Abstract
This study sought to investigate the inhibitory effect of some commonly consumed Nigerian green leafy vegetables (raw and blanched) on acetylcholinesterase and butyrylcholinesterase (key enzyme linked to Alzheimer’s disease) activities and some pro-oxidants (FeSO4, Sodium nitroprusside and Quinolinic acid) induced lipid peroxidation in rat brain in vitro. Three commonly consumed green leafy vegetables in Nigeria [Amarantus cruentus (Arowojeja), Struchium sparganophora (Ewuro-odo) and Telfairia occidentalis (Ugwu] were blanched in hot water for 10 min, and the extracts of the raw and blanched vegetables were prepared and used for subsequent analysis. The result revealed that all the vegetables inhibited acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) activity as well as the pro-oxidants induced lipid peroxidation in rat brain in a dose dependent manner; however, Amarantus cruentus extract (EC50 = 97.9 μg/ml) had the highest inhibitory effect on acetylcholinesterase activity while Telfairia occidentalis extract (EC50 = 52.7 μg/ml) had the highest inhibitory effect on butyrylcholinesterase activity. However, blanching of the vegetables caused a significant (P < 0.05) decrease in the inhibitory effect of the vegetables on AChE activities while it enhanced the inhibition of the pro-oxidants induced lipid peroxidation in rat brain in vitro. Therefore, some of the possible mechanism by which green leafy vegetables exert their neuroprotective activities could be through the inhibition of acetylcholinesterase and butyrylcholinesterase activities and prevention of lipid peroxidation in the brain. However, blanching of the vegetables could reduce their ability to inhibit acetylcholinesterase and butyrylcholinesterase activity.
Keywords: Vegetables, Blanching, Alzheimer’s disease, Acetylcholinesterase, Butyrylcholinesterase
Introduction
Alzheimer’s disease (AD) is the most common form of age-related dementia and is characterized by progressive and insidious neurodegeneration of the central nervous system that eventually leads to a gradual decline of cognitive function and dementia. In recent years, studies have implicated oxidative stress to play a crucial role in neurodegenerative diseases such as Alzheimer’s disease via lipid peroxidation of cell membrane of the neurons (Pratico and Delanty 2000). The brain and nervous system are thought to be particularly vulnerable to oxidative stress due to limited antioxidant capacity, consumes 20 percent of the metabolic oxygen, neurons cannot synthesize glutathione and contains more of polyunsaturated fatty acids (Marksberry and Lovell 2007; Oboh and Rocha 2007). Elements receiving the most attention in Alzheimer disease are aluminum (Al), mercury (Hg), and iron (Fe) (Fraga et al. 1990; Stacey and kappus 1982; Ehmann et al. 1986). Of these, iron may have the most important pathophysiologic role as a catalyst for free radical generation by virtue of having a loosely bound electron and the ability to exist in more than one valence (Ehmann et al. 1986). The mechanism by which iron can cause this deleterious effect is that Fe (II) can react with hydrogen peroxide (H2O2) to produce the hydroxyl radical (OH.) via the Fenton reaction, whereas superoxide can react with iron(III) to regenerate iron(II) that can participate in the Fenton reaction (Zago et al. 2000). The overproduction of ROS can directly attack the polyunsaturated fatty acids of the cell membranes and induce lipid peroxidation.
Nitric oxide (NO), a universal neuronal messenger in the central nervous system is known to play neurotransmitter and neuromodulator role in the brain (Moncada et al. 1991). However, it has also been implicated as an aggravating factor in Alzheimer’s disease and other neurodegenerative by reacting with superoxide radical to form peroxynitrite (Ahmad et al. 2008). Sodium nitroprusside (SNP) is an anti-hypertensive drug, which acts by relaxing smooth vascular muscle; consequently it dilates peripheral arteries and veins. However, it could cause cytotoxicity through the release of cyanide and/or nitric oxide (NO).
Quinolinic acid (2,3-pyridine-dicarboxylic acid, QA) is a neuroactive metabolite of the tryptophan-kinurenine pathway produced by macrophages and microglia in human and rat brain; it has been implicated in the pathogenesis of a variety of human neurological diseases (Guillemin and Brew 2002; Guillemin et al. 2006; Guillemin et al. 2004). There are two main mechanisms by which QA has been implicated in Alzheimer’s disease. The first is the activation of NMDA receptors by Quinolinic acid (Guillemin et al. 2004), which is already known to mediate neuronal apoptosis with caspase-3 activation (Tenneti and Lipton 2000; Stone 2001). The second possibility, which represent another important aspect of Quinolinic acid toxicity, is lipid peroxidation (Rios and Santamaria 1991). Quinolinic acid in concentration as low as 120 nM, induces lipid peroxidation and formation of free radicals leading to neuronal death (Behan et al. 1999).
Although the etiology of Alzheimer’s disease (AD) is not fully understood, nevertheless, inhibition of acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) activity has been accepted as an effective treatment/management strategy against AD (Arnold and Kumar 1993; Orhan et al. 2004). AChE inhibitors such as tacrine, donepezil and rivastigmine are commonly used synthetic drugs for the treatment of Alzheimer’s disease; however, these drugs are limited in use due to their adverse side-effects and are effective only against the mild type of AD. In addition, there is presently no drug available with BChE inhibitory activity (Schneider 2001). Hence, recent efforts have focused on plant phytochemicals as natural sources of effective acetylcholinesterase and butyrylcholinesterase inhibitors with little or no side effects which could be use as dietary intervention in the management of this disease (Orhan et al. 2004; conforti et al. 2007).
