Abstract
In our study effect of different end point temperature (51 °C, 65 °C, 71 °C and 79 °C) on physicochemical and storage stability of mutton chops were evaluated. The L* (lightness) value and b* (yellowness) increased (P < 0.05) in cooked mutton chops than the raw mutton. The a* value (redness) decreased (P < 0.05) as end point temperature increased. As internal cooking temperature increased soluble myoglobin content decreased with a corresponding increase in percent myoglobin denatured. Raw mutton chops (uncooked) had lower level of oxidation (less TBA values) than cooked mutton irrespective of storage length. Initial APC of raw and cooked mutton chops ranged from log 1.75 to log 3.73 and was lower in higher end point cooking temperature. It can be concluded that as end point temperature increased, mutton chops appear less red and raw mutton had lower level of oxidation than cooked mutton chops.
Keywords: Cooking, Colour, Mutton, Myoglobin, Storage
Introduction
Meat colour is determined primarily by the concentration and chemical state of myoglobin. Consumers do not accept products in which colour varies from the expected normal appearance (Sen et al. 2004). Variation in the colour of precooked meat products cooked to the same internal temperature has been a problem in the meat industry for over 30 years (Kropf and Hunt 1998). Many consumers use the internal cooked appearance to evaluate doneness. Persistent pinking and premature browning are two different occurrences that prevent cooked colour from being a reliable indicator of doneness.
Persistent pinking occurs when a pinkish internal colour remains after temperature ensuring safety from a microbial standpoint has been attained (Cornforth et al. 1991). However, the pink colour defect in cooked poultry white meat is perceived by consumers as an undercooked and unsafe to eat product (Holownia et al. 2004). Premature browning occurs when ground beef appear well done, with no pink remaining, even though temperatures that ensure microbial safety have not been attained. Though colour development in cooked meat depends on cooking temperatures and so many other factors including muscle pH and inherent meat quality. Moeller et al. (2010) reported that tenderness and juiciness scores were related to a greater extent to loin pH and to a lesser extent to cooked temperature. However, Crawford et al. (2010) suggested that pork loin tenderness (shear force) increased as cooked temperature increased. In heated muscle extracts, metmyoglobin can be reduced, producing a red colour similar to oxymyoglobin (Osborn et al. 2003).
It is fairly well accepted that cooking of meat and meat products increases the rate and extent of oxidation. McCarthy et al. (2001) reported cooking of pork patties significantly increased thiobarbituric acid reactive substances (TBARS) values with a 4-fold increase in oxidation levels being recorded in raw patties upon cooking. The rapid development of off flavour in cooked meat is commonly described as ‘warmed-over flavour’ and is generally considered to be due to the oxidation of phospholipids located in the cell membranes (Su et al. 1991). Patil et al. (2003) observed the increase in TBA values when the chevon was aged at 1 °C for 12 and 15 days. Much of the research concerning cooked colour and doneness has been done on beef and pork and no data are available for cooked mutton. Therefore, the objectives of our study were (1) to examine the effect of end point temperature on colour development of mutton chops, (2) to determine the effect of end point cooking temperature on myoglobin state of cooked mutton and (3) to assess the storage stability of raw and cooked mutton chops at refrigerated temperature.
Materials and methods
Mutton longissimus dorsi from each side of carcass were collected within 1–2 h after slaughter from a local processing plant for each of the trials. All animals were approximately 8–10 months old at slaughter and were slaughtered according to the traditional Halal method followed in India. The cuts packaged in low density polyethylene (LDPE) pouches were transported immediately to the laboratory. The cuts were trimmed off all external fat and cut into chops of uniform size (2 cm thick).
