | T* | CT | |
| IL-1(−) | T* | T* | TT |
| GG | T* | TT | |
| T* | CC | TT | |
| GG | CC | TT | |
| T* | CC | CT | |
| GG | CT | CT | |
| GG | CC | CT | |
T*indicates that the second allele in the genotype can be either a G or a T;
indicates that the genotype at that locus can be GG, GT, TT
RESULTS
Baseline Characteristics of the Study Group
Table 2 displays the baseline characteristics of the entire study group and of the IL-1(+) and IL-1(−) groups. IL-1(+) patients represented 59.9% of the population. There were no significant differences in any parameters between IL-1(+) and IL-1(−) patients, including extent of CAD, except a trend towards more previous myocardial infarction (18% vs. 12%, P=0.08) and higher hsCRP (3.1 mg/L vs. 2.3 mg/L, P=0.057) in the IL-1(+) patients. IL-1 status was not different between groups in the extent of CAD (no disease, mild disease, 1-, 2- and 3-vessel CAD) when analyzed by age ≤60 years old (P=0.88) and >60 years old (P=0.36). Similar results were obtained when IL-1 status was evaluated as >50% diameter stenosis (DS) and analyzed by age ≤60 years old (P=0.51) and >60 years old (P=0.47).
Table 2.
Baseline Characteristics of the Study Group
| Variable | All | IL-1(+) | IL-1(−) | P-value IL-1 effect |
|---|---|---|---|---|
| Number | 499 | 299 | 200 | - |
| Age - yr | 60.0±10.9 | 59.6±11.1 | 60.6±10.7 | 0.82 |
| Female sex - no. (%) | 190 (38) | 111 (37) | 79 (40) | 0.59 |
| White race - no. (%) | 485 (97) | 294 (98) | 191 (96) | 0.51 |
| Hypertension - no. (%) | 230 (46) | 140 (47) | 90 (45) | 0.69 |
| Current smoker - no. (%) | 40 (8) | 24 (8) | 16 (8) | 0.99 |
| Previous myocardial infarction -no. (%) | 77 (15) | 53 (18) | 24 (12) | 0.08 |
| Congestive heart failure -no. (%) | 59 (12) | 32 (11) | 27 (14) | 0.35 |
| Family history of CAD - no.(%) | 126 (25) | 76 (25) | 50 (25) | 0.92 |
| Extent of disease | 0.79 | |||
| No disease | 122(24) | 71(24) | 51(26) | |
| Mild disease | 109(22) | 68(23) | 41(20) | |
| 1-vessel | 84(17) | 46(15) | 38(19) | |
| 2-disease | 80(16) | 50(17) | 30(15) | |
| 3-disease | 104(21) | 64(21) | 40(20) | |
| Lipid levels - mg/dl | ||||
| Total cholesterol | 207±45 | 208±46 | 206±43 | 0.57 |
| LDL cholesterol | 125±37 | 124±35 | 125±37 | 0.81 |
| HDL cholesterol | 48±15 | 48±15 | 48±14 | 0.94 |
| Triglycerides | ||||
| Median | 153 | 154 | 151 | 0.48 |
| Interquartile range | 112–207 | 113–220 | 106–200 | |
| Apolipoprotein B-100 | 98±21 | 98±20 | 98±22 | 0.91 |
| Apolipoprotein AI | 132±26 | 132±27 | 131±24 | 0.48 |
| Lipoprotein (a) – mg/dl | ||||
| Median | 21.1 | 21.5 | 20.0 | 0.96 |
| Interquartile range | 8.7–39.6 | 9.2–38.4 | 7.7–42.3 | |
| OxPL/apoB - RLU | ||||
| Median | 6268 | 6268 | 6121 | 0.96 |
| Interquartile range | 3381–20829 | 3361–20620 | 3414–20843 | |
| hsCRP - mg/liter | ||||
| Median | 2.9 | 3.1 | 2.3 | 0.057 |
| Interquartile range | 1.2–6.7 | 1.3–7.3 | 1.0–6.1 | |
CAD Risk of OxPL is Mediated by IL-1 Genetic Differences
Odds ratios (OR) for CAD in each quartile of OxPL/apoB were calculated in all patients, in IL-1(+) and IL-1(−) patients, and further analyzed by age (all ages, ≤60 years old and >60 years old). In the entire cohort, a significant relationship was present between OxPL/apoB and CAD (>50% DS): the OR was 1.96 (95% confidence interval (CI), 1.18–3.26, P=0.009) for fourth quartile compared to first quartile (Table 3), and the OR for trend 1.25 (1.06–1.46, P=0.007].
Table 3.
Odds Ratios for CAD (>50% DS) According to Quartiles for Oxidized Phospholipid/ApoB in IL-1 Genotype Positive and IL-1 Genotype Negative Patients, According to All Ages, ≤60 years Old and ≥60 Years Old
| Patient Group | All Patients | IL-1 Genotype Positive | IL-1 Genotype Negative | ||||||
|---|---|---|---|---|---|---|---|---|---|
| All ages | Total No. | No. with CAD (%) | OR (95% CI) | Total No. | No. with CAD (%) | OR (95% CI) | Total No. | No. with CAD (%) | OR (95% CI) |
| Quartile I | 125 | 59 (47) | 1.00 | 77 | 35 (46) | 1.00 | 48 | 24 (50) | 1.00 |
| Quartile II | 125 | 62 (50) | 1.10 (0.67–1.81) | 73 | 36 (49) | 1.17 (0.62–2.22) | 52 | 26 (50) | 1.00 (0.46–2.19) |
| Quartile III | 125 | 68 (54) | 1.34 (0.81–2.19) | 75 | 37 (49) | 1.17 (0.62–2.21) | 50 | 31 (62) | 1.63 (0.73–3.65) |
| Quartile IV | 124 | 79 (64) | 1.96 (1.18–3.26) | 74 | 52 (70) | 2.84 (1.45–5.55) | 50 | 27 (54) | 1.17 (0.53–2.60) |
| OR (95%CI) for trend | 1.25 (1.06–1.46) | 1.35 (1.10–1.66) | 1.10 (0.86–1.42) | ||||||
| P for trend | 0.007 | 0.004 | 0.45 | ||||||
| Age ≤60 yr | |||||||||
| Quartile I | 60 | 19 (33) | 1.00 | 33 | 9 (27) | 1.00 | 27 | 12 (44) | 1.00 |
| Quartile II | 52 | 17 (32) | 0.75 (0.34 – 1.67) | 32 | 8 (25) | 0.89 (0.29 – 2.69) | 20 | 7 (35) | 0.67 (0.20–2.22) |
| Quartile III | 64 | 27 (45) | 1.64 (0.80 – 3.38) | 43 | 18 (42) | 1.92 (0.73 – 5.10) | 21 | 12 (57) | 1.67 (0.53–5.27) |
| Quartile IV | 63 | 41 (60) | 2.82 (1.36 – 5.87) | 40 | 29 (73) | 7.03 (2.50 – 19.77) | 23 | 9 (39) | 0.80 (0.26–2.49) |
| OR (95%CI) for trend | 1.48 (1.17–1.87) | 1.99 (1.43–2.78) | 1.01 (0.71–1.45) | ||||||
| P for trend | 0.001 | <0.001 | 0.94 | ||||||
| Age >60 yr | |||||||||
| Quartile I | 65 | 40 (59) | 1.00 | 44 | 26 (59) | 1.00 | 21 | 12 (57) | 1.00 |
| Quartile II | 73 | 47 (65) | 1.28 (0.65–2.55) | 41 | 28 (68) | 1.49 (0.61–3.64) | 32 | 19 (59) | 1.10 (0.36–3.35) |
| Quartile III | 61 | 41 (62) | 1.17 (0.57–2.40) | 32 | 19 (59) | 1.01 (0.40–2.56) | 29 | 19 (66) | 1.43 (0.45–4.52) |
| Quartile IV | 61 | 38 (67) | 1.46 (0.70–3.02) | 34 | 23 (68) | 1.45 (0.57–3.69) | 27 | 18 (67) | 1.50 (0.46–4.87) |
| OR (95%CI) for trend | 1.11 (0.88–1.39) | 1.08 (0.80–1.45) | 1.16 (0.81–1.68) | ||||||
| P for trend | 0.38 | 0.61 | 0.42 | ||||||
Analyzing patients by genotype, a significant association was present between increasing OxPL/apoB levels and risk for CAD in IL-1(+) patients [OR 2.84 (1.45–5.55, P=0.001) for fourth quartile compared to first quartile], whereas no significant relationship was present in IL-1(−) patients (Table 3). The genotype effect was strongly accentuated in IL-1(+) patients ≤60 years old (OR 7.03 (2.50–19.77, P<0.001) but not in IL-1(−) patients ≤60 years old (OR 0.80 (0.26–2.49, P=0.71). In patients >60 years old, the association between OxPL/apoB levels and risk for CAD was not significant in either IL-1(+) or IL-1(−) patients.
