Abstract
Objective
The physiology of the weight reduced (WR) state suggests that pharmacologic agents affecting energy homeostasis may have greater efficacy in WR individuals. Our aim was to establish a protocol that allows for evaluation of efficacy of weight maintenance agents and to assess the effectiveness of AZD2820, a novel melanocortin 4 receptor agonist in such a paradigm.
Design and Methods
MC4R agonist was administered in stratified doses to mice who were either fed high fat diet ad libitum (AL) throughout the study; or stabilized at a 20% reduced body weight (BW), administered the drug for four weeks, and thereafter released from caloric restriction while continuing to receive the drug (WR).
Results
After release of WR mice to AL feeding, the high-dose group (53.4nmol/day) regained 12.4% less BW than their vehicle-treated controls. In WR mice, 10.8nmol/day of the agonist was sufficient to maintain these animals at 95.1% of initial BW vs 53.4nmol/day required to maintain the BW of AL animals (94.5%).
Conclusions
In the WR state, the MC4R agonist was comparably efficacious to a 5-fold higher dose in the AL state. This protocol provides a model for evaluating the mechanisms and quantitative efficacy of weight-maintenance strategies and agents.
Introduction
Using diet and exercise many obese individuals are capable of losing weight but are unable to maintain this reduced body weight (BW) for extended periods of time [1]. Resistance to the maintenance of a weight reduced (WR) state results from the coordinate action of systems regulating energy intake and output to favor weight regain. WR individuals and rodents are hyperphagic and their energy expenditure is decreased beyond what would be predicted for their lower body mass and composition [2–4]. Changes in neuroendocrine axis, decreased circulating concentrations of leptin and bioactive thyroid hormones, and decreased sympathetic nervous system tone and increased parasympathetic nervous system tone promote the BW regain [2]. In rodents, weight reduction decreases the proportion of excitatory synapses on anorexigenic POMC neurons in the arcuate nucleus of the hypothalamus [3]. Behavioral studies indicate that WR humans are hungrier, have decreased perception of how many calories they have eaten and have reduced satiation [5]. These phenotypes are associated with characteristic changes in fMRI activity in regions of the brain mediating homeostatic and hedonic responses to food [6, 7].
Most of the physiological and neuro-functional changes that accompany maintenance of a reduced BW in either lean or obese individuals can be reversed with low doses of exogenous leptin sufficient to return the circulating leptin levels to those present at the usual BW [6–9]. These responses are similar to those seen in patients with congenital leptin deficiency [10–12]. Administration of exogenous leptin to obese or never-obese humans at their usual weights - even at doses sufficient to raise circulating leptin concentrations to supraphysiological levels - has modest or no effect on BW [13]. Similar to the effects of leptin, WR individuals may have increased sensitivity to other relevant molecules, influencing strategies for the design and application of weight-maintaining pharmacologic agents.
The melanocortin (MC) system is a downstream target of leptin [14]. The MC4 receptor (MC4R) is a G-protein-coupled receptor expressed primarily in paraventricular hypothalamus (PVH), dorsal motor nucleus of the vagus (DMV), thalamus and hippocampus [15, 16]. In addition, MC4Rs are also found outside of the CNS including in the nodose and dorsal root ganglia [17, 18]. Two major neuronal populations in the hypothalamus, the anorexigenic proopiomelanocortin (POMC) and orexigenic Agouti-related protein (AgRP)/Neuropeptide Y (NPY)/GABA neurons, are activated and inhibited by leptin, respectively, to reciprocally mediate energy balance through the MC4 receptors [14]. α-MSH, one of the peptides released from POMC neurons, is an agonist of MC4R whereas the AgRP is an inverse agonist of this receptor. Mc4r-null mice are obese due to both increased food intake and reduced energy expenditure [19]. Mice overexpressing α-MSH display increased energy expenditure [20]. Administration of MC4R agonists to mice or rats promotes weight loss by decreasing food intake and/or increasing energy expenditure, identifying MC4R as a potential pharmacological target [21, 22].
Physiologically, the weight-reduced state is one of relative leptin deficiency in which the expression of hypothalamic AgRP and NPY neuropeptides is increased [23, 24] and Pomc expression is decreased [25]. These changes can be largely normalized with administration of exogenous leptin [26]. In the WR state, treatment with MC4R agonist might be efficacious as well. Leptin has little or no efficacy when administered at a usual BW [13]; MC4R agonist might also be expected to have greater efficacy in the weight-reduced state.
In this study we examined the long term effects of a potent and selective cyclic peptide MC4R partial agonist (AZD2820; MC4R: EC50 1nM in cAMP generation assay, 38% efficacy vs. NDP-α-MSH, and MC3R: binding Ki 9nM, no agonist efficacy detected (AstraZeneca data on file) [27] on induction of weight loss and on maintenance of reduced BW in diet induced obese (DIO) mice. We found that, in the weight-reduced state, the dose required for equal efficacy of AZD2820 with regard to body weight maintenance was decreased nearly 5 fold.
Methods
Animals
18 week old male mice C57BL/6J fed high fat diet (HFD; Research Diets, Inc. D12492i, 60% kcal from fat) starting at six weeks of age were obtained from Jackson Laboratory (Bar Harbor, ME). Throughout the study, animals were maintained at room ambient 22–24°C with a 12-h dark-light cycle (lights on at 0700h) in a pathogen-free barrier facility. The protocol was approved by the Columbia University Institutional Animal Care and Use Committee.