Recent epidemiologic studies suggest that antioxidant phytochemicals from dietary fruits and vegetables, but not from supplements, play a role in delaying the onset of Alzheimer’s disease (Luchsinger et al. 2003; Zandi et al. 2004). Many phenolics have antioxidant capacities that are much stronger than those of vitamins C and E. Flavonols and flavones are flavonoids of particular importance because they have been found to possess antioxidant and free radical scavenging activity in foods (Amic et al. 2003). Some evidence showed that flavonoids could protect membrane lipids from oxidation (Amic et al. 2003).
In Nigeria, green leafy vegetables are not usually consumed in their fresh form unlike fruits; however, they are usually blanched before consumption or in soup preparation (Akindahunsi and Oboh 1999). Blanching stops the enzyme action, sets the colour, and shortens the drying and dehydration time. It is usually carried out in hot water or in steam; this technique is used by indigenous people to reduce or eliminate the bitterness of some vegetables and acid components that are common in leaves (Akindahunsi and Oboh 1999). Although a lot had been reported on the chemical characterization of phytoconstituents and antioxidant properties of tropical green leafy vegetables, however, there is still limited information on its potential use in the management/prevention of neurodegenerative diseases associated with oxidative stress. Hence, this study sought to investigate the interaction of leaf extracts (raw and blanched) of some Nigerian green leafy vegetables on acetylcholinesterase (key enzyme linked to Alzheimer’s disease) activity and some pro-oxidants induced lipid peroxidation in rat brain in vitro.
Materials and methods
Materials
Sample collection
Raw samples of three commonly consumed green leafy vegetables, Telfairia occidentalis (Ugwu), Amarantus cruentus (Arowojeja) and Struchium sparganophora (Ewuro Odo) were sourced from the University garden of The Federal University of Technology, Akure. Authentication of the vegetables was carried out in the Department of Biology, Federal University of Technology, Akure, Nigeria. Ten adult male wistar strain albino rats were purchased from the Biochemistry Department animal colony, University of Ilorin, Nigeria and acclimatized for 2 weeks, during which period they were maintained ad libitum on commercial diet and water.
Chemicals and reagents
Chemicals and reagents used such as thiobarbituric acid (TBA), gallic acid, Folin–Ciocalteau’s reagent were procured from Sigma-Aldrich, Inc., (St Louis, MO), trichloroacetic acid (TCA) was sourced from Sigma-Aldrich, Chemie GmbH (Steinheim, Germany), dinitrophenyl hydrazine (DNPH) from ACROS Organics (New Jersey, USA), methanol and acetic acid were sourced from BDH Chemicals Ltd., (Poole, England), thiourea, CuSO4.5H2O, H2SO4, sodium carbonate, AlCl3, potassium acetate, Tris-HCl buffer, sodium dodecyl sulphate, FeSO4, quinolinic acid and sodium nitropurriside were of analytical grade while the water was glass distilled.
Preparation of 70% ethanolic extract
The inedible parts of the vegetables were removed from the edible parts by hand picking. The edible parts were thoroughly washed in tap water to remove any dirts, chopped into small pieces by table knife. A portion of the chopped vegetables was then blanched for 10 min, while the other portion was not. The blanched portion was then drained of water. Both portions were then sun dried and milled to be obtained in a powder form. The powder was extracted with 70% ethanol then, the extract was filtered with Whatman filter paper and the filtrate was concentrated under reduced pressure to give a solid extract. The concentrated extract was further lyophilized. Then, the vegetable extract was reconstituted in distilled water and used for subsequent analysis.
Acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) inhibition assay
Inhibition of acetylcholinesterase (AChE) was assessed by a modified colorimetric method of Ellman (Perry et al. 2000). The AChE activity was determined in a reaction mixture containing 200 μL of brain AChE solution (EC 3.1.1.7) in 0.1 M phosphate buffer, pH 8.0, 100 μL of a solution of 5,5′-dithio-bis(2-nitrobenzoic) acid (DTNB 3.3 mM in 0.1 M phosphate buffered solution, pH 7.0, containing NaHCO3 6 mM), vegetable extracts (0–100 μl) and 500 μL of phosphate buffer, pH 8.0. After incubation for 20 min at 25 °C, acetylthiocholine iodide (100 μL of 0.05 mM water solution) was added as the substrate, and AChE activity was determined by UV spectrophotometry from the absorbance changes at 412 nm for 3.0 min at 25 °C.
Hundred microliters of butyrylthiocholine iodide was used as a substrate to assay butyrylcholinesterase enzyme, while all the other reagents and conditions were the same. The AChE and BChE inhibitory activity was expressed as percentage inhibition.
Preparation of brain homogenates
The rats were sacrificed using cervical dislocation and the cerebral tissue (whole-brain) was rapidly dissected and placed on ice and weighed. This tissue was subsequently homogenized in cold saline (1:10 w/v) with about 10-up-and-down strokes at approximately 1,200 rev/minute in a Teflon–glass homogenizer. The homogenate was centrifuged for 10 min at 3,000 g to yield a pellet that was discarded and the low-speed supernatant (S1) was kept for lipid peroxidation assay.
Lipid peroxidation and thiobarbibutric acid reactions
The lipid peroxidation assay was carried out using the modified method of Ohkawa et al. (1979). Briefly 100 μL S1 fraction was mixed with a reaction mixture containing 30 μL of 0.1 M Tris-HCl buffer (pH 7.4), vegetable extract (0–100 μL) and 30 μL of the pro-oxidant solution (25 μM FeSO4, 7 mM Sodium Nitroprussside and 15 mM Quinolinic acid). The volume was made up to 300 μL with water before incubation at 37 °C for 1 h. The color reaction was developed by adding 300 μL, 8.1% sodium dodecyl sulphate (SDS) to the reaction mixture containing S1; this was subsequently followed by the addition of 500 μL of acetic acid/HCl (pH 3.4) and 500 μL, 0.8% TBA (Thiobarbituric acid). This mixture was incubated at 100 °C for 1 h. The absorbance was measured at 532 nm and malondialdehyde (MDA) was used as standard.