Mutton chops were cooked in a grilling oven (Model MC-767, LG Electronics India Pvt. Ltd., New Delhi, India). Temperature was monitored for each sample using thermocouple thermometer (Okaton thermometer, Eutech Instruments, Ayer Rajah Crescent, China) with a needle probe inserted from the side to reach the geometric centre of each chop. When the chops reached the targeted end point temperature (51 °C, 65 °C, 71 °C and 79 °C) were removed from the cooking device and held at room temperature for 5 min. Each chop was analysed for instrumental colour, water activity, myoglobin denaturation. Raw and cooked mutton chops were then separately placed in laminated polyethylene pouches and analysed for physicochemical and microbial quality characteristics after 0, 4, 8 days of refrigerated (4 ± 1 °C) storage.
Instrumental colour
Colour measurements were conducted on the surface of samples with a Miniscan XE plus (Hunter Associated Labs, Inc, Reston, VA., USA) that had been calibrated against black and white reference tiles (X = 78.6, Y = 83.4, Z = 89.0). CIE L* (lightness), a* (redness) and b* (yellowness) values were obtained using a setting of D65 (daylight, 65-degree light angle). The hue angle (arc b*/a*) and chroma (a*2 + b*2)1/2 were calculated based on illuminant D65 and 10° standard observer. An average value from 4 random locations in each sample surface was used for statistical analysis.
pH determination
The pH of muscle was determined using a Thermo Orion KNI pH combo probe fitted in a Thermo Orion pH meter Model 420A + (Orion Research, Inc. Beverly, MA, USA). The probe was inserted in the center of each muscle perpendicular to the muscle fibers after standardized the pH meter at pH 4, 7 and 9.2.
Cook loss%
Each mutton chop for different end point cooking temperature was weighed before and after cooking. The loss after cooking was expressed in percentage.
Water holding capacity (WHC%)
The WHC of meat samples was measured as reported by Wardlaw et al. (1973). Minced meat samples (20 g) were stirred with 30 ml 0.6 M sodium chloride in a centrifuge tube. The tubes were kept at refrigeration temperature (4 ± 1)°C for 15 min, stirred for one min and then centrifuged at 3,000 g for 15 min (R-24, Remi Instrument, India). The supernatant was measured and WHC (as ml of 0.6 M NaCl retained by 100 g of meat) was expressed as a percentage.
Water activity (aw)
Water activity was determined with a Rotronic AG analyzer (Model Hygrolab 3, Grinddelstr. 6, CH-8303 Bassersdorf, UK) at 25 °C.
Moisture%
Moisture contents of raw and cooked mutton was determined by the method described in AOAC (1995).
Myoglobin denaturation
Myoglobin was extracted from raw or cooked samples in cold (1 °C) 0.04 M phosphate buffer, pH 6.8 (Warriss 1979). Total myoglobin and metmyoglobin (% of total) was calculated based on absorbance of clarified extract at 525, 572, and 700 nm (Krzynicki 1979) using a Model UV-2100 UV–VIS recording spectrophotometer (Shimadzu Co., Kyoto, Japan). Total myoglobin (Mb), metmyoglobin (MetMb,% of total), and percent Mb denatured during cooking (PMD) were calculated using the following formulas (Trout 1989):
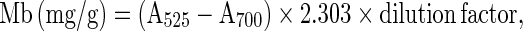 |
Where Mb = deoxy Mb + MbO2 + MetMb.