Study Design (schematized in Figure 1)
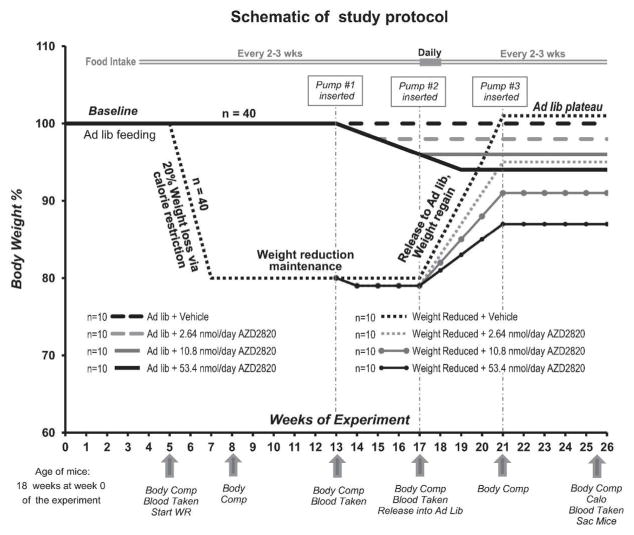
Figure 1.
Schematic of protocol to assess the effects of a novel MC4R agonist (AZD2820) on maintenance of reduced body weight in DIO mice. Timepoints of physiological measurements and pharmacological treatment are indicated by arrows. WR - weight reduction, Body Comp - Body Composition, Calo - Calorimetry, Sac – Sacrifice, Time 0 – mice arrived at the Columbia facility and began the 5-week acclimatization period.
After the 5-week acclimatization period, mice were randomly assigned to one of the following groups: 1.) Maintained on HFD ad lib (AL group; n=40) throughout the study or 2.) Weight reduced (WR group, n=40) to 80% of their initial weight by caloric restriction (CR). Eight weeks after the WR group entered the CR phase, both AL and WR groups were further randomized to receive either low (2.64 nmol/day), intermediate (10.8 nmol/day) or high (53.4 nmol/day) dose of AZD2820 dissolved in 5% mannitol solution or vehicle (5% mannitol solution; n=20 per treatment; n=10 WR and n=10 AL) via implanted subcutaneous mini-osmotic pumps (Alzet Model 2004) to allow for delivery at a steady infusion flow rate (0.25 μl/h). The pumps were replaced at 4 week intervals throughout the experiment for a total of 12 weeks of treatment. Four weeks following the initiation of drug administration (week 17; Fig. 1), WR mice were released from CR and returned to ad lib food access (weight re-gain phase). AL group continued ad lib HFD feeding. During the last week of drug administration (11–12 weeks following the onset of drug administration) mice were placed in metabolic cages for 72 hours to assess their energy expenditure (EE) and were then sacrificed immediately following removal from metabolic chambers.
Food intake was measured over 48 hours every 2–3 weeks in all AL mice throughout the study. The first week after release of WR mice from CR, food intake was measured daily (see Supplementary Methods).
Mini-pump implantation
Three mini-osmotic pump implantations (at 4 week intervals) were performed in each mouse over the 12 weeks of AZD2820 administration according to the manufacturer’s recommendations (see Supplementary Methods).
Body weight, body composition, and food intake
BW was measured (± 0.1 g) weekly in AL groups throughout the experiment and daily in the WR groups during CR phase using an Ohaus Scout Pro 200g scale (Nänikon Switzerland, 08:00–08:30h). Body composition was measured by time-domain-NMR (Minispec Analyst AD; Bruker Optics, Silberstreifen, Germany) [28] prior to the initiation of CR (week 5), before the insertion of each mini-pump (weeks 13, 17, and 21) and at the time of sacrifice (week 25). Food intake was recorded daily for the WR mice during the CR phase (week 5–17) and every 2–3 weeks for AL mice over 48 hours by weighing feeding baskets that were designed specifically to reduce the food spillage.
Serum or plasma assays
Blood was obtained by retro-orbital bleed following a 4.5-h fast at several time points [weeks 5, 13, 17, 24 and 25 (Fig. 1)].. The plasma concentrations of AZD2820 were determined after protein precipitation, by liquid chromatography with mass spectrometric detection (LLOQ 2 nmoles/L). Leptin was assayed using Quantikine ELISA kit (R&D Systems and insulin using the Crystal Chem Ultra Sensitive Mouse Insulin ELISA Kit (Crystal Chem). Glucose was measured at the same time as blood collection using the FreeStyle Lite Blood Glucose Monitoring System (Abbott Laboratories; see Supplementary Methods).
Indirect calorimetry
Energy expenditure and physical activity were measured with a LabMaster-CaloSys-Calorimetry System (TSE Systems, Bad Homburg, Germany) at the end of the study (week 25, immediately prior to sacrifice; see Supplementary Methods).
Results
Body weight and body composition
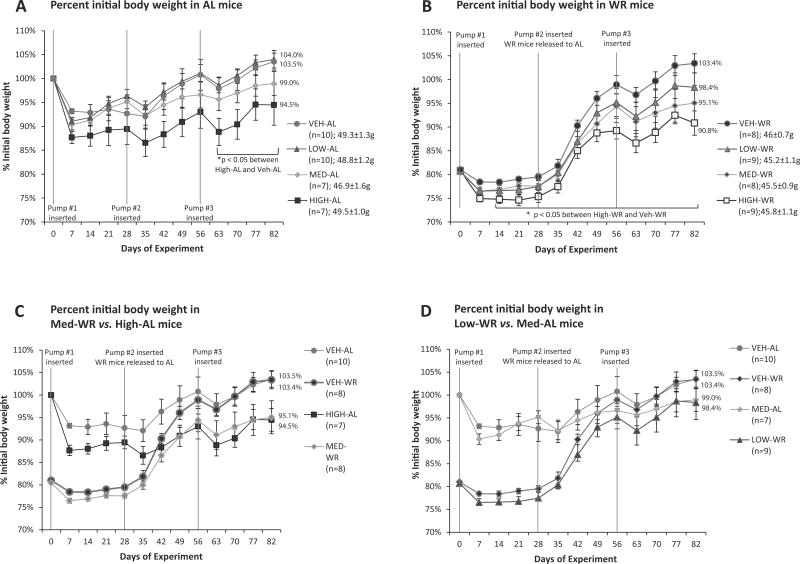
Prior to food restriction, BW of all groups were equal (by design). 40 mice were maintained on HFD ad lib throughout the study (AL group) and their weights are reported as percent initial BW - defined as BW (g) on any given day divided by BW (g) on day 0 of the experiment (when the first mini-pump was implanted; Fig. 2A). 40 mice fed a HFD were calorically restricted to reduce their weights by 20%. Weights are reported as percent initial BW which in the case of WR mice is defined as BW (g) on any given day divided by BW on the day prior to start of the calorie restriction (CR) phase (on day 0 when the first mini-pump is implanted; Fig. 2B).