ABTS radical scavenging ability
The ABTS˙+ (2,2′-azino-bis(3-ethylbenzthiazoline-6-sulphonic acid) scavenging ability of the vegetable extracts were determined according to the method described by Re et al. (1999). The ABTS˙+ was generated by reacting an (7 mmol/L) ABTS aqueous solution with K2S2O8 (2.45 mmol/L, final concentration) in the dark for 16 h and adjusting the Absorbance at 734 nm to 0.700 with ethanol. 0.2 mL of appropriate dilution of the extract was added to 2.0 mL ABTS˙+ solution and the absorbance were measured at 734 nm after 15 min. Trolox was used as standard and trolox equivalent antioxidant capacity (TEAC) was subsequently calculated.
Determination of total phenol content
The total phenol content was determined according to the method of Singleton et al. (1999). Briefly, appropriate dilutions of the vegetable extracts were oxidized with 2.5 mL 10% Folin-Ciocalteau’s reagent (v/v) and neutralized by 2.0 mL of 7.5% sodium carbonate. The reaction mixture was incubated for 40 min at 45 °C and the absorbance was measured at 765 nm in the UV-Visible spectrophotometer (Model 6305; Jenway, Bar loworld Scientific, Dunmow, United Kingdom). The total phenol content was subsequently calculated as gallic acid equivalent.
Determination of total flavonoid content
The total flavonoid content was determined using a slightly modified method reported by Meda et al. (2005). Briefly 0.5 mL of appropriately diluted sample was mixed with 0.5 mL methanol, 50 μL 10% AlCl3, 50 μL 1 M Potassium acetate and 1.4 mL water, and allowed to incubate at room temperature for 30 min. The absorbance of the reaction mixture was subsequently measured at 415 nm in the UV-Visible spectrophotometer (Model 6305; Jenway, Barloworld Scientific, Dunmow, United Kingdom). The total flavonoid content was subsequently calculated using quercetin as standard.
Data analysis
The result of three replicate experiments were pooled and expressed as mean ± standard deviation (SD) (Zar 1984). A one-way analysis of variance (ANOVA) and Positive analysis was done using Duncan multiple test. Significance was accepted at P ≤ 0.05.
Results and discussion
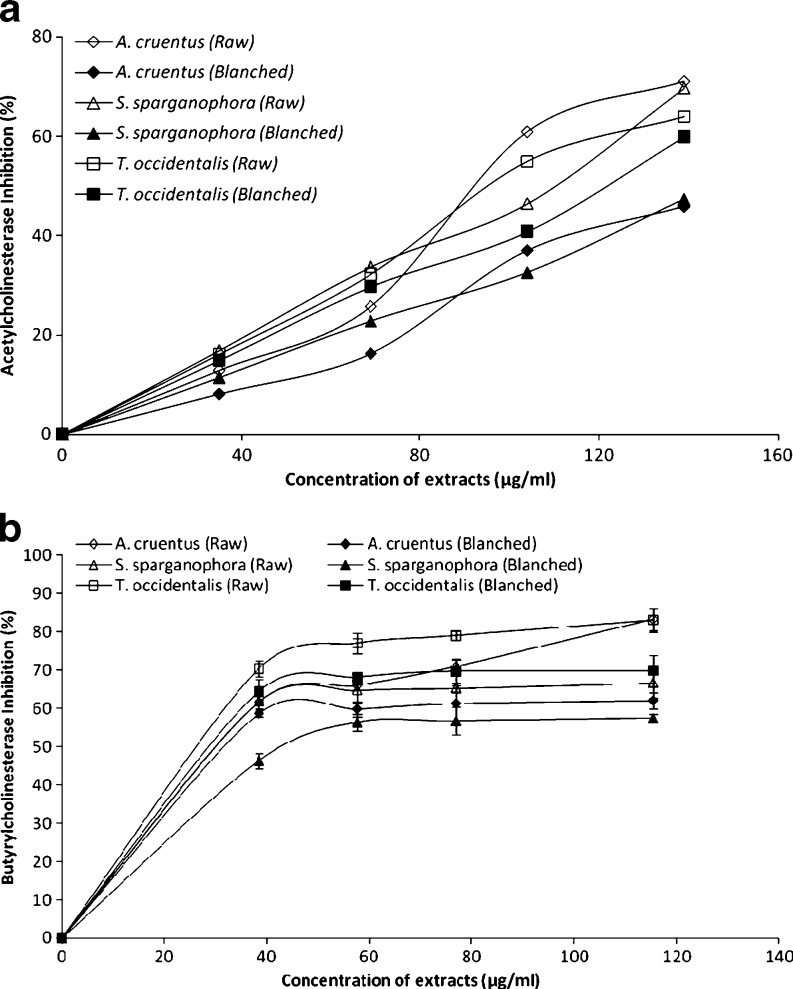
Inhibition of acetylcholinesterase (AChE) activity has been accepted as an effective treatment/management strategy against AD (Howes et al. 2003). Inhibition of AChE activity prevents it from breaking down acetylcholine in the brain and consequent increased concentrations of acetylcholine leads to increased communication between the nerve cells that use acetylcholine as a chemical messenger, which may in turn temporarily improve or stabilize the symptoms of Alzheimer’s disease (Howes et al. 2003). The ability of the vegetable extracts to inhibit acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) activity in vitro was investigated and the result is presented in Fig. 1a and b. The results revealed that all the vegetable extracts inhibited AChE and BChE in a dose-dependent manner. However, as revealed by the EC50 (extract concentration causing 50% enzyme inhibition) values (Table 1), Amarantus cruentus (97.9 μg/mL) had the highest AChE inhibitory activity while Telfairia occidentalis (52.7 μg/mL) had the highest BChE inhibitory activity. The determined acetylcholinesterase (AChE) inhibitory activity agreed with some earlier reports where plant phytochemicals from Citrus medica inhibited acetylcholinesterase (AChE) and plants extracts of G. biloba and S. lavandulaefolia, showed a significant improvement in cognitive performance and memory (Mazza et al. 2006; Maruyama et al. 2006; Akhondzadeh and Abbasi 2006). This also agreed with a recent worked reported by Oboh et al. (2010a) where red and white ginger inhibited acetylcholinesterase (AChE) activity in vitro. However, the blanched vegetables caused a significant (P < 0.05) decrease in the inhibition of acetylcholinesterase (AChE) activity than raw vegetables in all the three vegetables tested. This could be attributed to the damage/loss of physiologically active phytochemicals having AChE inhibitory activities during the heat processes involved in blanching such as observed in Table 1.