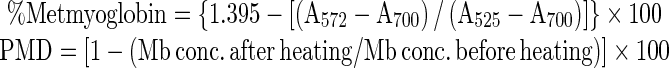 |
TBA number
Thiobarbituric acid reactive substances (TBARS) assay was performed as described by Buege and Aust (1978), as modified by Lee et al. (1999). After cooking to the four end point temperatures, streaks were sliced horizontally and duplicate 0.5 g samples were taken from the steak interior. Meat samples (0.5 g) were then mixed with 2.5 ml of stock solution containing 0.375% thiobarbituric acid, 15% trichloro-acetic acid and 0.25 N HCl. The mixture was heated for 10 min in a boiling water bath (100 °C) to develop pink color, cooled in tap water, and then centrifuged at 5,500 rpm for 25 min. The absorbance of the supernatant was measured spectrophotometrically at 532 nm against a blank that contained all the reagents minus the meat. The malonaldehyde (MDA) concentration was calculated using an extinction coefficient of 1.56 × 105 M−1 cm−1 for the pink TBA-MDA pigment. The MDA concentration was converted to TBA number (mg MDA/kg meat sample) as follows:
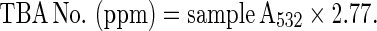 |
Microbiological analysis
Bacterial counts were determined by the pour plate method. Meat sample (10 g) was homogenized with 90 ml, 0.1% sterile peptone water. Serial 10-fold dilutions were prepared by diluting 1 ml of homogenate in 9 ml of 0.1% peptone water. Appropriate serial dilutions were duplicate plated with plate count agar for aerobic plate count (APC) and pshychrotropic count (PPC) and violet red bile agar (VRBA) for coliform counts. Plates were incubated at 37 °C for 48 h for APC and coliforms and 7 °C for 10 days for PPC (ICMSF 1983).
Sensory analysis
Sensory odour score during storage at refrigerated temperature was judged by a five member expert panelists in a 5-point scale (5 = Highly desirable and 1 = Undesirable off odour).
Statistical analysis
All data were analysed using SPSS (version 10.0 for Windows, SPSS, Chicago, Ill., USA). Three trials were conducted and the measurements were made in duplicate. One way ANOVA was applied for colour values, pH, WHC%, water activity, moisture and myoglobin denaturation. However, in case of storage data (TBA and microbial counts) a two-way ANOVA was performed. Significant means were separated using the least significance difference (LSD) test (P < 0.05).
Results and discussion