Figure 2.
Percent initial body weight post pump #1 implantation of mice fed a HFD throughout the study (A) and treated with MC4R agonist or vehicle and weight reduced by 20% prior to administration of the drug (B). Percent initial body weight in Medium-WR mice compared to High-AL group (C) and in Low-WR mice compared to Medium-AL group (D). Medium and low dose in WR mice is sufficient to maintain them at the same percentage initial body weight as AL mice treated with a high and medium dose of MC4R agonist, respectively.
Mean initial body weight (g) [SEM] is given for each group in the figure legend (A, B)
Nominal doses of AZD2820 - High : 53.4 nmol/day; Medium : 10.8 nmol/day; Low : 2.64 nmol/day. Each data point represents mean percent body weight for each group with SEM.
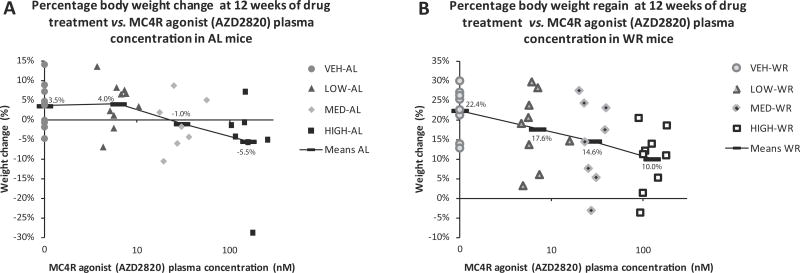
Mice maintained on the ad lib feeding regimen for the duration of the study lost weight when treated with high drug dose (53.4 nmol/day) compared to their vehicle-treated controls (5% mannitol). The high dose-AL lost 5.5 ± 4.2% vs. control-AL which gained 3.5 ± 1.8% (p= 0.042; Fig. 2A). Intermediate drug dose (medium; 10.8 nmol/day) treated mice also lost weight (1.0 ± 2.5%) but were not significantly different from the vehicle treated controls (Fig. 2A). There was a dose-response relationship regarding drug efficacy in WR mice (Fig. 2B). Eight weeks after WR mice were released from CR and allowed AL access to HFD, the high dose group regained 10.0 ± 2.6% of their BW since the beginning of the drug treatment compared to 22.4 ± 2.2% in the vehicle-treated control group (p= 0.0027; Fig. 2B). In WR mice, an intermediate dose of the agonist was sufficient to maintain these animals at 95.1 ± 3.7% of initial BW vs. 94.5 ± 4.2% in the high dose-AL mice (Fig. 2C). Similarly, low dose AZD2820 (2.64 nmol/day) in WR mice kept them at 98.4 ± 3.0% of initial BW compared to 99.0 ± 2.5% in the medium dose-AL mice (neither group was significantly lower than their respective controls, but both show a trend towards lower % initial BW; Fig. 2D). Whereas weight-reduced animals are sensitive to the agonist even in the lowest concentration rate (Fig. 3B), mice fed AL require an agonist concentration of >10 nM to produce weight loss (Fig. 3A). Thus, mice in the WR state were more sensitive to the MC4R agonist and required an ~5-fold lower dose of the agonist to achieve a response comparable to that required in the AL state (Fig. 2C–D).
Figure 3.
Percent body weight change (A) or regain (B) at 12 weeks of drug treatment plotted individually for each mouse against plasma concentration of MC4R agonist. AL (A) and WR (B) mice are plotted separately. Black bars are % BW change means for each group. Note, the drug concentration (x-axis) is plotted on a log scale.
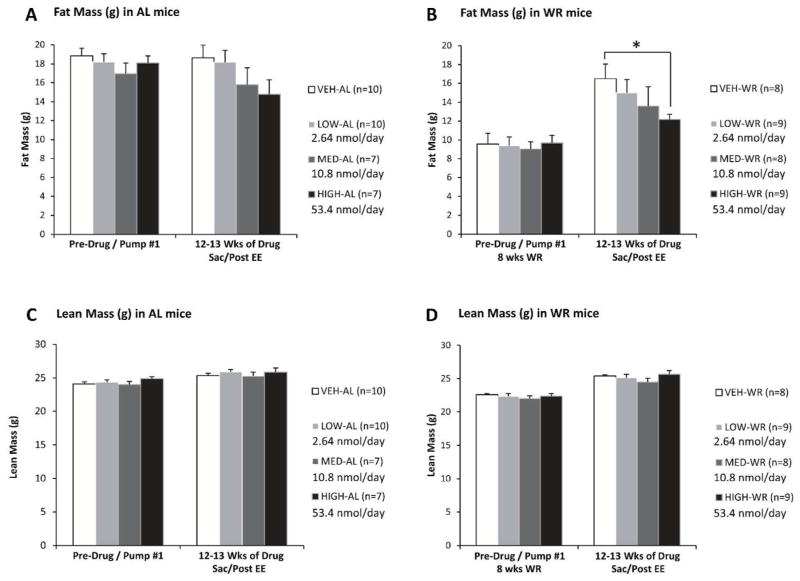