Similar to the OxPL/apoB results, the association between Lp(a) and risk for CAD was observed primarily in IL-1(+) patients (Table 4), with the strongest genotype effect present in patients ≤60 years of age (OR 9.00 (3.00–27.03, P<0.001).
Table 4.
Odds Ratios for CAD (>50% DS) According to Quartiles for Lp(a) in IL-1 Genotype Positive and IL-1 Genotype Negative Patients, According to All Ages, ≤60 years Old and ≥60 Years Old
| Patient Group | All Patients | IL-1 Genotype Positive | IL-1 Genotype Negative | ||||||
|---|---|---|---|---|---|---|---|---|---|
| All ages | Total No. | No. with CAD (%) | OR (95% CI) | Tot al No. | No. with CAD (%) | OR (95% CI) | Total N | No. with CAD (%) | OR (95% CI) |
| Quartile I | 125 | 55 (44) | 1.00 | 70 | 30 (43) | 1.00 | 55 | 25 (46) | 1.00 |
| Quartile II | 125 | 62 (50) | 1.25 (0.76–2.06) | 77 | 37 (48) | 1.23 (0.64–2.37) | 48 | 25 (52) | 1.30 (0.60–2.83) |
| Quartile III | 125 | 68 (54) | 1.52 (0.92–2.50) | 84 | 42 (50) | 1.33 (0.70–2.52) | 41 | 26 (63) | 2.08 (0.91–4.76) |
| Quartile IV | 124 | 83 (67) | 2.58(1.54–4.31) | 68 | 51 (75) | 4.00 (1.94–8.26) | 56 | 32 (57) | 1.60 (0.76–3.39) |
| OR (95%CI) for trend | 1.35 (1.15–1.59) | 1.49 (1.20–1.85) | 1.20 (0.94–1.52) | ||||||
| P for trend | <0.001 | <0.001 | 0.14 | ||||||
| Age >60 yr | |||||||||
| Quartile I | 65 | 38 (58) | 1.00 | 38 | 22 (58) | 1.00 | 27 | 15 (56) | 1.00 |
| Quartile II | 68 | 43 (62) | 1.30 (0.65–2.61) | 41 | 27 (66) | 1.40 (0.56–3.49) | 27 | 16 (59) | 1.16 (0.40–3.42) |
| Quartile III | 67 | 42 (61) | 1.21 (0.56–2.24) | 40 | 23 (58) | 0.98 (0.40–2.42) | 27 | 17 (63) | 1.36 (0.46–4.04) |
| Quartile IV | 60 | 44 (72) | 2.08 (0.98–4.42) | 32 | 24 (75) | 2.18 (0.78–6.09) | 28 | 20 (71) | 2.00 (0.65–6.11) |
| OR (95%CI) for trend | 1.21 (0.96–1.53) | 1.19 (0.87–1.62) | 1.25 (0.88–1.77) | ||||||
| P for trend | P=0.10 | P=0.27 | P=0.22 | ||||||
Interaction tests were performed comparing IL-1(+) vs. IL-1(−) patients. P-values for the interaction of OxPL/apoB were P=0.007 for IL-1(+) vs. IL-1(−) patients ≤60 years of age and P=0.70 for IL-1(+) vs. IL-1(−) patients >60 years of age. P-values for the interaction of Lp(a) were P=0.019 for IL-1(+) vs. IL-1(−) patients ≤60 years of age and P=0.77 for IL-1(+) vs. IL-1(−) patients >60 years of age.
Multivariable Analysis of CAD Risk in the Different Genetic Strata
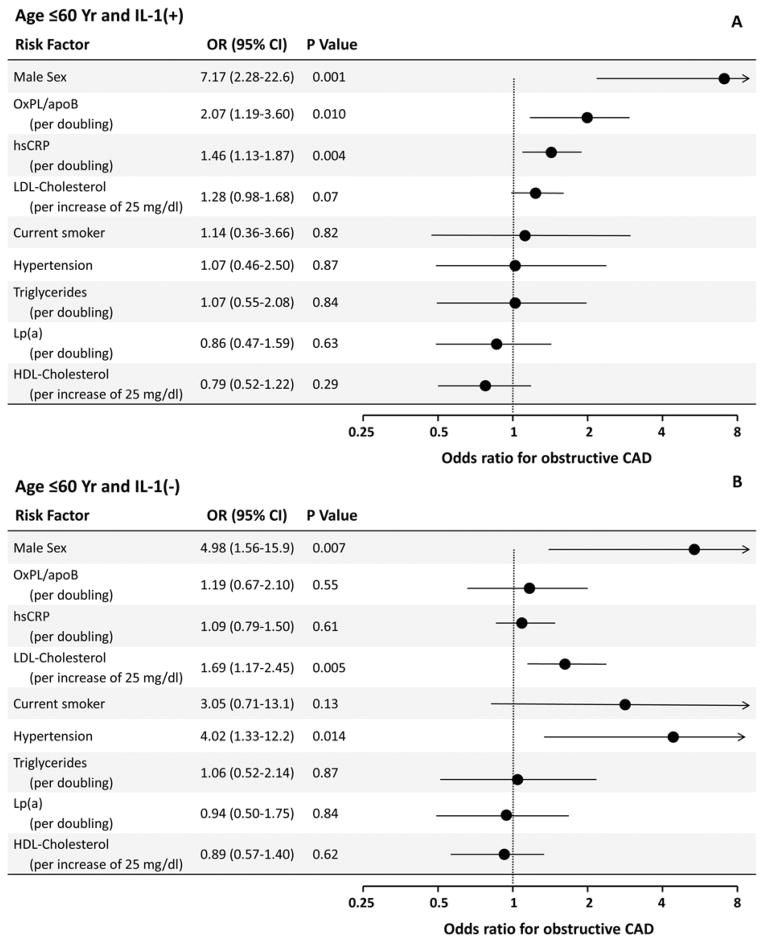
Multivariate logistic regression analysis was performed to adjust for factors known to affect risk of CAD. Figure 1 shows ORs for sex, OxPL/apoB, hsCRP, current smoking, LDL-C, hypertension, triglycerides, Lp(a), and HDL-C when all are included in a single logistic binary regression model. In patients ≤60 years old and IL-1(+), OxPL/apoB (log2), male sex and hsCRP (log2) were independent predictors of CAD, whereas Lp(a) was not a significant predictor (Figure 1A). The association of OxPL/apoB [(OR 1.83 (1.10–3.05, P=0.02] to CAD remained similar without hsCRP in the model. When the 44 patients with myocardial infarction within 60 days prior to coronary angiography were excluded, the data remained qualitatively similar, except that hsCRP was no longer a predictor of CAD (OR 1.23 (0.89–1.68, P=0.21). Baseline hsCRP levels in these patients were significantly elevated compared to patients without myocardial infarction, as described previously (33). In patients ≤60 years old and IL-1(−), male sex, LDL-C, and hypertension were independent predictors (Figure 1B).