Means for the instrumental colour values of raw and cooked mutton chops are shown in Table 1. The L* (lightness) value and b* (yellowness) increased (P < 0.05) in cooked mutton chops with the increase in end point cooking temperature. Although data for lightness were not significant at each point temperature, the values increased as temperature increased. The a* value (redness) decreased (P < 0.05) as end point temperature increased. Lien et al. (2001) also reported that the internal cooked color of pork loin chops became progressively less reddish pink as end point temperature increased. Yancey et al. (2011) also reported that steaks cooked to a 65.5 °C were the reddest internally and those cooked to 76.7 °C were the least red. The values for saturation index and hue angle increased (less red and more yellow) as end point temperature increased. For redness and chroma, more differences were noted up to 65 °C end point temperature, once 71 °C was reached, differences became less apparent.
Table 1.
Effect of end point temperature on some quality characteristics of raw and cooked mutton chops
| Treatment | ||||||
|---|---|---|---|---|---|---|
| Raw | T51(51 °C) | T65 (65 °C) | T71 (71 °C) | T79 (79 °C) | SEM | |
| Instrumental Colour x (n = 12) | ||||||
| L* | 36.1b | 44.4a | 46.0a | 46.7a | 47.8a | 1.14 |
| a* | 11.4a | 11.4a | 10.3a | 8.9b | 8.2b | 0.44 |
| b* | 13.1b | 19.9a | 19.5a | 19.7a | 20.7a | 1.30 |
| Huey | 48.8d | 55.5c | 61.9b | 64.8b | 68.4a | 1.49 |
| Chromaz | 17.4b | 21.6a | 22.1a | 21.7a | 22.1a | 1.00 |
| Physicochemical (n = 6) | ||||||
| pH | 5.9b | 5.9b | 6.4a | 6.4a | 6.4a | 0.06 |
| Cook loss% | – | 5.5d | 12.0c | 16.5b | 31.1a | 3.88 |
| WHC% | 48c | 46.7c | 53.3b | 58.3a | 58.3a | 2.45 |
| Water activity | 0.91 | 0.90 | 0.93 | 0.93 | 0.93 | 0.01 |
| Moisture% | 75.2a | 74.1a | 71.8b | 70.9b | 69.9b | 0.60 |
| Myoglobin Denaturation (n = 9) | ||||||
| Myoglobin | 5.1a | 3.7b | 2.7c | 1.9cd | 1.1d | 0.35 |
| Metmyoglobin | 64.6 | 70.8 | 59.1 | 54.0 | 63.7 | 7.01 |
| Denatured myoglobin | – | 25.7c | 45.7b | 62.6a | 77.3a | 6.24 |
xHunter Lab Spectrocolorimeter, illuminant D65, 10 0 observer, 2.5 cm port
yHue angle = tan−1(b*/a*), L*- Lightness, a*- Redness, b*-Yellowness
zChroma = (a* 2 + b*2)1/2
SEM Standard error of means
Means with same letters are not different (P > 0.05)
T51, T65, T71, T79 -end point temperature 51 °C, 65 °C, 71 °C, 79 °C respectively
The pH increased significantly (P < 0.05) as the end point temperature increased (Table 1). After 65 °C end point temperature it remains almost constant. The cook loss% also showed the similar trend. However, as each end point temperature increase, the cook loss% increased significantly. It is expected that due to higher temperature, more water and meat juices will be expelled as cook loss. At lower end point temperature (51 °C) no significant difference was noted in Water holding capacity (WHC) of raw and cooked mutton chops. As the temperature