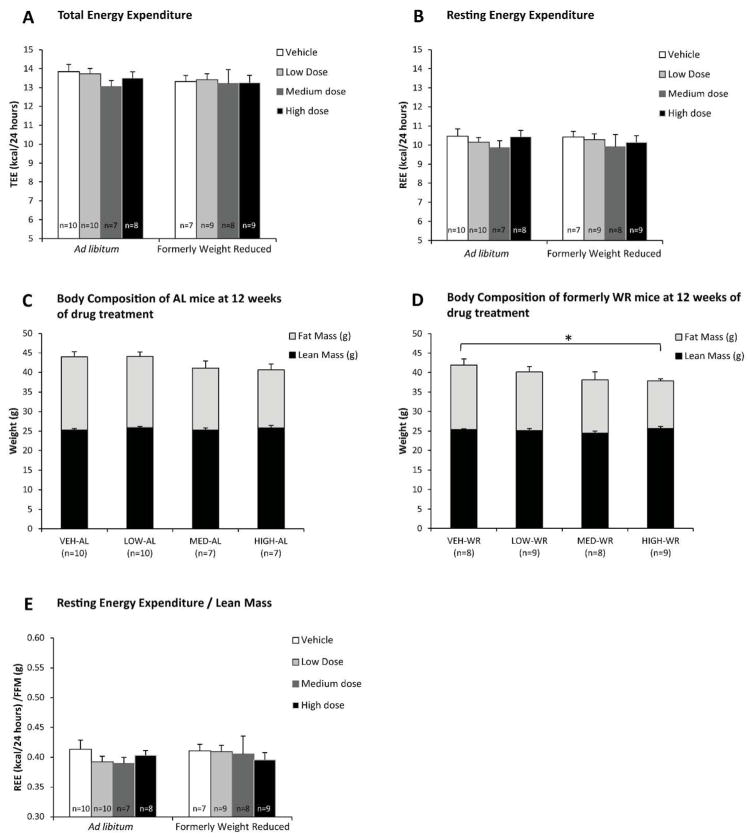
The change in BW in response to MC4R agonist administration was primarily accounted for by changes in fat mass. Following the MC4R agonist treatment, fat mass stratified according to changes in BW (Fig. 4A–B). The WR group treated with high drug dose had a significantly lower body fat mass after 8 weeks of drug administration compared to their vehicle-treated controls (at 8 weeks of drug: high-WR: 11.9 ± 0.6 g vs. control-WR: 15.8 ± 1.5 g; p= 0.030; data not shown; at 12 weeks of drug: high-WR: 12.2 ± 0.5 g vs. control-WR: 16.5 ± 1.6 g; p= 0.015; Fig. 4B). Mice fed AL and given high dose, as well as medium dose-treated mice (in both AL and formerly WR state), showed a trend towards reduced fat mass at 8 and 12 weeks of drug treatment (Fig. 4A). Lean mass of WR mice was lower than AL mice only during the CR phase; no difference was noted among any of the groups in response to AZD2820 treatment after the release of WR mice to AL feeding (Fig. 4C–D).
Figure 4.
Fat Mass (A, B) and lean mass (C, D) in mice maintained on HFD ad lib throughout the study (A, C) or weight reduced by 20% prior to drug administration (B, D) measured prior to and 12 weeks after MC4R agonist treatment. Formerly WR mice given high nominal drug dose (53.4 nmol/day) achieved a significantly lower body fat than the controls after an 8 week body weight regain period.
Nominal doses of AZD2820 - High : 53.4 nmol/day; Medium : 10.8 nmol/day; Low : 2.64 nmol/day. Each data point represents mean fat mass or lean (fat free mass) for each group with SEM. *P < 0.05 between vehicle and high dose treated groups by Student’s t-test.
Plasma hormones and glucose
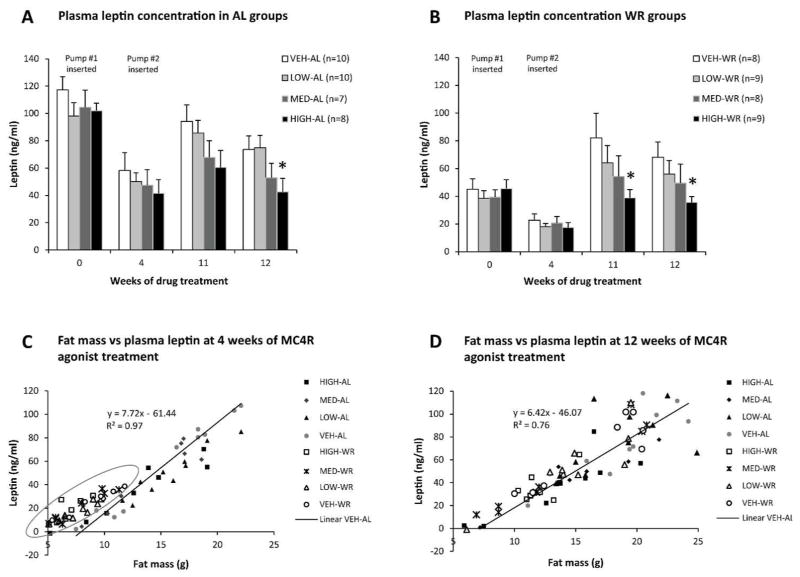
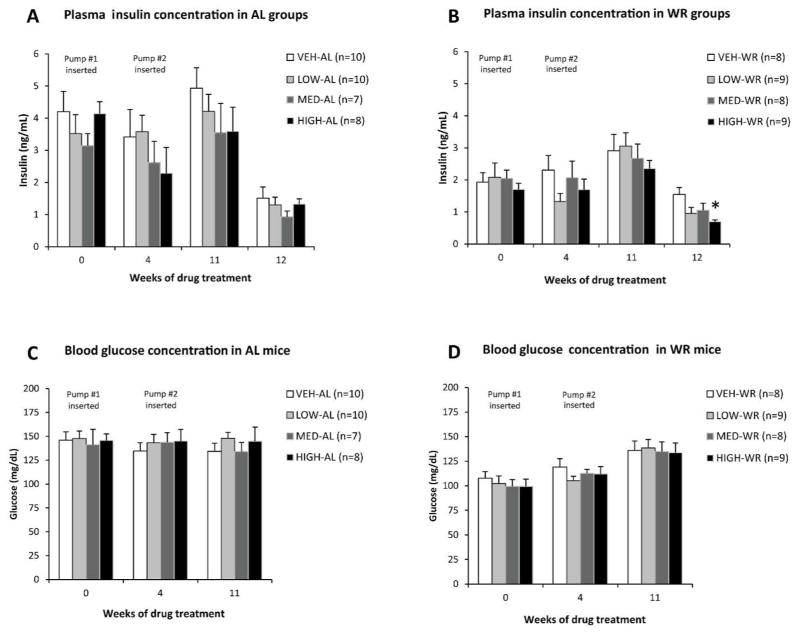
As expected, fasting plasma leptin and insulin levels maintained proportionality to fat mass following weight loss (Fig. 5–6). By the end of the study both AL and formerly WR mice treated with high-dose MC4R agonist had significantly lower circulating leptin levels compared to their respective vehicle-treated controls (Fig. 5A–B). Circulating insulin concentrations were significantly reduced in high dose-WR mice compared to WR controls (Fig. 6B). Medium dose-treated mice showed a trend towards a decrease in circulating leptin and insulin levels (Fig. 5A–B, 6A–B). At 4 weeks of drug treatment, while WR mice were still calorie restricted, their plasma leptin concentrations were increased relative to fat mass when compared to a regression of leptin vs. fat mass in the vehicle treated AL fed mice (Fig. 5C). This increase in leptin levels was not correlated with MC4R agonist dose, occurred only in WR animals, and was reversed after formerly WR mice had returned to AL feeding for 8 weeks (Fig. 5D). Fasting blood glucose levels were not different among groups throughout the study (Fig. 6C–D).