Figure 1. Multivariable analysis derived odds ratios for CAD associated with selected risk factors among patients ≤60 years old stratified by genotype.
CI=confidence interval, LDL=low-density lipoprotein (per increase of 25 mg/dl), hsCRP=C-reactive protein (per doubling), OxPL/apoB (per doubling), Lp(a) (per doubling), HDL=high-density lipoprotein (per increase of 10 mg/dl), and triglycerides (per doubling).
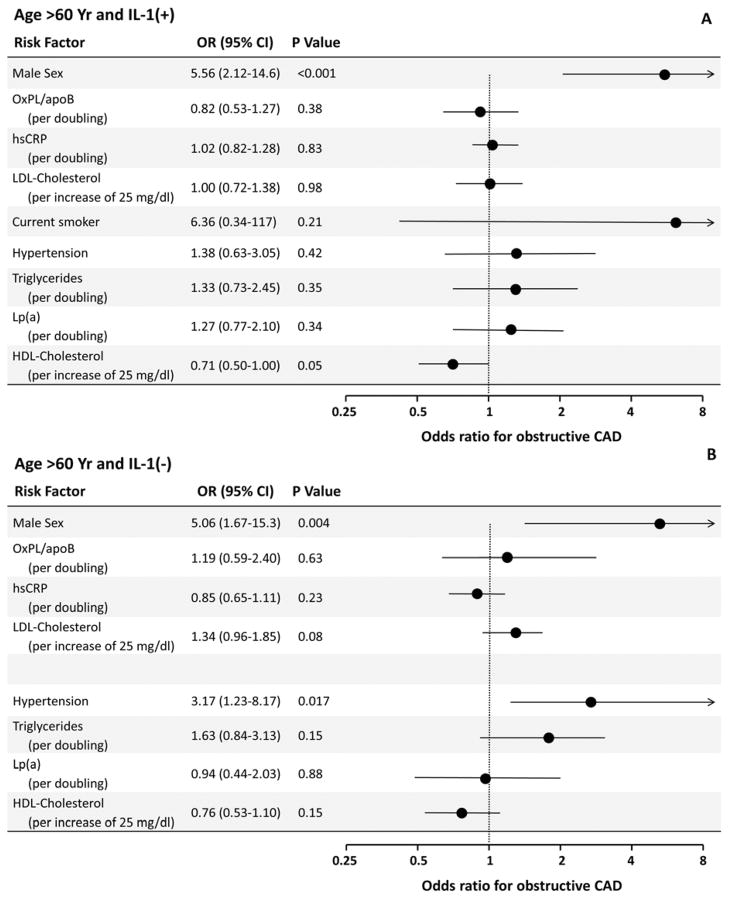
In patients >60 years old and IL-1(+), male sex and HDL-C were independent predictors of CAD, but not OxPL/apoB (Figure 2A). In patients >60 years old and IL-1(+), male sex, and hypertension were associated with higher risk (Figure 2B).
Figure 2. Multivariable analysis derived odds ratios for CAD associated with selected risk factors among patients >60 years old stratified by genotype.
CI=confidence interval, LDL=low-density lipoprotein (per increase of 25 mg/dl), hsCRP=C-reactive protein (per doubling), OxPL/apoB (per doubling), Lp(a) (per doubling), HDL=high-density lipoprotein (per increase of 10 mg/dl), and triglycerides (per doubling). Age is measured per decade. Current smoking was deleted as a factor for those >60 years old because of negligible sample size of smokers in this category.
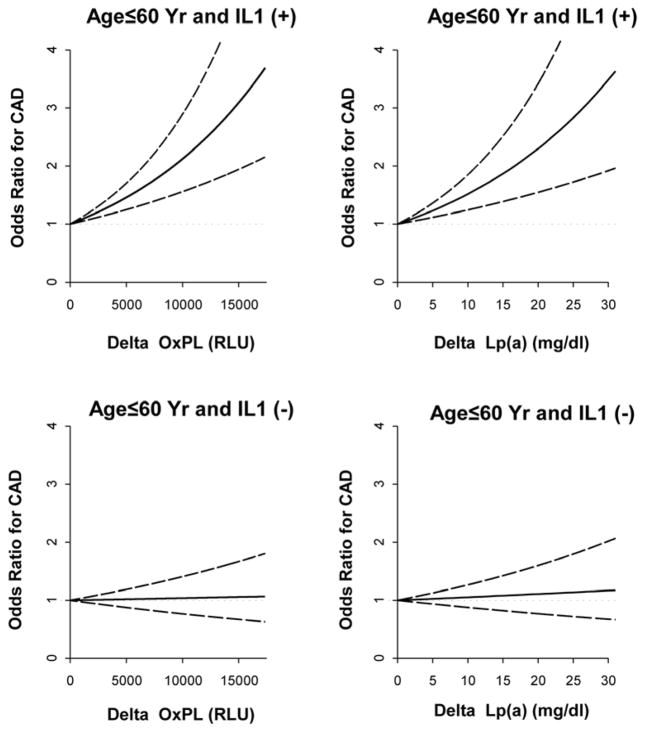
To further explore further the relationship between OxPL/apoB or Lp(a) levels and IL-1 genotype relative to risk for CAD, we stratified patients by IL-1 genotype and developed regression models to assess the relationship of OxPL/apoB and Lp(a) levels to CAD risk. The relationship of the OR values for CAD is expressed as function of the magnitude of differences in OxPL/apoB and Lp(a) levels in IL-1(+) and IL-1(−) patients (Figure 3). The OR for CAD was highly sensitive to differences in levels of both OxPL/apoB and Lp(a) in IL-1(+) patients, but no association was present in IL-1(−) patients.
Figure 3. Odds ratios (OR) (solid line) and 95% confidence intervals (dashed lines) for CAD were calculated in a logistic regression model.
In this model, risk associated with an incremental increase of each risk factor ranging from 0 (i.e., an odds ratio of 1) to the value equal to the difference between the 75th and 25th percentiles of the risk factors. The analysis was performed on patients ≤60 years of age stratified as IL-1(+) or IL-1(−).
IL-1 Genotype Effect on OxPL Risk for CAD and C-Reactive Protein Levels
Since some of the IL-1 gene variations included in the genetic patterns used in this study have been previously associated with elevated CRP (7,10) we evaluated whether the IL-1 genotype influence on the OxPL association with CAD was influenced by hsCRP levels. The relationship of OxPL/apoB to CAD in IL-1 (+) individuals ≤60 years of age was analyzed in the multivariate logistic regression framework for patients with hsCRP above and below the median hsCRP level in this study population (2.86 mg/L). The OR for the OxPL/apoB association with CAD in IL-1(+) patients with hsCRP >2.86 mg/L was 3.36 (1.21–9.40, P=0.02] and in those with hsCRP <2.86 mg/L the OR was 1.43 (0.62–3.31, P=0.40). Removing the 44 patients with recent MI yielded similar results, with an OR of 4.57 (CI 1.06–19.67, P=0.042] for the OxPL/apoB association with CAD in IL-1(+) patients with hsCRP above the median and 1.21 (CI 0.50–2.90, P=0.68] for those below the median.
Relationship of IL-1 Genotype to Age at Presentation to the Cardiac Catheterization Laboratory
Having established the relationship of OxPL/apoB and Lp(a) with CAD in IL-1(+) but not IL-1(−) individuals, particularly those at a younger age, we evaluated whether age at the time of cardiac catheterization was related to IL-1 composite genotype. IL-1(+) patients above the median of OxPL/apoB presented to the cardiac catheterization laboratory a mean of 3.9 years younger than IL-1(−) patients (58.0 vs. 61.9, P=0.006). Similarly, IL-1(+) patients above the median of Lp(a) presented a mean of 3.5 years younger than IL-1(−) patients (58.8 vs. 62.1, P=0.019). In contrast, there was no significant IL-1 genotype effect on the age at presentation to the cardiac catheterization laboratory for patients below the median of OxPL/apoB (58.9 vs. 60.7, P=0.18) or Lp(a) (58.7 vs. 59.9, P=0.40).