increased WHC% increased and once temperature reached to 71 °C the value was almost constant. Here we can infer that to get optimum juiciness in mutton chops, higher end point temperature is desirable and reached to be at least 71 °C. The water activity was more in mutton chops cooked at higher end point temperature though the differences were minimal and not significant. Moisture% significantly (P < 0.05) reduced as the end point cooking temperature increased. Though, after attaining the end point temperature at 65 °C, no significant change was observed in moisture% of cooked mutton chops.
The myoglobin and denatured myogobin% of mutton chops at different end point temperature are shown in Table 1. As internal cooking temperature increased soluble myoglobin content decreased with a corresponding increase in percent myoglobin denatured. Percent myoglobin denaturation values ranged from 0 (raw chops) to 77.30% in mutton chops cooked to 79 °C internal temperature. The increase in percent myoglobin denaturation with increased temperature was also reported by John et al. (2005). The metmyoglobin decreased as end point cooking temperature increased and the maximum metmyoglobin was observed in mutton chops with end point temperature of 79 °C. The metmyoglobin content at this temperature was almost similar as in raw mutton chops.
Other researchers have shown that increased reddening may become particularly important when the end point temperature is below 71 °C and suggested that myoglobin will partially be denatured upon cooking (Deniz and Serdaroglu 2003).
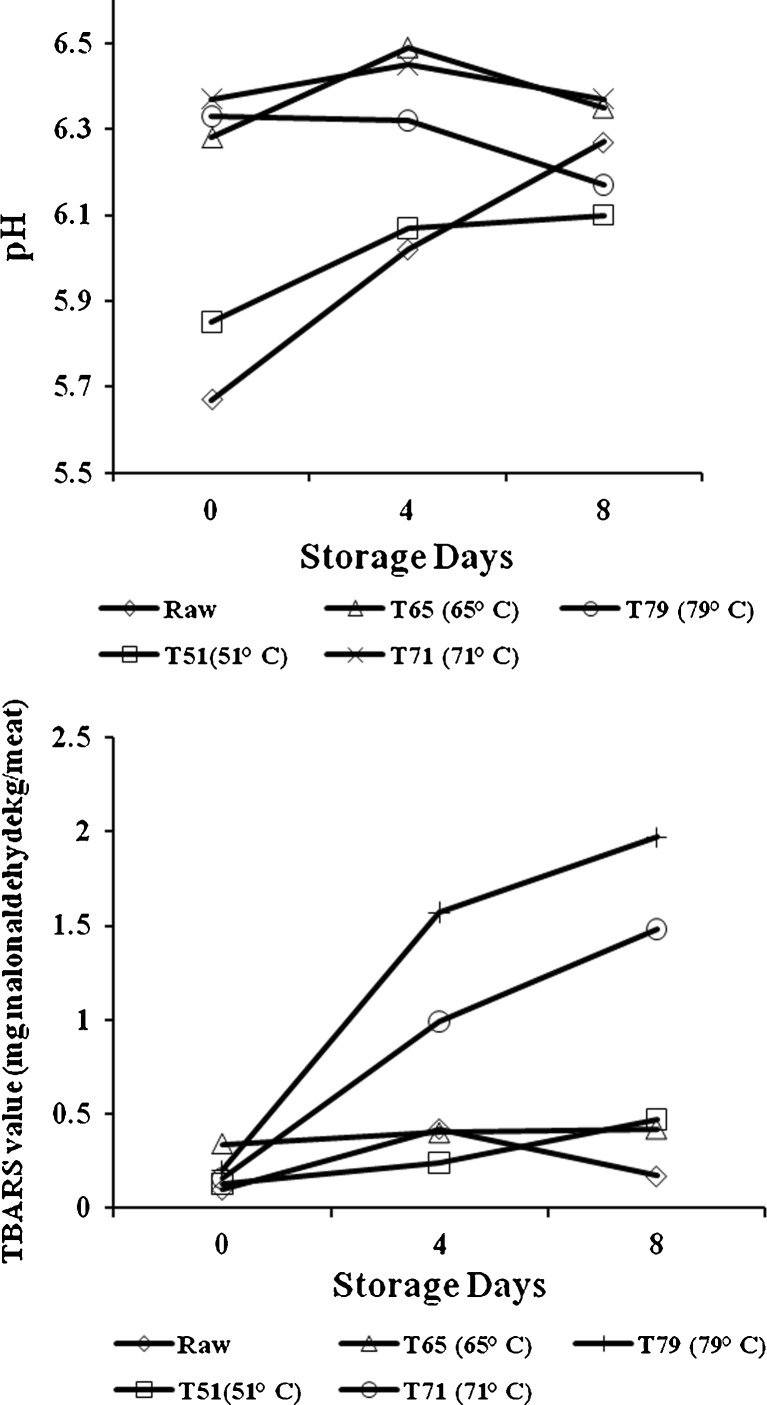
The pH and TBA values of raw and cooked mutton during storage are shown in Fig. 1. During storage, the pH of mutton chops increased though the increase was more prominent in raw and cooked mutton chops at 51 °C end point temperature. In case of higher end point temperature this change was very small and non-significant. The storage length and meat condition significantly influenced (P < 0.01) the extent of lipid oxidation. Raw mutton chops (uncooked) had lower level of oxidation (less TBA values) than cooked mutton irrespective of storage length. Similar findings in broiler chicken were also reported by Onibi and Osho (2007). This may be due to the fact that heating accelerates lipid oxidation and production of volatiles in meat (Beltran et al. 2003) by disrupting muscle cell structure, inactivating antioxidant enzymes and other antioxidant compounds and releasing iron from heme pigments (Min et al. 2008). Therefore, cooking had a significant pro oxidant effect in meat as measured by TBA values.
Fig. 1.