Fig. 1.
Anticholinesterase inhibitory activity (a) Acetylcholinesterase inhibitory activity and (b) Butyrylcholonesterase inhibitory activity; of three commonly consumed green leafy vegetables in Nigeria observation mean (n = 3)
Table 1.
The total phenol, flavonoid content, ABTS˙+ scavenging ability, EC50 of acetylcholinesterase and butyrylcholinesterase inhibitory activity of three commonly consumed green leafy vegetables as affected by blanching
| Samples | Raw | Blanched |
|---|---|---|
| Total Phenol (mg/100 g) | ||
| Amarantus cruentus | 9.3a ± 0.40 | 10.2a ± 0.30 |
| Struchium sparganophora | 7.3c ± 0.20 | 3.1b ± 0.40 |
| Telfairia occidentalis | 13.0b ± 0.30 | 5.8a ± 0.80 |
| Total flavonoid (mg/100 g) | ||
| Amarantus cruentus | 3.6b ± 0.10 | 1.4c ± 0.20 |
| Struchium sparganophora | 0.6c ± 0.00 | 0.7c ± 0.00 |
| Telfairia occidentalis | 7.3b ± 0.30 | 1.1d ± 0.10 |
| ABTS˙+ scavenging ability (μmol. TEAC/100 g) | ||
| Amarantus cruentus | 44.0b ± 2.00 | 51.0c ± 3.00 |
| Struchium sparganophora | 59.0c ± 3.00 | 58.0c ± 1.00 |
| Telfairia occidentalis | 53.0b ± 3.50 | 61.0d ± 3.00 |
| AChE inhibitory activity (μg/ml) | ||
| Amarantus cruentus | 97.9b ± 0.70 | 155.5a ± 10.09 |
| Struchium sparganophora | 103.6c ± 0.89 | 151.3b ± 10.12 |
| Telfairia occidentalis | 103.8b ± 0.92 | 119.3a ± 10.04 |
| BChE inhibitory activity (μg/ml) | ||
| Amarantus cruentus | 56.7b ± 2.01 | 68.4a ± 1.45 |
| Struchium sparganophora | 53.8c ± 1.20 | 75.0b ± 1.12 |
| Telfairia occidentalis | 52.7b ± 0.92 | 60.6a ± 1.04 |
Values represent mean ± standard deviation of triplicate experiments
Values with the same superscript letter along the same row are not significantly different (P < 0.05)
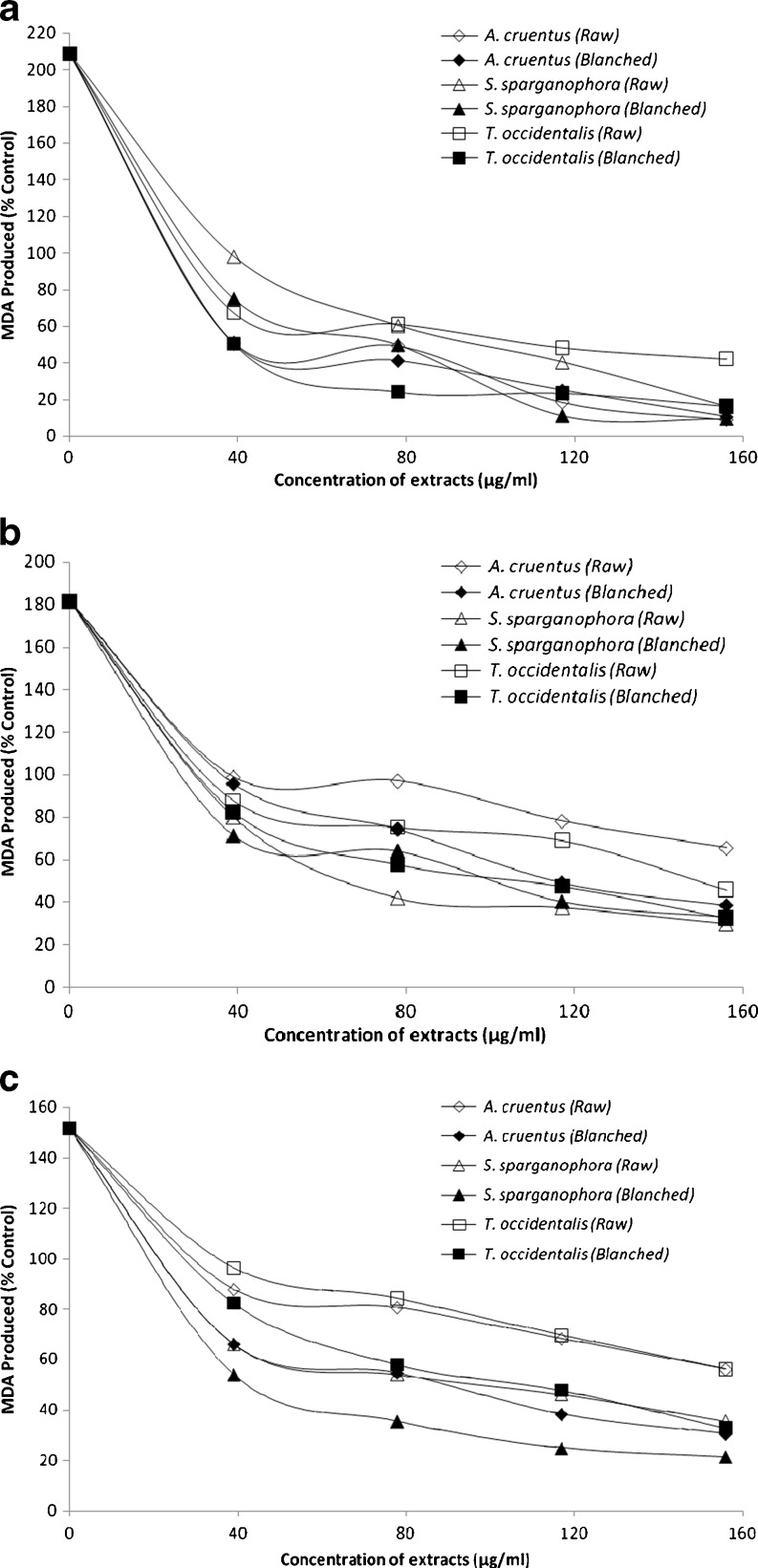
Incubation of rat brain tissues in the presence of 250 μM FeSO4 caused a significant (P < 0.05) increase in the MDA content of the brain (208.8%) as presented in Fig. 2a. This finding agreed with earlier report by Oboh et al. (2010b) where significant increase in MDA production in rat brain was observed in the presence of Fe2+. The increased lipid peroxidation in the presence of Fe2+ could be attributed to the fact that Fe2+ can catalyze one-electron transfer reactions that generate reactive oxygen species, such as the reactive OH˙, which is formed from H2O2 through the Fenton reaction. Iron also decomposes lipid peroxides, thus generating peroxyl and alkoxyl radicals, which favors the propagation of lipid oxidation (Zago et al. 2000). Elevated Fe2+ content in the brain had been linked to a host of neurodegenerative diseases and high Fe contents have been localized to degenerate regions of brains from Alzheimer’s disease patients, a finding also demonstrated in animal models of the disease (Martinez et al. 2003). However, the introduction of the vegetable extracts inhibited MDA production in rat brain in a dose-dependent manner (39–156 μg/ml) as shown in Fig. 2a with A. cruentus having the highest inhibitory effect and T. occidentalis the least. However, the blanched vegetables had a significantly (P < 0.05) higher inhibitory effect on Fe2+ - induced lipid peroxidation in rat brain than the raw unprocessed vegetables. This finding is consistent with our earlier report where plant extracts inhibited Fe2+ induced lipid peroxidation in rat brain in vitro (Oboh et al. 2010b).