Figure 5.
Circulating leptin concentrations (ng/ml) in mice maintained on HFD ad lib throughout the study (A) or weight reduced by 20% prior to drug administration (B) measured at different timepoints during the study. As expected from the fat mass data, circulating leptin levels are lower in high dose treated animals (both AL and WR) after at least 11 weeks of treatment. Regression of circulating leptin (ng/ml) against fat mass (g) in all mice at 4 weeks (C) and 12 weeks (D) of MC4R agonist treatment. The circle denotes WR mice which have an increased plasma leptin concentrations relative to fat mass compared to the vehicle treated AL mice. This effect is independent of MC4R agonist treatment and is reversed after formerly WR mice had returned to AL feeding for 8 weeks (D).
Nominal doses of AZD2820 - High : 53.4 nmol/day; Medium : 10.8 nmol/day; Low : 2.64 nmol/day. Each data point represents mean value for each group with SEM. *P < 0.05 between vehicle and high dose treated groups by Student’s t-test.
Figure 6.
Circulating insulin (from plasma; A–B) and glucose (from venous tail blood; C–D) concentrations in mice maintained on HFD ad lib throughout the study (A, C) or weight reduced by 20% prior to drug administration (B, D) measured at different timepoints during the study.
Nominal doses of AZD2820 - High : 53.4 nmol/day; Medium : 10.8 nmol/day; Low : 2.64 nmol/day. Each data point represents mean value for each group with SEM. *P < 0.05 between vehicle and high dose treated groups by Student’s t-test.
Food Intake
No difference was found between any of the groups in the 24hr food intake measurements taken one week after the first mini-pump implantation and every 2–3 weeks thereafter (data not shown). Food intake was also measured more precisely at the end of the study when mice were housed in metabolic chambers for 48hr (Fig. 7). Similarly, there were no differences detected in food intake.
Figure 7.
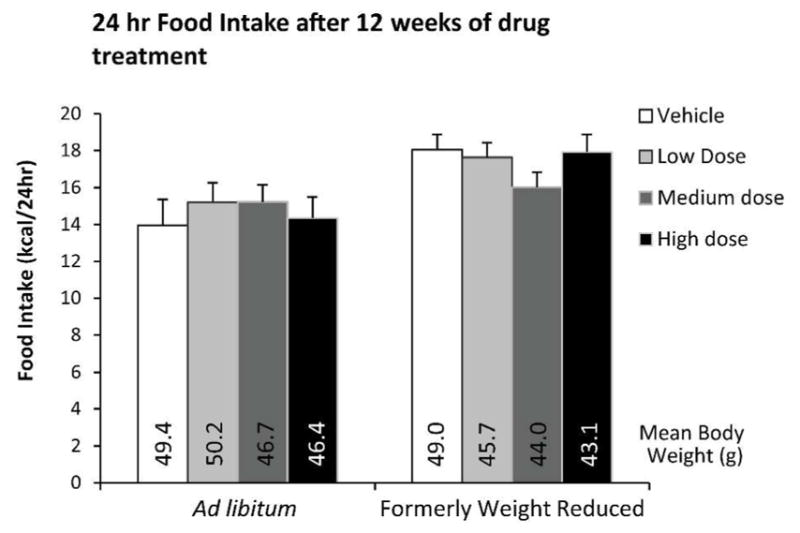
24 hour food intake in mice maintained on HFD diet ad lib throughout the study or weight reduced by 20% prior to drug administration measured at 12–13 weeks of drug treatment during calorimetry assessment (TSE LabMaster system). Mean body weight is provided for each group.
Nominal doses of AZD2820 - High : 53.4 nmol/day; Medium : 10.8 nmol/day; Low : 2.64 nmol/day. Each data point represents mean 24 hr food intake for each group with SEM.