Relationship of IL-1 Genotype to CAD events
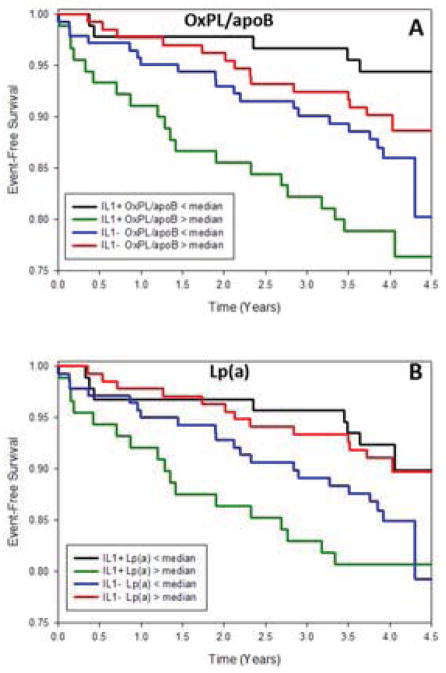
In the overall group, cardiovascular events were not significantly different between IL-1(+) and IL-1(−) patients (p=0.56). However, Kaplan-Meier curves revealed that IL-1(+) patients with OxPL/apoB above the median had the worst 4-year event-free survival (death, MI, stroke, revascularization) (P=0.002 compared to other 3 groups, Figure 4A). The p-value among 4 groups for death was P=0.069 and for death/MI P=0.016. The interaction test for IL-1 group by OxPL/apoB group was P=0.002 for event-free survival. For Lp(a), IL-1(+) patients with Lp(a) above the median exhibited worst 4-year event-free survival (P=0.034 compared to other 3 groups, Figure 4B). The p-value among 4 groups for death was P=0.054 and for death/MI P=0.011. The interaction test for IL-1 group by Lp(a) group was P=0.014 for event-free survival. There were not enough events to analyze by age cutoffs.
Figure 4. Event-free survival free period in years of death, MI, stroke and revascularization.
Event-free survival free period in years of death, MI, stroke and revascularization was plotted for 4 groups based on IL-1 genotype and OxPL/apoB (A) and Lp(a) (B) median split by the Kaplan-Meier method and compared with a log-rank test.
DISCUSSION
This study demonstrates that genetic differences in the IL-1 gene cluster, known to be associated with inflammatory responsiveness, strongly influence the presence of angiographically-determined CAD and CAD events mediated by OxPL/apoB and Lp(a). Patients with pro-inflammatory IL-1(+) genotypes were at a continuum of risk for the presence of CAD whereas patients with IL-1(−) genotypes seemed to be insensitive to risk for CAD mediated by increasing OxPL/apoB or Lp(a) levels. These findings were accentuated in subjects with elevated hsCRP levels. This study provides evidence of a biological link between genetic predisposition to inflammation, oxidation of phospholipids and genetically-mediated elevated Lp(a) levels. It also highlights a possible effect of specific genetic factors in accelerating atherogenesis, development of CAD on angiography and mediation of cardiovascular events.
The genes encoding the pro-inflammatory cytokines interleukin-1 alpha (IL-1α) and interleukin-1 beta (IL-1β) are among the first to be activated in the course of an inflammatory response, and play a major role in both acute and chronic inflammation(4). Plasma levels of IL-1α and IL-1β show reproducible inter-individual differences. Furthermore, IL-1 gene patterns that are highly prevalent in the population, 60% of Caucasians as noted in this study, have been associated with variations in the levels or expression of IL-1α(39), IL-1β(8) and the endogenous antagonist, IL-1 receptor antagonist (IL-1Ra)(40). The IL-1 composite genotypes used in this study were derived from combinations of the predominant functional haplotypes in the promoter region of the gene for IL-1β (6) and other SNPs in the IL-1α and β genes that have been associated with pro-inflammatory responses(10,41). IL-1β haplotypes exhibit allele-specific differences in nuclear protein binding and transcription rates(6). IL-1(+) genotypes are associated with enhanced generation of IL-1β when mononuclear cells are stimulated(8) and have been associated with higher IL-1β levels in plasma(40). Some of the three composite genotypes that comprise the IL-1(+) pattern for this study have been associated with significantly elevated hsCRP levels in plasma compared with IL-1(−) pattern(7,8,10,42,43). It should be noted that although the IL-1 genotype association with elevated IL-1β expression is also significant in gastric mucosa, the genotypes associated with elevated expression appear to be different from those reported for peripheral blood mononuclear cells(44,45).
L-1Ra is an important component of the net IL-1 biological activity of this system, as signaled through the IL-1 receptor type 1, and has been implicated in atherosclerotic cardiovascular diseases(3). Variants in the gene for IL-1Ra (IL1RN) have been associated with lower expression and circulating levels of IL-1Ra(9,46,47)) and with cardiovascular disease outcomes in some but not all studies(14,20,48). Several variants in IL1RN and other genes in the IL-1 cluster on chromosome 2q13-14 are in linkage disequilibrium with the specific IL1A and IL1B markers used in this study. For example in a population cohort of 839 unrelated Caucasians who tested positive for the IL-1 genotype patterns used in the current study, 64.5% were also homozygous for the T allele of IL1RN (+2018; rs419598) compared to 35.0% of those who tested negative (data not shown). Future studies with larger data sets may allow the analysis of contributions by other variants in the IL-1 region, in light of the strong linkage disequilibrium.
Vascular wall cells, such as endothelial cells and smooth muscle cells, as well as macrophages and monocytes can produce IL-1β and IL-1Ra and these cytokines are also present in human atherosclerotic lesions(3,17,19,49). It was shown that IL-1β promoter haplotype pairs are associated with higher levels of IL-1β in plasma and from stimulated peripheral blood monocytes from patients, as well as elevated levels of CRP(10). In addition, the IL-1(+) genotype patterns used in the current study tag haplotypes that include the T allele of IL1A(-889; rs1800587), which has been shown to alter transcription factor binding sites in the IL1A gene (50) and was associated with increased levels of IL-1α protein in human gingival fluid samples (51). Transgenic mouse models with variations in IL-1 genotypes further support the causal role of IL-1 in atherogenesis(reviewed in (3,26)). In IL-1 receptor antagonist knockout mice, unopposed IL-1 biological activity resulted in spontaneous arterial inflammation with massive infiltration of macrophages and CD4+, interferon γ+ T-cells at branch points in mid and large arteries(52,53). Decreases in IL-1 biological activity in apoE-deficient mice decreased the rate and extent of atherosclerosis formation(54,55). In contrast, increases in IL-1 activity increased atherosclerotic lesion size with more macrophages within lesions(56). Furthermore, anakinra, a recombinant form of human IL-1Ra improves vascular function in patients with rheumatoid arthritis(57).
In experimental studies, oxidized phospholipids interact with cells in the vessel wall and promote pro-inflammatory and pro-atherogenic properties. For example, in a large-scale gene expression analysis involving 9,600 cDNA targets, IL-1β was one of the differentially over-expressed genes when macrophages were loaded with OxLDL, which is known to be enriched in OxPL detected by E06, compared to acetylated-LDL loading(58). OxLDL stimulation of coronary artery smooth muscle cells also led to significant over-expression of IL-1β(59). More specifically stimulation of endothelial cells and macrophages with OxPL leads to prominent expression of IL-1α and IL-1β(60,61), and in turn, stimulation of endothelial cells with IL-1α leads to the generation of such OxPL(62). Thus, it is reasonable to hypothesize that the polymorphisms of the IL-1 family might influence the expression of inflammatory responses to OxPL. Indeed, supporting data shows that IL-1 genetic variations have been associated with acute coronary events, CAD and stroke(11–16,20–23,25,57,63,64).