Fig. 2.
Some antioxidant parameters (a) Inhibition of Fe2+ induced Lipid peroxidation (b) Inhibition of SNP induced lipid peroxidation (c) Inhibition of QA induced lipid peroxidation; of three commonly consumed green leafy vegetables in Nigeria observation mean (n = 3)
In addition, incubation of rat brain tissues in the presence of 7 mM sodium nitroprusside (SNP) caused a significant (P < 0.05) increase in the MDA production in the brain (181.6%) as presented in Fig. 2b. However, the extracts of all the vegetables inhibited MDA production in rat brain in a dose-dependent manner (39–156 μg/mL) with S. sparganophora having the highest inhibitory effect while A. cruentus had the least. However, in all the three vegetables the blanched had significantly (P < 0.05) higher inhibitory effect on SNP-induced lipid peroxidation in rat brain than raw vegetables as tested. NO has been reported to contribute to degenerative diseases by reacting with superoxide radical (O−2) produced in Fenton reaction to form the powerful peroxynitrite (ONOO−). The ONOO− can then induce lipid peroxidation, oxidation of proteins and DNA which leads to ATP-dependent PARP (poly ADP-ribose polymerase) over activation causing neuronal ATP depletion, mitochondrial dysfunction as well as inflammation and ultimately, cell death (Parihar and Hemnani 2004).
Furthermore, incubating rat brain tissue homogenates in the presence of QA (a well-known excitotoxin that induces oxidative stress and damage) caused a significant (P < 0.05) increase in the MDA production in the brain (151.8%) as shown in Fig. 2c. This finding is in agreement with Oboh et al. (2010b) where QA caused a significant increase in the MDA content of rat brain in vitro. However, the vegetable extracts inhibited MDA production in rat brain in a dose-dependent manner (39–156 μg/mL) with S. sparganophora having the highest inhibitory effect while A. cruentus had the least. Also, the blanched vegetables had a significantly (P < 0.05) higher inhibitory effect on QA-induced lipid peroxidation in rat brain than raw vegetables for all the vegetables tested. Quinolinic acid (QA) had been reported to activate neurons expressing NMDA receptors and glutamate-type excitotoxicity (Stone and Perkins 1981). The mechanism through which QA induces lipid peroxidation has been linked to free radical generation resulting from overstimulation of NMDA receptors. Increases in QA concentration are known to be associated with several neurodegenerative diseases including Alzheimer’s disease (Guillemin et al. 2006). Free radical scavengers and antioxidant enzyme inducers can protect neuronal tissue against the oxidotoxicity of QA under in vitro and in vivo conditions (Cabrera et al. 2000; Stone 1993; Santamaria et al. 1999).