Energy Expenditure
Immediately prior to sacrifice, mice were placed in metabolic chambers for assessment of energy expenditure. Absolute total and resting EE did not differ among treatment groups which did not differ significantly in lean body mass; fat mass was lower in the WR high dose treated mice compared to the controls (Fig. 8D) but their TEE and REE was the same (REE in high-WR: 10.1 ± 0.3 kcal/24h vs. control-WR: 10.4 ± 0.3 kcal/24h; TEE in high-WR 13.3 ± 0.4 kcal/24h vs. control-WR: 13.3 ± 0.3 kcal/24h; Fig. 8A–B). The limited number of mice in the control group (n=10) and the low variation in lean mass among all mice limited power to build a significant regression model to calculate the residual EE values for the treatment groups. Ratio of TEE and REE (Fig. 8E) to lean mass was not different among the treatment groups. However, the ratios of TEE and REE to BW were higher in high dose (WR and AL combined) treated animals (REE in high dose: 0.226 ± 0.005 kcal/g/24h vs. control: 0.209 ± 0.005 kcal/g/24h; p=0.02; TEE in high dose 0.294 ± 0.006 kcal/g/24h vs. control: 0.272 ± 0.006 kcal/g/24h; p=0.012; data not shown). Respiratory exchange ratio (RER or respiratory quotient, RQ) and physical activity were not different among treatment groups (data not shown).
Figure 8.
Absolute total (A) and resting (B) energy expenditure (kcal/24h) measured at 12–13 weeks of drug treatment and immediately prior to sacrifice by indirect calorimetry (TSE LabMaster system). No significant difference was found in absolute total and resting EE among treatment groups. Body Composition in AL mice (C) and formerly WR mice (D) at 12 weeks of drug treatment. Formerly WR mice treated with high dose of MC4R agonist have a significantly lower BW and fat mass but show the same TEE and REE. (E) Resting energy expenditure (kcal/24h) per unit of lean mass (g) measured at 12–13 weeks of drug treatment and immediately prior to sacrifice by indirect calorimetry (TSE LabMaster system). Nominal doses of AZD2820 - High : 53.4 nmol/day; Medium : 10.8 nmol/day; Low : 2.64 nmol/day. *P < 0.05 between vehicle and high dose treated groups by Student’s t-test.
Drug delivery verification
AZD2820 plasma concentrations measured after 4, 11, and 12–13 weeks of drug treatment were indistinguishable between AL and WR groups. AZD2820 treatment caused penile erection in all mice regardless of drug dose but was not observed in vehicle-treated mice. Every mouse was assessed visually three times throughout the study. There was no sign of drug tolerance as the penile erection persisted until the end of the study.
Discussion
The purpose of this study was to establish a study protocol to enable evaluation of the efficacy of weight maintenance agents, and to assess the effectiveness of a novel MC4R partial agonist (AZD2820) [27] in the maintenance of reduced BW. We found that in the WR state a 5× lower drug exposure is sufficient to maintain mice at a reduced weight versus the dose required to induce that degree of weight loss. Mice in the WR state are consequently more sensitive to the MC4R partial agonist.
We were unable to detect quantitatively significant differences in food intake or energy expenditure related to the AZD2820 administration. However, these studies were conducted relatively late in the treatment (11–12 weeks post start of agent). Also, subtle differences in food intake too small to measure (especially in mice) can accumulate into significant weight change. The ultimate differences in body mass and composition indicate that differences in energy balance must have occurred.
The effects of MC4R agonists on energy homeostasis have been assessed in animal studies [21, 29–31]. Food intake is initially suppressed - most strongly in the first day of treatment, and lasting for about a week - but then returns to control levels. In longer term studies [21, 22, 32], weight loss occurs initially as a result of decreased food intake but persistence of weight loss throughout the drug treatment period suggests that relative elevation of energy expenditure plays a role in sustaining the reduced BW. Hamilton, et al. showed that, in DIO rats, FI was significantly suppressed during the first week of continuous 28-day MTII (MC4R and MC3R full agonist) treatment, whereas EE was increased 3 days after the start of agonist administration [22]. An eight-week treatment with a novel MC4R agonist in DIO Rhesus macaques caused weight loss (13.5%), due to decreased food intake (~25–35%) for the first two weeks. EE was measured at baseline and at 8 weeks after drug treatment and showed a 14% increase suggesting that reduced food intake is an initial transient response, and that elevated EE may explain the persistence of reduced BW and contribute to the weight loss as well [32].
Using a paradigm similar to the one used here, DIO rats were either fed ad libitum throughout the study or fed calories reduced (CR) by 30% or 60% (versus ad lib) for 2 weeks, followed by 4 weeks of MTII treatment (at either low [0.3 mg/kg/day] or 10 fold higher dose) [33]. In the AL state, both MTII doses were equally efficacious in inducing weight loss; but in the CR groups (both 30% and 60% CR), the effectiveness of MTII in maintaining reduced BW was greater with the higher dose; the drug was more dynamically efficacious in the WR state. This is similar to the current study where AZD2820 was more efficacious in the WR state. The lack of difference in efficacy of low or high doses of the MTII in AL rats suggests that the low dose induced maximal weight loss in the AL state [33].
Circulating leptin concentrations were, as expected, significantly lower in all WR groups compared to control AL mice when measured at 4 weeks of AZD2820 treatment, while WR mice were still calorie restricted. Interestingly, leptin concentration per fat mass was increased in WR mice compared to AL control group, independent of drug dose, found only in WR animals, and reversed after mice had returned to ad lib feeding for 8 weeks. High dose-treated mice showed decreases in leptin and insulin plasma concentrations; these changes are likely a reflection of weight loss and not drug itself. Blood glucose concentration was only lower during the CR phase while WR mice were maintaining 80% of their initial BW. It was not affected by the MC4R agonist per se.
Therapies for obesity are focused primarily on inducing weight loss. Since weight regain, and not the inability to lose weight, is what defeats most efforts at obesity treatment [1], sustaining the weight-reduced state deserves more attention. Induction of weight loss is physiologically different than maintenance of a reduced BW [2, 8, 34]. The weight-reduced state in both humans and mice is characterized by hyperphagia and reduction in energy expenditure greater than predicted by lower body size [2, 4, 8], phenotypes that are largely reversed by administration of low “replacement” doses of leptin [6, 7]. These observations, congruent with those described in this report, suggest that the pharmacology of the weight-reduced state deserves greater attention as a therapeutic target in obesity treatment. Similarly to effects seen in WR individuals treated with low levels of leptin, MC4R agonists might be efficacious in maintenance of weight loss. However, MC4R agonists would be expected to be less effective due to increased levels of endogenous MC4R antagonist/inverse agonist (AgRP) which could potentially interfere with MC4R agonism.