Effect of end point temperature and storage days on pH and TBA values of raw and cooked mutton chops, n = 6
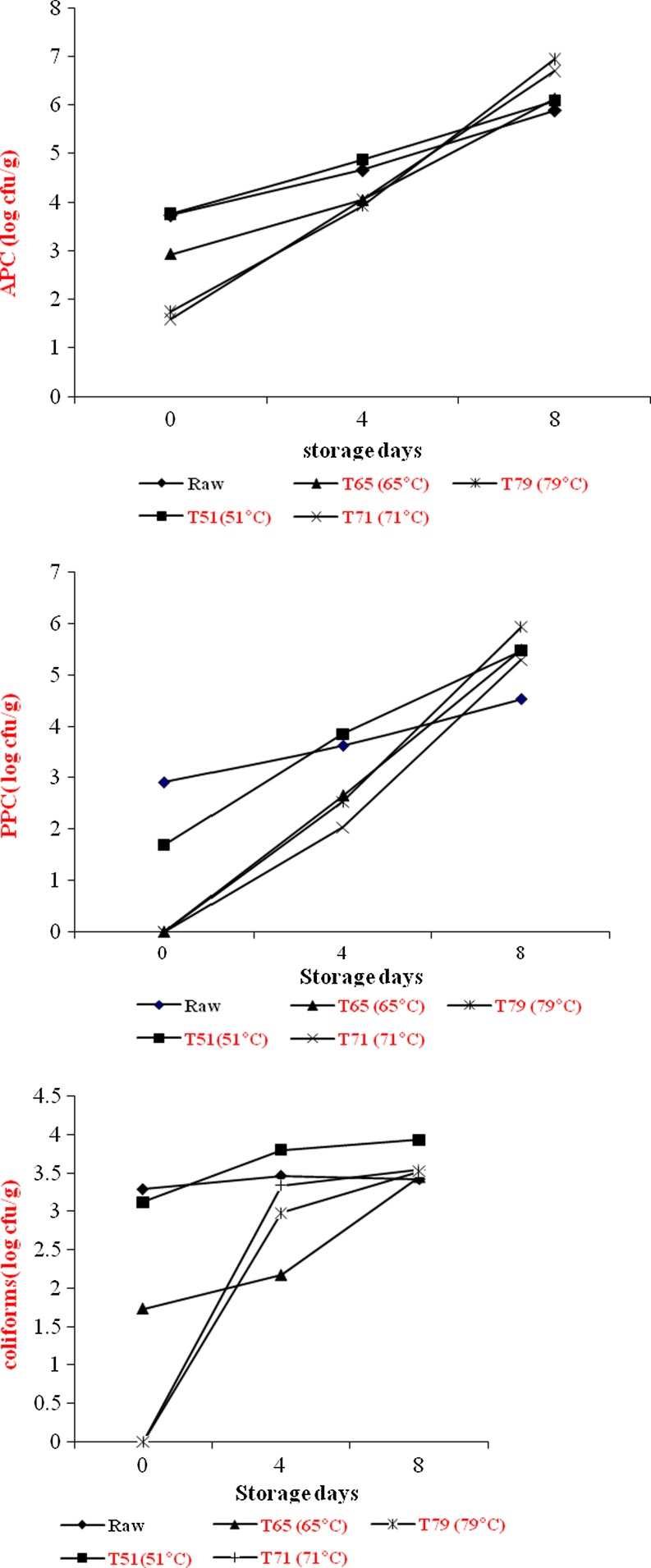
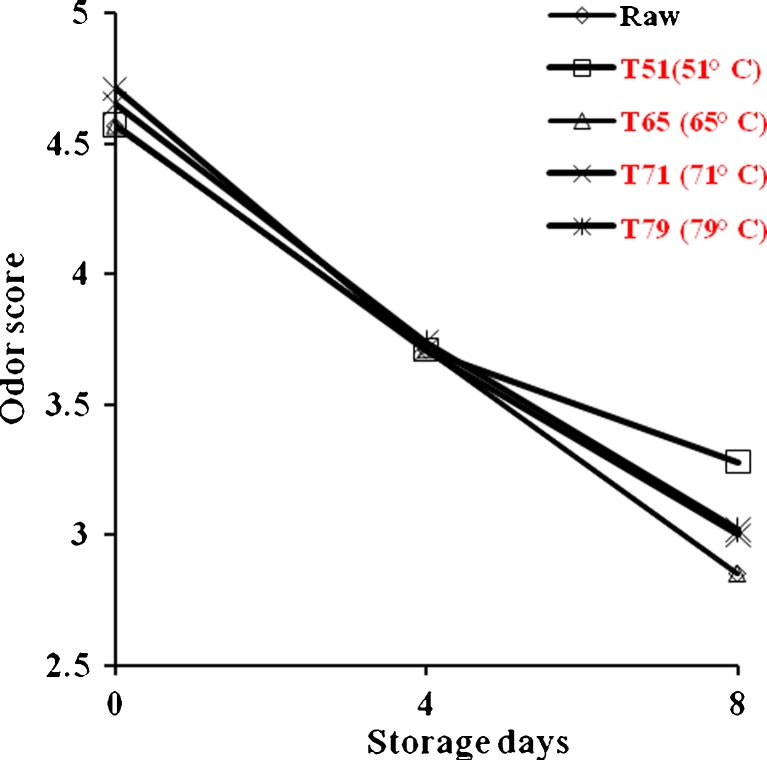
Aerobic plate counts (APC), Pshychrotrphic counts (PPC) and coliforms of raw and cooked mutton chops during refrigerated storage are shown in Fig. 2. Initial APC of raw and cooked mutton chops ranged from log 1.75 to log 3.73 and was lower in higher end point cooking temperature. However, after 8 days of refrigerated storage the cooked meat showed higher APC counts than raw mutton chops. But the APC levels were well within the acceptable limits (Kumudavally et al. 2011). Similarly, the rate of growth of PPC was more in cooked mutton chops than raw. However, no PPC was noted in mutton chops after attaining the end point cooking temperature of 65 °C. The coliforms also showed the similar trend. Initially (0 day), the lower bacterial load of the cooked meat than raw (uncooked) mutton chops may be attributed to the fact that some of the bacteria might be thermolabile. Also the surface cover of raw meat is soft for easy penetration of bacteria while cooked meat is a little toughened and drier thus making penetration of bacteria more difficult. Though with the progressive storage, the picture was different and cooked meat showed higher bacterial load than the raw mutton chops. No off odor was detected after 8 days of refrigerated storage (Fig. 3). Samples were rated very good to good in odor score and no significant difference was observed in raw and cooked mutton chops at different end point temperature.
Fig. 2.
Effect of end point temperature and storage days on microbial counts (log cfu/g) of raw and cooked mutton chops, n = 6
Fig. 3.
Effect of end point temperature and storage days on odor score of raw and cooked mutton chops, n = 12
Conclusions
It can be concluded that as end point temperature increased, mutton chops appear less red and myoglobin denaturation increased as end point cooking temperature increases. Raw mutton had lower level of oxidation than cooked mutton chops. Further, raw (uncooked) mutton contains more bacterial load than cooked mutton chops. It needs further study for mutton to optimize cooking temperature for proper myoglobin denaturation and produce safe precooked mutton without much deterioration in color during refrigerated storage.
Acknowledgement
Authors are thankful to the Officer on Special Duty, NRC on Meat, Hyderabad for providing necessary facilities.