Antioxidants carry out their protective properties on cells either by preventing the production of free radicals or by neutralizing/scavenging free radicals produced in the body (Oboh et al. 2007). Therefore, the free radical scavenging ability of the vegetable extracts was studied using a moderately stable nitrogen-centred radical species—ABTS radical (Re et al. 1999). The ABTS˙+ scavenging ability of the three vegetables is presented in Table 1 as trolox equivalent antioxidant capacity (TEAC). The results revealed that the extracts of S. sparganophora (0.06 mmol TEAC/100 g) had the highest ABTS˙+ scavenging ability while A. cruentus (0.04 mmol TEAC/100 g) had the least. However, blanching of vegetables caused a significant (P < 0.05) decrease in ABTS˙+ scavenging ability except in A. cruentus, where there was an increase. The decrease in ABTS˙+ scavenging ability for both S. sparganophora and T. occidentalis agrees with the total phenol content (Table 1) where blanching caused a reduction in phenol content. Numerous reports have shown strong correlation between antioxidant properties of plant foods and their phenolic content (Chu et al. 2002).
Chu et al. (2002) had earlier reported that many plants are rich sources of phytochemicals and that intake of these plant chemicals has a protective potential against degenerative diseases such as diabetes, Alzheimer’s disease etc. The result of the total phenol and flavonoid content in the green leafy vegetables is presented in Table 1. The phenolic content of the vegetables ranges from 9.3 mg/100 g for A. cruentus to 13.0 mg/100 g for T. occidentalis while the flavonoid content of the vegetables ranges from 0.6 mg/100 g for S. sparganophora to 7.3 mg/100 g for T. occidentalis. The values were lower than what Oboh (2005) reported for some tropical green leafy vegetables (1–3 mg/g). The difference in phenolic value is as a result of the extraction medium used in the study. However, there was a decrease in the phenolic content due to blanching except in A. cruentus, where the increase was insignificant. The basis of the decrease could not be categorically stated, however, it could be that during blanching some of the phenols would have been leached into the water. However, the result was in agreement with Chen and Lin (2007) that phenolics content in cooked yams prepared at different temperatures (50–100 °C) was lower compared to the raw ones. Also, this result was in line with Chung et al. (2008) that more than 40% of phenolic content in yam peels were lost after blanching at 85 °C for 30 s.
Phenolic compounds can protect the human body from free radicals, whose formation is associated with the normal metabolism of aerobic cells. They are strong antioxidants capable of removing free radicals, chelate metal catalysts, activate antioxidant enzymes, reduce α-tocophenol radicals and inhibit oxidases (Amic et al. 2003). The presence of derivatives of flavonoids has been found in many fruits and vegetables; moreover, numerous studies have conclusively shown that the majority of the antioxidant activity maybe from compounds such as flavonoids, isoflavones, flavones, anthocyanins, catechin and isocatechin rather than from vitamins C, E and β-carotene (Marin et al. 2004; Oboh et al. 2007). Flavonoids have antioxidant activity and could therefore lower cellular oxidative stress (Oboh et al. 2007). Polyphenols are considered to be strong antioxidants due to the redox properties of their hydroxyl groups (Materska and Perucka 2005).
Conclusion
In the light of these findings, we can conclude that all the three vegetable extracts showed inhibitory activity against both of the enzymes (AChE and BChE) in dose-dependent manner and they could be considered for further studies in the treatment of AD. However, blanching of these vegetables could reduce their acetylcholinesterase and butyrylcholinesterase inhibitory activity.
References
- Ahmad B, Siddig I, Adel SA, Manal MET, Syam MM. Zerumbone: a natural compound with anti-cholinesterase activity. Am J Pharm and Toxicol. 2008;3(3):209–211. doi: 10.3844/ajptsp.2008.209.211. [DOI] [Google Scholar]
- Akhondzadeh S, Abbasi SH. Herbal medicine in the treatment of Alzheimer’s disease. Am J Alzheimers Dis Other Demen. 2006;21:113–118. doi: 10.1177/153331750602100211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akindahunsi AA, Oboh G (1999) Effect of some post-harvest treatments on the bio-availability of zinc from some selected tropical vegetables. La Rivista Italiana Delle Sostanze Grasse LXXVI:285–287
- Amic D, Davidovic-Amic D, Beslo D, Trinajstic N. Structure-radical scavenging activity relationship of flavonoids. Croatia Chem Act. 2003;76(1):55–61. [Google Scholar]
- Arnold SE, Kumar A. Reversible dementias. Med Clin Nort Am. 1993;77:215–225. doi: 10.1016/s0025-7125(16)30280-2. [DOI] [PubMed] [Google Scholar]