Drugs found to be modestly efficacious in the induction of weight loss, may be more useful in the maintenance of reduced BW; those agents effective in producing weight loss, but problematic by virtue of off-target effects, may be helpful in maintaining reduced BW at much lower doses. This protocol could be used to examine agents for efficacy in maintenance of reduced BW.
Supplementary Material
What is already known about this subject?
Difficulty in maintaining a reduced body weight is a major clinical problem in the treatment of obesity;
The weight-reduced state is accompanied by decreased energy expenditure and increased hunger;
Administration of exogenous leptin can reverse these phenotypes.
What does this study add?
AZD2820, an MC4R agonist, is significantly more efficacious in sustaining than in inducing weight loss in DIO mice;
Weight- reduced DIO mice are more sensitive to the MC4R agonist than mice at initial body weight;
This study emphasizes the utility of a pharmacologic approach to the maintenance of reduced body weight and provides a prototype for evaluating the efficacy of such agents.
Acknowledgments
The AZD2820 compound was generously provided by AstraZeneca. We gratefully acknowledge the financial and technical assistance of AstraZeneca.
This work was supported by research grants from AstraZeneca, National Institutes of Health Grants RO1-DK-64773, P30-DK-26687, T32 DK 7647-23 and the Japan Society for the Promotion of Science.
Footnotes
Conflicts of Interest
The authors AL and SH are or were employees of AstraZeneca and may hold stock in that company.
References
- 1.McGuire MT, Wing RR, Klem ML, Hill JO. Behavioral strategies of individuals who have maintained long-term weight losses. Obes Res. 1999;7:334–41. doi: 10.1002/j.1550-8528.1999.tb00416.x. [DOI] [PubMed] [Google Scholar]
- 2.Leibel RL, Rosenbaum M, Hirsch J. Changes in energy expenditure resulting from altered body weight. N Engl J Med. 1995;332:621–8. doi: 10.1056/NEJM199503093321001. [DOI] [PubMed] [Google Scholar]
- 3.Ravussin Y, Gutman R, Diano S, Shanabrough M, Borok E, Sarman B, et al. Effects of chronic weight perturbation on energy homeostasis and brain structure in mice. Am J Physiol Regul Integr Comp Physiol. 2011;300:R1352–62. doi: 10.1152/ajpregu.00429.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosenbaum M, Vandenborne K, Goldsmith R, Simoneau JA, Heymsfield S, Joanisse DR, et al. Effects of experimental weight perturbation on skeletal muscle work efficiency in human subjects. Am J Physiol Regul Integr Comp Physiol. 2003;285:R183–92. doi: 10.1152/ajpregu.00474.2002. [DOI] [PubMed] [Google Scholar]
- 5.Rosenbaum M, Kissileff HR, Mayer LE, Hirsch J, Leibel RL. Energy intake in weight-reduced humans. Brain Res. 2010;1350:95–102. doi: 10.1016/j.brainres.2010.05.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosenbaum M, Sy M, Pavlovich K, Leibel RL, Hirsch J. Leptin reverses weight loss-induced changes in regional neural activity responses to visual food stimuli. J Clin Invest. 2008;118:2583–91. doi: 10.1172/JCI35055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hinkle W, Cordell M, Leibel R, Rosenbaum M, Hirsch J. Effects of reduced weight maintenance and leptin repletion on functional connectivity of the hypothalamus in obese humans. PLoS One. 2013;8:e59114. doi: 10.1371/journal.pone.0059114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosenbaum M, Goldsmith R, Bloomfield D, Magnano A, Weimer L, Heymsfield S, et al. Low-dose leptin reverses skeletal muscle, autonomic, and neuroendocrine adaptations to maintenance of reduced weight. J Clin Invest. 2005;115:3579–86. doi: 10.1172/JCI25977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kissileff HR, Thornton JC, Torres MI, Pavlovich K, Mayer LS, Kalari V, et al. Leptin reverses declines in satiation in weight-reduced obese humans. Am J Clin Nutr. 2012;95:309–17. doi: 10.3945/ajcn.111.012385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Williamson DA, Ravussin E, Wong ML, Wagner A, Dipaoli A, Caglayan S, et al. Microanalysis of eating behavior of three leptin deficient adults treated with leptin therapy. Appetite. 2005;45:75–80. doi: 10.1016/j.appet.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 11.Baicy K, London ED, Monterosso J, Wong ML, Delibasi T, Sharma A, et al. Leptin replacement alters brain response to food cues in genetically leptin-deficient adults. Proc Natl Acad Sci U S A. 2007;104:18276–9. doi: 10.1073/pnas.0706481104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harris RB, Zhou J, Redmann SM, Jr, Smagin GN, Smith SR, Rodgers E, et al. A leptin dose-response study in obese (ob/ob) and lean (+/?) mice. Endocrinology. 1998;139:8–19. doi: 10.1210/endo.139.1.5675. [DOI] [PubMed] [Google Scholar]
- 13.Heymsfield SB, Greenberg AS, Fujioka K, Dixon RM, Kushner R, Hunt T, et al. Recombinant leptin for weight loss in obese and lean adults: a randomized, controlled, dose-escalation trial. JAMA. 1999;282:1568–75. doi: 10.1001/jama.282.16.1568. [DOI] [PubMed] [Google Scholar]