In clinical studies elevated OxPL/apoB levels predict new CVD events(29,33,35,65). This study has expanded our understanding of the underlying mechanisms behind this risk by showing that the enhanced risk of CAD and CAD events mediated by OxPL/apoB and Lp(a) is particularly potent in IL-1 (+) patients. Interestingly, this risk of CAD persisted despite Lp(a) in the model, suggesting that in certain patient populations, such as patients <60 years old, OxPL/apoB may be a better predictor than Lp(a). Patients with underlying genetic predisposition to inflammation and dyslipidemia have exposure to cardiovascular risk from birth, which may explain why IL-1(+) patients ≤60 years old with elevated Lp(a) and OxPL/apoB levels are at particularly elevated risk for premature CAD. Consistent with the role of life-long exposure to genetic predisposition to inflammation and genetically determined Lp(a) levels, it was demonstrated in this study that IL-1(+) patients with Lp(a) or OxPL/apoB levels above the median presented for coronary angiography several years earlier than those in the lowest quartiles.
It is noteworthy that in this population, the IL-1 genotype effect on risk of CAD was more pronounced in patients above the median of hsCRP, a biomarker of inflammation generated secondary to cytokines such as IL-6 and IL-1. Similarly, the LPA gene contains an IL-6 response element(66) and patients with inflammatory disorders such as rheumatoid arthritis have elevated Lp(a) levels, which are reduced on treatment with the IL-6 receptor antagonist antibody tocilizumab(67,68). The CANTOS trial will be instrumental in testing whether inhibition of inflammatory responses leads to a lower rate of cardiovascular events. The data from this study suggests that patients with the IL-1(+) genotype and elevated OxPL/apoB and/or Lp(a) levels are a particularly high risk subset that may maximally benefit from therapies aimed at inhibiting IL-1β and IL-1α responses.
Limitations of this study include that patients were selected from a population referred for coronary angiography for clinical indications and thus the data may not be generalizable to broader populations. This study also included predominantly Caucasian patients whose IL-1 genotypes and genetic associations may differ in other ethnic groups and it will be important to study these associations in other populations.
In conclusion, this study demonstrates that the previously demonstrated contribution of OxPL/apoB and Lp(a) on angiographically documented CAD and CAD events is conditional on pro-inflammatory IL-1 genotypes. This novel paradigm links the etiology of atherogenesis attributed to OxPL and Lp(a) from genetics to clinical expression of CAD. If confirmed and validated in prospective populations, these findings may facilitate our understanding of atherogenesis and provide enhanced tools for diagnosis and treatment of cardiovascular disease.
METHODS
Study design
The study design has been described previously in detail(1). Briefly, 504 eligible, consecutive patients (>97% Caucasian), age 18 to 75, undergoing clinically indicated coronary angiography who consented for the study were recruited between June and December 1998. The study was prospectively designed to test the association of CAD with specific IL-1 genotype groups known to be associated with higher inflammatory responses. Patients with prior coronary revascularization and diabetes mellitus were excluded to avoid potential enrichment with cases that may have confounding etiological factors. The angiographic analysis was previously described in detail(1). The extent of angiographically documented CAD was quantified as follows: normal coronary arteries (smooth, with either no stenosis or a stenosis of <10 percent of the luminal diameter), mild disease (10–50% diameter stenosis (DS) in one or more coronary arteries or their major branches), or one vessel, two-vessel, or three-vessel disease, defined as DS> 50% of the luminal diameter in one, two, or three coronary arteries or their major branches. We focused some of our analyses on angiographically significant disease defined as DS>50%. For some of the analyses (Tables 2 and 3 and figures 1–3), we compared patients with no or mild disease to obstructive disease. Two patients had incomplete OxPL/apoB data and 3 patients incomplete IL-1 data, therefore 499 patients were available for the present analysis.
Four hundred sixty-six patients (92.5%) were contacted by a follow-up questionnaire or by telephone in September 2002 [median follow-up of 4.0 years (interquartile range, 3.9–4.2 years)]. The remaining 38 patients either refused to participate in the follow-up (n=18) or could not be contacted (n=20). The medical records of the patients who had an event were obtained and reviewed to ascertain the type of event or the cause of death. The follow-up events of these patients were described previously (2,3)and consisted of 20 deaths (6 cardiac), 14 myocardial infarctions, 26 coronary revascularizations (15 percutaneous intervention only, 9 coronary artery bypass surgery only, and 2 with both), and 10 strokes.
Laboratory analyses
Apolipoproteins B-100 and AI, Lp(a), total cholesterol, HDL cholesterol (HDL-C) and triglycerides were measured with commercially available kits. LDL cholesterol (LDL-C) was estimated using the Friedewald formula. High sensitivity CRP (hsCRP) (lower range 0.15 mg/L) was measured as previously described(1). The content of OxPL per apoB-100 particle (OxPL/apoB) and Lp(a) were measured as previously described(1,4).
Genetic analyses
DNA was extracted and genotyping was performed at the Division of Genomic Medicine, University of Sheffield, UK. All genetic analyses were performed blinded to clinical and angiographic data. Genotyping was performed by a 5′ nuclease assay (Taqman™; Hoffman-LaRoche, Inc.) based on the 5′ nuclease activity of Taq Polymerase and the detection by FRET of the cleavage of two probes, designed to match and hybridize to either allele copy during PCR. The probe and primer sequences and cycling conditions have been previously described(5). Single nucleotide polymorphisms (SNPs) were genotyped at two loci in the gene for IL-1β, IL1B (−511; C>T; rs16944) and IL1B(+3954; C>T; rs1143634); and at one locus in the gene for IL-1α, IL1A (+4845; G>T; rs17561)(6).
Approximately 20% of the samples were evaluated as duplicates, which were blinded to laboratory personnel to test reproducibility of the genotyping methods. There was 100% concordance between duplicate samples.
IL-1 composite genotype patterns used for association with biochemical and clinical parameters
We designed the study to evaluate the relationship between CAD and IL-1 genotypes that are associated with differential expression of interleukin-1β (IL-1β). Four single-nucleotide polymorphisms (SNPs) in the promoter region of IL1B have been shown to be functional at the molecular level and operate in haplotype context to alter transcriptional activity of IL-1β(7). The functional IL1B SNPs define four predominant haplotypes that as pairs observed together account for significantly different clinical levels of IL-1β protein in tissue fluid samples(8). All possible composite genotype combinations of the 3 SNPs used in the study provided an efficient tagging of the composite genotypes resulting from combinations of the functional IL1B promoter haplotypes that define differential expression of IL-1β protein(8). Table 1 shows the composite genotypes in this study that defined the IL-1(+) group, which are associated with over-expression of IL-1β, and the IL-1(−) group, which are the composite genotypes that have not been associated with over-expression of IL-1β. The IL-1(+) and IL-1(−) groups were defined and published (Francis S.E., Crossman D.C., Duff G.W., Kornman K. S., Stephenson K. 2003. Diagnostics for cardiovascular disorders. United States Patent # 6,524,795; Filed November 1, 1999; granted February 25, 2003) prior to data analysis.
Acknowledgments
This study was supported by NIH grants HL055798 and HL088093 and a grant from Interleukin Genetics, Inc. to the MAYO Clinic Foundation.