- Behan WM, McDonald M, Darlington LG, Stone TW. Oxidative stress as a mechanism for quinolinic acid-induced hippocampal damage: protection by melatonin and deprenyl. Br J Pharmacol. 1999;128:1754–1760. doi: 10.1038/sj.bjp.0702940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrera J, Reiter RJ, Tan D, Qi W, Sainz RM, Mayo JC, Garcia JJ, Kim SJ, El-Sokkary G. Melatonin reduces oxidative neurotoxicity due to quinolinic acid: in vitro and in vivo findings. Neuropharm. 2000;39:507–514. doi: 10.1016/S0028-3908(99)00128-8. [DOI] [PubMed] [Google Scholar]
- Chen YT, Lin KW. Effects of heating temperature on the total phenolic compound, antioxidative ability and the stability of dioscorin of various yam cultivars. Food Chem. 2007;101(3):955–963. doi: 10.1016/j.foodchem.2006.02.045. [DOI] [Google Scholar]
- Chu Y, Sun J, Wu X, Liu RH. Antioxidant and antiproliferative activity of common vegetables. J Agric Food Chem. 2002;50:6910–6916. doi: 10.1021/jf020665f. [DOI] [PubMed] [Google Scholar]
- Chung YC, Chiang BH, Wei JH, Wang CK, Chen PC, Hsu CK. Effects of blanching, drying and extraction processes on the antioxidant activity of yam (Dioscorea alata) Intl J of Food Sci and Technol. 2008;43(5):859–864. doi: 10.1111/j.1365-2621.2007.01528.x. [DOI] [Google Scholar]
- Conforti F, Statti GA, Tundis R, Loizzo MR, Menichini F. In vitro activities of Citrus medica L. cv. Diamante (Diamante citron) relevant to treatment of diabetes and Alzheimer’s disease. Phytother Res. 2007;21:427–433. doi: 10.1002/ptr.2077. [DOI] [PubMed] [Google Scholar]
- Ehmann WD, Markesbery WR, Alauddin M. Brain trace elements in alzheimer’s disease. Neurotoxicol. 1986;7:195–206. [PubMed] [Google Scholar]
- Fraga CG, Oteiza PI, Golub MS, Gershwin ME, Keen CL. Effect of aluminum on brain lipid peroxidation. Toxicol Lett. 1990;51:213–9. doi: 10.1016/0378-4274(90)90212-5. [DOI] [PubMed] [Google Scholar]
- Guillemin GJ, Brew BJ. Implications of the kynurenine pathway and quinolinic acid in Alzheimer’s disease. J neuroinfl. 2002;7:199–206. doi: 10.1179/135100002125000550. [DOI] [PubMed] [Google Scholar]
- Guillemin GJ, Kerr SJ, Brew BJ. Involvement of quinolinic acid in AIDS dementia complex. Neurotox Res. 2004;7:103–124. doi: 10.1007/BF03033781. [DOI] [PubMed] [Google Scholar]
- Guillemin G, Meininger V, Brew B. Implications for the kynurenine pathway and quinolinic acid in amyotrophic lateral sclerosis. Neurodeg dis. 2006;2:166–176. doi: 10.1159/000089622. [DOI] [PubMed] [Google Scholar]
- Howes MJR, Perry NSL, Houghton PJ. Plants with traditional uses and activities, relevant to the management of Alzheimer’s disease and other cognitive disorders. Phytother Res. 2003;17:1–18. doi: 10.1002/ptr.1280. [DOI] [PubMed] [Google Scholar]
- Luchsinger JA, Tang MX, Shea S, Mayeux R. Antioxidant vitamin intake and risk of Alzheimer disease. Arch Neurol. 2003;60(2):203–208. doi: 10.1001/archneur.60.2.203. [DOI] [PubMed] [Google Scholar]
- Marin A, Ferreres F, Tomas-Barberan FA, Gil MJ. Characterization and quantitation of antioxidant constituents of sweet pepper (Capsicum annuum L) J Agric and Food Chem. 2004;52:3861–3869. doi: 10.1021/jf0497915. [DOI] [PubMed] [Google Scholar]
- Marksberry WR, Lovell MA. Damage to lipids, proteins, DNA and RNA in mild cognitive impairment. Arch Neurology. 2007;64:954–956. doi: 10.1001/archneur.64.7.954. [DOI] [PubMed] [Google Scholar]
- Martinez GR, Loureiro AP, Marques SA, Miyamoto S, Yamaguchi LF, Onuki J, Almeida EA, Garcia CC, Barbosa LF, Medeiros MH, Di Mascio P. Oxidative and alkylating damage in DNA. Mut Res. 2003;544:115–127. doi: 10.1016/j.mrrev.2003.05.005. [DOI] [PubMed] [Google Scholar]
- Maruyama M, Tomita N, Iwasaki K. Benefits of combining donepezil plus traditional Japanese herbal medicine on cognition and brain perfusion in Alzheimer’s disease: a 12-week observer-blind, donepezil monotherapy controlled trial. J Am Geriatr Soc. 2006;54:869–871. doi: 10.1111/j.1532-5415.2006.00722.x. [DOI] [PubMed] [Google Scholar]
- Materska M, Perucka I. Antioxidant activity of the main phenolic compounds isolated from hot pepper fruits (Capsicum annuum L.) J Agric and Food Chem. 2005;53:1730–1758. doi: 10.1021/jf035331k. [DOI] [PubMed] [Google Scholar]
- Mazza M, Capuano A, Bria P, Mazza S. Ginkgo biloba and donepezil: a comparison in the treatment of Alzheimer’s dementia in a randomized placebo-controlled double-blind study. Eur J Neurol. 2006;9:981–985. doi: 10.1111/j.1468-1331.2006.01409.x. [DOI] [PubMed] [Google Scholar]
- Meda A, Lamien CE, Romito M, Millogo J, Nacoulma OG. Determination of the total phenolic, flavonoid and proline contents in Burkina Fasan honey, as well as their radical scavenging activity. Food Chem. 2005;91:571–7. doi: 10.1016/j.foodchem.2004.10.006. [DOI] [Google Scholar]