- 14.Schwartz MW, Woods SC, Porte D, Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404:661–71. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- 15.Gantz I, Miwa H, Konda Y, Shimoto Y, Tashiro T, Watson SJ, et al. Molecular cloning, expression, and gene localization of a fourth melanocortin receptor. J Biol Chem. 1993;268:15174–9. [PubMed] [Google Scholar]
- 16.Liu H, Kishi T, Roseberry AG, Cai X, Lee CE, Montez JM, et al. Transgenic mice expressing green fluorescent protein under the control of the melanocortin-4 receptor promoter. J Neurosci. 2003;23:7143–54. doi: 10.1523/JNEUROSCI.23-18-07143.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gautron L, Lee CE, Lee S, Elmquist JK. Melanocortin-4 receptor expression in different classes of spinal and vagal primary afferent neurons in the mouse. J Comp Neurol. 2012;520:3933–48. doi: 10.1002/cne.23137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gautron L, Lee C, Funahashi H, Friedman J, Lee S, Elmquist J. Melanocortin-4 receptor expression in a vago-vagal circuitry involved in postprandial functions. J Comp Neurol. 2010;518:6–24. doi: 10.1002/cne.22221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ste Marie L, Miura GI, Marsh DJ, Yagaloff K, Palmiter RD. A metabolic defect promotes obesity in mice lacking melanocortin-4 receptors. Proc Natl Acad Sci U S A. 2000;97:12339–44. doi: 10.1073/pnas.220409497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee M, Kim A, Chua SC, Jr, Obici S, Wardlaw SL. Transgenic MSH overexpression attenuates the metabolic effects of a high-fat diet. Am J Physiol Endocrinol Metab. 2007;293:E121–31. doi: 10.1152/ajpendo.00555.2006. [DOI] [PubMed] [Google Scholar]
- 21.Pierroz DD, Ziotopoulou M, Ungsunan L, Moschos S, Flier JS, Mantzoros CS. Effects of acute and chronic administration of the melanocortin agonist MTII in mice with diet-induced obesity. Diabetes. 2002;51:1337–45. doi: 10.2337/diabetes.51.5.1337. [DOI] [PubMed] [Google Scholar]
- 22.Hamilton BS, Doods HN. Chronic application of MTII in a rat model of obesity results in sustained weight loss. Obes Res. 2002;10:182–7. doi: 10.1038/oby.2002.28. [DOI] [PubMed] [Google Scholar]
- 23.Yu Y, Deng C, Huang XF. Obese reversal by a chronic energy restricted diet leaves an increased Arc NPY/AgRP, but no alteration in POMC/CART, mRNA expression in diet-induced obese mice. Behav Brain Res. 2009;205:50–6. doi: 10.1016/j.bbr.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 24.Hahn TM, Breininger JF, Baskin DG, Schwartz MW. Coexpression of Agrp and NPY in fasting-activated hypothalamic neurons. Nat Neurosci. 1998;1:271–2. doi: 10.1038/1082. [DOI] [PubMed] [Google Scholar]
- 25.Kinzig KP, Hargrave SL, Tao EE. Central and peripheral effects of chronic food restriction and weight restoration in the rat. Am J Physiol Endocrinol Metab. 2009;296:E282–90. doi: 10.1152/ajpendo.90523.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Korner J, Savontaus E, Chua SC, Jr, Leibel RL, Wardlaw SL. Leptin regulation of Agrp and Npy mRNA in the rat hypothalamus. J Neuroendocrinol. 2001;13:959–66. doi: 10.1046/j.1365-2826.2001.00716.x. [DOI] [PubMed] [Google Scholar]
- 27.Melanocortin receptor-specific peptides. Vol. 8. U.P Office; 2013. p. 455.p. 617B2. [Google Scholar]
- 28.Halldorsdottir S, Carmody J, Boozer CN, Leduc CA, Leibel RL. Reproducibility and accuracy of body composition assessments in mice by dual energy x-ray absorptiometry and time domain nuclear magnetic resonance. Int J Body Compos Res. 2009;7:147–154. [PMC free article] [PubMed] [Google Scholar]
- 29.Hoggard N, Rayner DV, Johnston SL, Speakman JR. Peripherally administered [Nle4, D-Phe7]-alpha-melanocyte stimulating hormone increases resting metabolic rate, while peripheral agouti-related protein has no effect, in wild type C57BL/6 and ob/ob mice. J Mol Endocrinol. 2004;33:693–703. doi: 10.1677/jme.1.01632. [DOI] [PubMed] [Google Scholar]
- 30.Chen AS, Metzger JM, Trumbauer ME, Guan XM, Yu H, Frazier EG, et al. Role of the melanocortin-4 receptor in metabolic rate and food intake in mice. Transgenic Res. 2000;9:145–54. doi: 10.1023/a:1008983615045. [DOI] [PubMed] [Google Scholar]
- 31.McMinn JE, Wilkinson CW, Havel PJ, Woods SC, Schwartz MW. Effect of intracerebroventricular alpha-MSH on food intake, adiposity, c-Fos induction, and neuropeptide expression. Am J Physiol Regul Integr Comp Physiol. 2000;279:R695–703. doi: 10.1152/ajpregu.2000.279.2.R695. [DOI] [PubMed] [Google Scholar]
- 32.Kievit P, Halem H, Marks DL, Dong JZ, Glavas MM, Sinnayah P, et al. Chronic treatment with a melanocortin-4 receptor agonist causes weight loss, reduces insulin resistance, and improves cardiovascular function in diet-induced obese rhesus macaques. Diabetes. 2013;62:490–7. doi: 10.2337/db12-0598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seeley RJ, Burklow ML, Wilmer KA, Matthews CC, Reizes O, McOsker CC, et al. The effect of the melanocortin agonist, MT-II, on the defended level of body adiposity. Endocrinology. 2005;146:3732–8. doi: 10.1210/en.2004-1663. [DOI] [PubMed] [Google Scholar]
- 34.Rosenbaum M, Nicolson M, Hirsch J, Murphy E, Chu F, Leibel RL. Effects of weight change on plasma leptin concentrations and energy expenditure. J Clin Endocrinol Metab. 1997;82:3647–54. doi: 10.1210/jcem.82.11.4390. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.