Footnotes
Disclosure: Drs. Tsimikas and Witztum are named as co-inventors of patents owned by the University of California, San Diego on the clinical use of oxidation-specific antibodies and interleukin genotypes. Dr. Tsimikas has received investigator-initiated grants from Pfizer and is a consultant to ISIS, Quest, Sanofi and Genzyme, Inc. Dr. Witztum is a consultant to ISIS, Quest and Regulus, Inc. Dr. Kornman is an officer and shareholder of Interleukin Genetics, which has patents covering the use of IL-1 genetic variations in multiple diseases. Dr. Duff is a scientific advisor to Interleukin Genetics and Drs. Rogus and Huttner were former employees of Interleukin Genetics.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Miller YI, Choi SH, Wiesner P, et al. Oxidation-specific epitopes are danger-associated molecular patterns recognized by pattern recognition receptors of innate immunity. Circ Res. 2011;108:235–48. doi: 10.1161/CIRCRESAHA.110.223875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Duff GW. Peptide regulatory factors in non-malignant disease. Lancet. 1989;1:1432–5. doi: 10.1016/s0140-6736(89)90134-7. [DOI] [PubMed] [Google Scholar]
- 3.Fearon WF, Fearon DT. Inflammation and cardiovascular disease: Role of the interleukin-1 receptor antagonist. Circulation. 2008;117:2577–2579. doi: 10.1161/CIRCULATIONAHA.108.772491. [DOI] [PubMed] [Google Scholar]
- 4.Dinarello CA, Simon A, van der Meer JW. Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nature reviews Drug discovery. 2012;11:633–52. doi: 10.1038/nrd3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duff GW. Influence of genetics on disease susceptibility and progression. Nutrition reviews. 2007;65:S177–81. doi: 10.1111/j.1753-4887.2007.tb00359.x. [DOI] [PubMed] [Google Scholar]
- 6.Chen H, Wilkins LM, Aziz N, et al. Single nucleotide polymorphisms in the human interleukin-1B gene affect transcription according to haplotype context. Hum Mol Genet. 2006;15:519–529. doi: 10.1093/hmg/ddi469. [DOI] [PubMed] [Google Scholar]
- 7.Berger P, McConnell JP, Nunn M, et al. C-reactive protein levels are influenced by common IL-1 gene variations. Cytokine. 2002;17:171–174. doi: 10.1006/cyto.2001.0974. [DOI] [PubMed] [Google Scholar]
- 8.Iacoviello L, Di Castelnuovo A, Gattone M, et al. Polymorphisms of the interleukin-1beta gene affect the risk of myocardial infarction and ischemic stroke at young age and the response of mononuclear cells to stimulation in vitro. Arterioscler Thromb Vasc Biol. 2005;25:222–227. doi: 10.1161/01.ATV.0000150039.60906.02. [DOI] [PubMed] [Google Scholar]
- 9.Reiner AP, Wurfel MM, Lange LA, et al. Polymorphisms of the IL1-receptor antagonist gene (IL1RN) are associated with multiple markers of systemic inflammation. Arterioscler Thromb Vasc Biol. 2008;28:1407–12. doi: 10.1161/ATVBAHA.108.167437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rogus J, Beck JD, Offenbacher S, et al. IL1B gene promoter haplotype pairs predict clinical levels of interleukin-1beta and C-reactive protein. Hum Genet. 2008;123:387–98. doi: 10.1007/s00439-008-0488-6. [DOI] [PubMed] [Google Scholar]
- 11.Stegger JG, Schmidt EB, Tjønneland A, et al. Single nucleotide polymorphisms in IL1B and the risk of acute coronary syndrome: A Danish case-cohort study. PloS one. 2012;7:e36829. doi: 10.1371/journal.pone.0036829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Gaetano M, Quacquaruccio G, Di Castelnuovo A, et al. Haplotypes and haplotype-pairs of IL-1 beta and IL-6 genes and risk of non fatal myocardial infarction in the Western New York Acute MI Study. Thromb Haem. 2011;106:1231–3. doi: 10.1160/TH11-06-0377. [DOI] [PubMed] [Google Scholar]
- 13.Fragoso JM, Delgadillo H, Llorente L, et al. Interleukin 1 receptor antagonist polymorphisms are associated with the risk of developing acute coronary syndrome in Mexicans. Immunol Lett. 2010;133:106–11. doi: 10.1016/j.imlet.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 14.van Minkelen R, Wettinger SB, de Visser MC, et al. Haplotypes of the interleukin-1 receptor antagonist gene, interleukin-1 receptor antagonist mRNA levels and the risk of myocardial infarction. Atherosclerosis. 2009;203:201–5. doi: 10.1016/j.atherosclerosis.2008.06.029. [DOI] [PubMed] [Google Scholar]
- 15.Latella MC, de Gaetano M, Di Castelnuovo A, et al. Interleukin 1 gene cluster, myocardial infarction at young age and inflammatory response of human mononuclear cells. Immunol Invest. 2009;38:203–19. doi: 10.1080/08820130902766142. [DOI] [PubMed] [Google Scholar]
- 16.Bis JC, Heckbert SR, Smith NL, et al. Variation in inflammation-related genes and risk of incident nonfatal myocardial infarction or ischemic stroke. Atherosclerosis. 2008;198:166–73. doi: 10.1016/j.atherosclerosis.2007.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Olofsson PS, Sheikine Y, Jatta K, et al. A functional interleukin-1 receptor antagonist polymorphism influences atherosclerosis development. The interleukin-1beta:interleukin-1 receptor antagonist balance in atherosclerosis. Circulation journal. 2009;73:1531–6. doi: 10.1253/circj.cj-08-1150. [DOI] [PubMed] [Google Scholar]
- 18.Waehre T, Yndestad A, Smith C, et al. Increased expression of interleukin-1 in coronary artery disease with downregulatory effects of HMG-CoA reductase inhibitors. Circulation. 2004;109:1966–72. doi: 10.1161/01.CIR.0000125700.33637.B1. [DOI] [PubMed] [Google Scholar]
- 19.Galea J, Armstrong J, Gadsdon P, Holden H, Francis SE, Holt CM. Interleukin-1 beta in coronary arteries of patients with ischemic heart disease. Arterioscler Thromb Vasc Biol. 1996;16:1000–1006. doi: 10.1161/01.atv.16.8.1000. [DOI] [PubMed] [Google Scholar]
- 20.Francis SE, Camp NJ, Dewberry RM, et al. Interleukin-1 receptor antagonist gene polymorphism and coronary artery disease. Circulation. 1999;99:861–866. doi: 10.1161/01.cir.99.7.861. [DOI] [PubMed] [Google Scholar]
- 21.Tuttolomondo A, Di Raimondo D, Forte GI, et al. Single nucleotide polymorphisms (SNPs) of pro-inflammatory/anti-inflammatory and thrombotic/fibrinolytic genes in patients with acute ischemic stroke in relation to TOAST subtype. Cytokine. 2012;58:398–405. doi: 10.1016/j.cyto.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 22.Um JY, Moon KS, Lee KM, et al. Association of interleukin-1 alpha gene polymorphism with cerebral infarction. Brain Res Mol Brain Res. 2003;115:50–54. doi: 10.1016/s0169-328x(03)00179-7. [DOI] [PubMed] [Google Scholar]
- 23.Dziedzic T, Slowik A, Pera J, Szczudlik A. Interleukin 1 beta polymorphism (−511) and risk of stroke due to small vessel disease. Cerebrovasc Dis. 2005;20:299–303. doi: 10.1159/000087928. [DOI] [PubMed] [Google Scholar]
- 24.Kastrati A, Koch W, Berger PB, et al. Protective role against restenosis from an interleukin-1 receptor antagonist gene polymorphism in patients treated with coronary stenting. J Am Coll Cardiol. 2000;36:2168–73. doi: 10.1016/s0735-1097(00)01014-7. [DOI] [PubMed] [Google Scholar]