- Moncada S, Palmer M, Higgs EA. Nitric oxide: physiology, pathophysiology and pharmacology. Pharmacol Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- Oboh G. Effect of blanching on the antioxidant property of some tropical green leafy vegetables. Lebensm Wiss Technol. 2005;38:513–517. doi: 10.1016/j.lwt.2004.07.007. [DOI] [Google Scholar]
- Oboh G, Rocha JBT. Distribution and antioxidant activity of polyphenols in ripe and unripe tree pepper (Capsicum pubescens) J Food Biochem. 2007;31:456–473. doi: 10.1111/j.1745-4514.2007.00123.x. [DOI] [Google Scholar]
- Oboh G, Puntel RL, Rocha JBT. Hot pepper (Capsicum annuum, Tepin & Capsicum chinese, Habanero) prevents Fe2+—induced lipid peroxidation in Brain—In vitro. Food Chem. 2007;102(1):178–185. doi: 10.1016/j.foodchem.2006.05.048. [DOI] [Google Scholar]
- Oboh G, Ademiluyi AO, Akinyemi AJ (2010a) Inhibition of acetylcholinesterase inhibition activities and some prooxidant induced lipid peroxidation in rat brain by two varieties of ginger (Zingiber officinale). Exp Toxicol Pathol doi:10.1016/j.etp.2010.09.004 [DOI] [PubMed]
- Oboh G, Akinyemi AJ, Ademiluyi AO (2010b) Antioxidant and inhibitory effect of red ginger (Zingiber officinale var. Rubra) and white ginger (Zingiber officinale Roscoe) on Fe2+ induced lipid peroxidation in rat brain in vitro. Exp Toxicol Pathol doi:10.1016/j.etp.2010.06.002 [DOI] [PubMed]
- Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- Orhan I, Sener B, Choudhary MI, Khalid A. Acetylcholinesterase and butyrylcholinesterase inhibitory activity of some Turkish medicinal plants. J Ethnopharm. 2004;91:57–60. doi: 10.1016/j.jep.2003.11.016. [DOI] [PubMed] [Google Scholar]
- Parihar MS, Hemnani T. Alzheimer’s disease pathogenesis and therapeutic interventions. J Clin Neurosci. 2004;11:456–467. doi: 10.1016/j.jocn.2003.12.007. [DOI] [PubMed] [Google Scholar]
- Perry NS, Houghton PJ, Theobald A, Jenner P, Perry EK. In-vitro activity of S. lavandulaefolia (Spanish sage) relevant to treatment of Alzheimer’s disease. J Pharm Pharmacol. 2000;52:895–902. doi: 10.1211/0022357001774598. [DOI] [PubMed] [Google Scholar]
- Pratico D, Delanty N. Oxidative injury in diseases of the central nervous system: focus on Alzheimer’s disease. Am J Med. 2000;109:577–585. doi: 10.1016/S0002-9343(00)00547-7. [DOI] [PubMed] [Google Scholar]
- Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorisation assay. Free Rad in Biol and Med. 1999;26:1231–1237. doi: 10.1016/S0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- Rios C, Santamaria A. Quinolinic acid is a potent lipid peroxidant in rat-brain homogenates. Neurochem Res. 1991;16:1139–1143. doi: 10.1007/BF00966592. [DOI] [PubMed] [Google Scholar]
- Santamaria D, Espinoza-Gonzalez V, Rios C, Santamaria A. N Omega- nitro-L- arginine, a nitric oxide synthase inhibitor, antagonizes quinolinic acid-induced neurotoxicity and oxidative stress in rat striatal slices. Neurochem Res. 1999;24:843–848. doi: 10.1023/A:1020949812581. [DOI] [PubMed] [Google Scholar]
- Schneider LJ. Treatment of Alzheimer’s disease with cholinesterase inhibitors. Clin Geriatr Med. 2001;17:337–339. doi: 10.1016/S0749-0690(05)70072-0. [DOI] [PubMed] [Google Scholar]
- Singleton VL, Orthofer R, Lamuela-Raventos RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin–Cioalteau reagent. Met in Enzymol. 1999;299:152–78. doi: 10.1016/S0076-6879(99)99017-1. [DOI] [Google Scholar]
- Stacey NH, Kappus H. Cellular toxicity and lipid peroxidation in response to mercury. Toxicol Appl Pharmacol. 1982;63:29–35. doi: 10.1016/0041-008X(82)90023-0. [DOI] [PubMed] [Google Scholar]
- Stone TW. The neuropharmacology of quinolinic acid and kynurenic acids. Pharmacol Rev. 1993;45:309–379. [PubMed] [Google Scholar]
- Stone TW. Kynurenines in the CNS: from endogenous obscurity to therapeutic importance. Prog Neurobiol. 2001;64:185–218. doi: 10.1016/S0301-0082(00)00032-0. [DOI] [PubMed] [Google Scholar]
- Stone TW, Perkins MN. Quinolinic acid: a potent endogenous excitant at amino acid receptors in CNS. Eur J Pharmacol. 1981;72:411–412. doi: 10.1016/0014-2999(81)90587-2. [DOI] [PubMed] [Google Scholar]
- Tenneti L, Lipton SA. Involvement of activated caspase-3-like proteases in N- methyl-D-aspartate-induced apoptosis in cerebrocortical neurons. J Neurochem. 2000;74:134–142. doi: 10.1046/j.1471-4159.2000.0740134.x. [DOI] [PubMed] [Google Scholar]
- Zago MP, Verstraeten SV, Oteiza PI. Zinc in the prevention of Fe2+ initiated lipid and protein oxidation. Biological Res. 2000;33:143–150. doi: 10.4067/S0716-97602000000200014. [DOI] [PubMed] [Google Scholar]
- Zandi PP, Anthony JC, Khachaturian AS. Reduced risk of Alzheimer disease in users of antioxidant vitamin supplements: the Cache County Study. Arch Neurol. 2004;61(1):82–88. doi: 10.1001/archneur.61.1.82. [DOI] [PubMed] [Google Scholar]
- Zar JH. Biostatistical analysis. USA: Prentice-Hall Inc; 1984. p. 620. [Google Scholar]