- 25.van Minkelen R, de Visser MCH, Houwing-Duistermaat JJ, Vos HL, Bertina RM, Rosendaal FR. Haplotypes of IL1B, IL1RN, IL1R1, and IL1R2 and the Risk of Venous Thrombosis. Arterioscler Thromb Vasc Biol. 2007;27:1486–1491. doi: 10.1161/ATVBAHA.107.140384. [DOI] [PubMed] [Google Scholar]
- 26.Ridker PM, Thuren T, Zalewski A, Libby P. Interleukin-1 beta inhibition and the prevention of recurrent cardiovascular events: rationale and design of the Canakinumab Anti-inflammatory Thrombosis Outcomes Study (CANTOS) Am Heart J. 2011;162:597–605. doi: 10.1016/j.ahj.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 27.Lee S, Birukov KG, Romanoski CE, Springstead JR, Lusis AJ, Berliner JA. Role of phospholipid oxidation products in atherosclerosis. Circ Res. 2012;111:778–799. doi: 10.1161/CIRCRESAHA.111.256859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Dijk RA, Kolodgie F, Ravandi A, et al. Differential expression of oxidation-specific epitopes and apolipoprotein(a) in progressing and ruptured human coronary and carotid atherosclerotic lesions. J Lipid Res. 2012;53:2773–90. doi: 10.1194/jlr.P030890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bertoia ML, Pai JK, Lee JH, et al. Oxidation-specific biomarkers and risk of peripheral artery disease. J Am Coll Cardiol. 2013;61:2169–79. doi: 10.1016/j.jacc.2013.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsimikas S, Bergmark C, Beyer RW, et al. Temporal increases in plasma markers of oxidized low-density lipoprotein strongly reflect the presence of acute coronary syndromes. J Am Coll Cardiol. 2003;41:360–70. doi: 10.1016/s0735-1097(02)02769-9. [DOI] [PubMed] [Google Scholar]
- 31.Tsimikas S, Lau HK, Han KR, et al. Percutaneous coronary intervention results in acute increases in oxidized phospholipids and lipoprotein(a): short-term and long-term immunologic responses to oxidized low-density lipoprotein. Circulation. 2004;109:3164–70. doi: 10.1161/01.CIR.0000130844.01174.55. [DOI] [PubMed] [Google Scholar]
- 32.Kiechl S, Willeit J, Mayr M, et al. Oxidized phospholipids, lipoprotein(a), lipoprotein-associated phospholipase A2 activity, and 10-year cardiovascular outcomes: prospective results from the Bruneck study. Arterioscler Thromb Vasc Biol. 2007;27:1788–95. doi: 10.1161/ATVBAHA.107.145805. [DOI] [PubMed] [Google Scholar]
- 33.Tsimikas S, Willeit P, Willeit J, et al. Oxidation-specific biomarkers, prospective 15-year cardiovascular and stroke outcomes, and net reclassification of cardiovascular events. J Am Coll Cardiol. 2012;60:2218–29. doi: 10.1016/j.jacc.2012.08.979. [DOI] [PubMed] [Google Scholar]
- 34.Tsimikas S, Mallat Z, Talmud PJ, et al. Oxidation-specific biomarkers, lipoprotein(a), and risk of fatal and nonfatal coronary events. J Am Coll Cardiol. 2010;56:946–55. doi: 10.1016/j.jacc.2010.04.048. [DOI] [PubMed] [Google Scholar]
- 35.Taleb A, Witztum JL, Tsimikas S. Oxidized phospholipids on apolipoprotein B-100 (OxPL/apoB) containing lipoproteins: A biomarker predicting cardiovascular disease and cardiovascular events. Biomarkers Med. 2011;5:673–694. doi: 10.2217/bmm.11.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leibundgut G, Arai K, Orsoni A, et al. Oxidized phospholipids are present on plasminogen, affect fibrinolysis, and increase following acute myocardial infarction. J Am Coll Cardiol. 2012;59:1426–1437. doi: 10.1016/j.jacc.2011.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tsimikas S, Brilakis ES, Miller ER, et al. Oxidized phospholipids, Lp(a) lipoprotein, and coronary artery disease. N Engl J Med. 2005;353:46–57. doi: 10.1056/NEJMoa043175. [DOI] [PubMed] [Google Scholar]
- 38.di Giovine FS, Takhsh E, Blakemore AI, Duff GW. Single base polymorphism at -511 in the human interleukin-1 beta gene (IL1 beta) Hum Mol Genet. 1992;1:450. doi: 10.1093/hmg/1.6.450. [DOI] [PubMed] [Google Scholar]
- 39.Shirodaria S, Smith J, McKay IJ, Kennett CN, Hughes FJ. Polymorphisms in the IL-1A gene are correlated with levels of interleukin-1 alpha protein in gingival crevicular fluid of teeth with severe periodontal disease. J Dent Res. 2000;79(11):1864–1869. doi: 10.1177/00220345000790110801. [DOI] [PubMed] [Google Scholar]
- 40.Hurme M, Santtila S. IL-1 receptor antagonist (IL-1Ra) plasma levels are co-ordinately regulated by both IL-1Ra and IL-1beta genes. Eur J Immunol. 1998;28:2598–2602. doi: 10.1002/(SICI)1521-4141(199808)28:08<2598::AID-IMMU2598>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 41.Kornman KS. Interleukin 1 genetics, inflammatory mechanisms, and nutrigenetic opportunities to modulate diseases of aging. Am J Clin Nutr. 2006;83:475S–483S. doi: 10.1093/ajcn/83.2.475S. [DOI] [PubMed] [Google Scholar]
- 42.Barber MD, Powell JJ, Lynch SF, Fearon KC, Ross JA. A polymorphism of the interleukin-1 beta gene influences survival in pancreatic cancer. Br J Cancer. 2000;83:1443–1447. doi: 10.1054/bjoc.2000.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.D’Aiuto F, Casas JP, Shah T, Humphries SE, Hingorani AD, Tonetti MS. C-reactive protein (+1444C>T) polymorphism influences CRP response following a moderate inflammatory stimulus. Atherosclerosis. 2005;179:413–417. doi: 10.1016/j.atherosclerosis.2004.10.036. [DOI] [PubMed] [Google Scholar]
- 44.Vilaichone RK, Mahachai V, Tumwasorn S, Wu JY, Graham DY, Yamaoka Y. Gastric mucosal cytokine levels in relation to host interleukin-1 polymorphisms and Helicobacter pyloricagA genotype. Scand J Gastroenterol. 2005;40(5):530–539. doi: 10.1080/00365520510012299. [DOI] [PubMed] [Google Scholar]
- 45.Hwang IR, Kodama T, Kikuchi S, et al. Effect of interleukin 1 polymorphisms on gastric mucosal interleukin 1beta production in Helicobacter pylori infection. Gastroenterology. 2002;123(6):1793–1803. doi: 10.1053/gast.2002.37043. [DOI] [PubMed] [Google Scholar]
- 46.Carrol ED, Payton A, Payne D, et al. The IL1RN promoter rs4251961 correlates with IL-1 receptor antagonist concentrations in human infection and is differentially regulated by GATA-1. J Immunol. 2011;186:2329–35. doi: 10.4049/jimmunol.1002402. [DOI] [PubMed] [Google Scholar]
- 47.Rafiq S, Stevens K, Hurst AJ, et al. Common genetic variation in the gene encoding interleukin-1-receptor antagonist (IL-1RA) is associated with altered circulating IL-1RA levels. Genes Immun. 2007;8:344–51. doi: 10.1038/sj.gene.6364393. [DOI] [PubMed] [Google Scholar]
- 48.Olsson S, Holmegaard L, Jood K, et al. Genetic variation within the interleukin-1 gene cluster and ischemic stroke. Stroke. 2012;43:2278–82. doi: 10.1161/STROKEAHA.111.647446. [DOI] [PubMed] [Google Scholar]
- 49.Dewberry R, Holden H, Crossman D, Francis S. Interleukin-1 receptor antagonist expression in human endothelial cells and atherosclerosis. Arterioscler Thromb Vasc Biol. 2000;20:2394–400. doi: 10.1161/01.atv.20.11.2394. [DOI] [PubMed] [Google Scholar]
- 50.Moerman-Herzog AM, Barger SW. A polymorphism in the upstream regulatory region of the interleukin-1alpha gene confers differential binding by transcription factors of the AP-1 family. Life Sci. 2012;90:975–9. doi: 10.1016/j.lfs.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shirodaria S, Smith J, McKay IJ, Kennett CN, Hughes FJ. Polymorphisms in the IL-1A gene are correlated with levels of interleukin-1alpha protein in gingival crevicular fluid of teeth with severe periodontal disease. J Dent Res. 2000;79:1864–9. doi: 10.1177/00220345000790110801. [DOI] [PubMed] [Google Scholar]
- 52.Nicklin MJ, Hughes DE, Barton JL, Ure JM, Duff GW. Arterial inflammation in mice lacking the interleukin 1 receptor antagonist gene. J Exp Med. 2000;191:303–312. doi: 10.1084/jem.191.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shepherd J, Nicklin MJ. Elastic-vessel arteritis in interleukin-1 receptor antagonist-deficient mice involves effector Th1 cells and requires interleukin-1 receptor. Circulation. 2005;111:3135–3140. doi: 10.1161/CIRCULATIONAHA.104.519132. [DOI] [PubMed] [Google Scholar]
- 54.Kirii H, Niwa T, Yamada Y, et al. Lack of interleukin-1beta decreases the severity of atherosclerosis in ApoE-deficient mice. Arterioscler Thromb Vasc Biol. 2003;23:656–660. doi: 10.1161/01.ATV.0000064374.15232.C3. [DOI] [PubMed] [Google Scholar]
- 55.Elhage R, Maret A, Pieraggi MT, Thiers JC, Arnal JF, Bayard F. Differential effects of interleukin-1 receptor antagonist and tumor necrosis factor binding protein on fatty-streak formation in apolipoprotein E-deficient mice. Circulation. 1998;97:242–244. doi: 10.1161/01.cir.97.3.242. [DOI] [PubMed] [Google Scholar]
- 56.Isoda K, Sawada S, Ishigami N, et al. Lack of interleukin-1 receptor antagonist modulates plaque composition in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2004;24:1068–1073. doi: 10.1161/01.ATV.0000127025.48140.a3. [DOI] [PubMed] [Google Scholar]
- 57.Ikonomidis I, Lekakis JP, Nikolaou M, et al. Inhibition of interleukin-1 by anakinra improves vascular and left ventricular function in patients with rheumatoid arthritis. Circulation. 2008;117:2662–2669. [Google Scholar]
- 58.Hung YC, Hong MY, Huang GS. Cholesterol loading augments oxidative stress in macrophages. FEBS Lett. 2006;580:849–861. doi: 10.1016/j.febslet.2005.12.102. [DOI] [PubMed] [Google Scholar]
- 59.Deng DX, Spin JM, Tsalenko A, et al. Molecular signatures determining coronary artery and saphenous vein smooth muscle cell phenotypes: distinct responses to stimuli. Arterioscler Thromb Vasc Biol. 2006;26:1058–1065. doi: 10.1161/01.ATV.0000208185.16371.97. [DOI] [PubMed] [Google Scholar]
- 60.Romanoski CE, Che N, Yin F, et al. Network for activation of human endothelial cells by oxidized phospholipids. Circ Res. 2011;109:E27–U52. doi: 10.1161/CIRCRESAHA.111.241869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kadl A, Sharma PR, Chen W, et al. Oxidized phospholipid-induced inflammation is mediated by Toll-like receptor 2. Free Rad Biol Med. 2011;51:1903–1909. doi: 10.1016/j.freeradbiomed.2011.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Subbanagounder G, Wong JW, Lee H, et al. Epoxyisoprostane and epoxycyclopentenone phospholipids regulate monocyte chemotactic protein-1 and interleukin-8 synthesis. Formation of these oxidized phospholipids in response to interleukin-1beta. J Biol Chem. 2002;277:7271–81. doi: 10.1074/jbc.M107602200. [DOI] [PubMed] [Google Scholar]
- 63.Ray KK, Camp NJ, Bennett CE, Francis SE, Crossman DC. Genetic variation at the interleukin-1 locus is a determinant of changes in soluble endothelial factors in patients with acute coronary syndromes. Clin Sci (Lond) 2002;103:303–10. doi: 10.1042/cs1030303. [DOI] [PubMed] [Google Scholar]
- 64.Carter KW, Hung J, Powell BL, et al. Association of Interleukin-1 gene polymorphisms with central obesity and metabolic syndrome in a coronary heart disease population. Hum Genet. 2008;124:199–206. doi: 10.1007/s00439-008-0540-6. [DOI] [PubMed] [Google Scholar]
- 65.Leibundgut G, Scipione C, Yin H, et al. Determinants of binding of oxidized phospholipids on apolipoprotein (a) and lipoprotein (a) J Lipid Res. 2013;54:2815–30. doi: 10.1194/jlr.M040733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wade DP, Clarke JG, Lindahl GE, et al. 5′ control regions of the apolipoprotein(a) gene and members of the related plasminogen gene family. PNAS. 1993;90:1369–1373. doi: 10.1073/pnas.90.4.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Berthold HK, Laudes M, Krone W, Gouni-Berthold I. Association between the interleukin-6 promoter polymorphism −174G/C and serum lipoprotein(a) concentrations in humans. PLoS One. 2011;6:e24719. doi: 10.1371/journal.pone.0024719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schultz O, Oberhauser F, Saech J, et al. Effects of inhibition of interleukin-6 signalling on insulin sensitivity and lipoprotein (a) levels in human subjects with rheumatoid diseases. PLoS One. 2010;5:e14328. doi: 10.1371/journal.pone.0014328. [DOI] [PMC free article] [PubMed] [Google Scholar]
References
- 1.Tsimikas S, Brilakis ES, Miller ER, et al. Oxidized phospholipids, Lp(a) lipoprotein, and coronary artery disease. N Engl J Med. 2005;353:46–57. doi: 10.1056/NEJMoa043175. [DOI] [PubMed] [Google Scholar]
- 2.Brilakis ES, Khera A, McGuire DK, et al. Influence of race and sex on lipoproteinassociated phospholipase A2 levels: Observations from the Dallas Heart Study. Atherosclerosis. 2007 doi: 10.1016/j.atherosclerosis.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tsimikas S, Brilakis ES, Lennon RJ, et al. Relationship of IgG and IgM autoantibodies to oxidized low density lipoprotein with coronary artery disease and cardiovascular events. J Lipid Res. 2007;48:425–33. doi: 10.1194/jlr.M600361-JLR200. [DOI] [PubMed] [Google Scholar]
- 4.Taleb A, Witztum JL, Tsimikas S. Oxidized phospholipids on apolipoprotein B-100 (OxPL/apoB) containing lipoproteins: A biomarker predicting cardiovascular disease and cardiovascular events. Biomarkers Med. 2011;5:673–694. doi: 10.2217/bmm.11.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.di Giovine FS, Camp NJ, Cox A, et al. Detection and population analysis of IL-1 and TNF gene polymorhpisms in Cytokine Molecular Biology--A Practical Approach. In: Blackwell F, editor. Cytokine Molecular Biology. London: Oxford University Press; 2000. pp. 21–46. [Google Scholar]
- 6.di Giovine FS, Takhsh E, Blakemore AI, Duff GW. Single base polymorphism at −511 in the human interleukin-1 beta gene (IL1 beta) Hum Mol Genet. 1992;1:450. doi: 10.1093/hmg/1.6.450. [DOI] [PubMed] [Google Scholar]
- 7.Chen H, Wilkins LM, Aziz N, et al. Single nucleotide polymorphisms in the human interleukin-1B gene affect transcription according to haplotype context. Hum Mol Genet. 2006;15:519–529. doi: 10.1093/hmg/ddi469. [DOI] [PubMed] [Google Scholar]
- 8.Rogus J, Beck JD, Offenbacher S, et al. IL1B gene promoter haplotype pairs predict clinical levels of interleukin-1beta and C-reactive protein. Hum Genet. 2008;123:387–98. doi: 10.1007/s00439-008-0488-6. [DOI] [PubMed] [Google Scholar]