References
- Official methods of analysis. 16. Washington: Association of Official Analytical Chemists; 1995. pp. 391–399. [Google Scholar]
- Beltran E, Pla R, Yuste J, Mor-mur M. Lipid oxidation of pressurized and cooked chicken: role of sodium chloride and mechanical processing on TBARS and hexanal values. Meat Sci. 2003;64:19–25. doi: 10.1016/S0309-1740(02)00132-8. [DOI] [PubMed] [Google Scholar]
- Buege JA, Aust SD. Microsomal lipid peroxidation. Methods Enzymol. 1978;52:302–304. doi: 10.1016/S0076-6879(78)52032-6. [DOI] [PubMed] [Google Scholar]
- Cornforth D, Calkins CR, Faustman C (1991) Methods for identification and prevention of pink color in cooked meat. In: Proc. Reciprocal Meat Conf., American Meat Science Association, Savoy, IL, pp 53–58
- Crawford SM, Moeller SJ, Zerby HN, Irvin KM, Leeds TD. Effects of cooked temperature on pork tenderness and relationships among muscle physiology and pork quality traits in loin from Landrace and Berkshire swine. Meat Sci. 2010;84:607–612. doi: 10.1016/j.meatsci.2009.10.019. [DOI] [PubMed] [Google Scholar]
- Deniz EE, Serdaroglu M. Effects of nitrite levels, end point temperature and storage on pink color development in turkey rolls. Eur Food Res Tech. 2003;217:471–474. doi: 10.1007/s00217-003-0774-4. [DOI] [Google Scholar]
- Holownia K, Chinnan MS, Reynolds AE. Pink color defects in poultry white meat as affected by endogenous conditions. J Food Sci. 2004;68:742–747. doi: 10.1111/j.1365-2621.2003.tb08235.x. [DOI] [Google Scholar]
- Elliott RP, Clark DS, Lewis KH, Lundbeck H, Olson JC, Simonsen, editors. Microorganisms in foods. Their significance and methods of enumeration. Toronto: University of Toronto Press; 1983. International commission of microbiological specifications for foods. [Google Scholar]
- John L, Cornforth D, Carpenter CE, Sorheim O, Pettee BC, Whittier DR. Color and thiobarbituric acid values of cooked top sirloin steaks packaged in modified atmospheres of 80% oxygen or 0.4% carbon monoxide or vacuum. Meat Sci. 2005;69:441–449. doi: 10.1016/j.meatsci.2004.08.013. [DOI] [PubMed] [Google Scholar]
- Kropf DH, Hunt MC. End point cooking temperature and meat color. Proc Recip Meat Conference. 1998;51:144–148. [Google Scholar]
- Krzynicki K. Assessment of relative content of myoglobin, oxymyoglobin and metmyoglobin at the surface of beef. Meat Sci. 1979;3:1–5. doi: 10.1016/0309-1740(79)90019-6. [DOI] [PubMed] [Google Scholar]
- Kumudavally KV, Tabassum A, Radhakrishna K, Bawa AS. Effect of ethanolic extract of clove on the keeping quality of fresh mutton during storage at ambient temperature (25 ± 2 °C) J Food Sci Tech. 2011;48:466–471. doi: 10.1007/s13197-010-0181-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B, Hendricks DG, Cornforth DP. A comparison of carnosine and ascorbic acid on color and lipid stability in a ground beef patties model system. Meat Sci. 1999;51:245–253. doi: 10.1016/S0309-1740(98)00121-1. [DOI] [PubMed] [Google Scholar]
- Lien R, Hunt MC, Anderson S, Kropf DH, Loughin TM, Dikeman ME, Velazco J. Effects of end point temperature on the internal color of pork patties of different myoglobin form, initial cooking state and quality. J Food Sci. 2001;67:1011–1015. doi: 10.1111/j.1365-2621.2002.tb09445.x. [DOI] [Google Scholar]
- McCarthy JP, Kerry JF, Lynch PB, Buckley DJ. Evaluation of the antioxidant potential of natural food/plant extracts as compared with synthetic antioxidants and vitamin E in raw and cooked pork patties. Meat Sci. 2001;57:45–52. doi: 10.1016/S0309-1740(00)00129-7. [DOI] [PubMed] [Google Scholar]
- Min B, Nam KC, Cordray J, Ahn DU. Endogenous factors affecting oxidative stability of beef loin, pork loin and chicken breast and thigh meats. J Food Sci. 2008;73:C439–C446. doi: 10.1111/j.1750-3841.2008.00805.x. [DOI] [PubMed] [Google Scholar]
- Moeller SJ, Miller RK, Edwards KK, Zerby HN, Boggess M, Box-Steffensmeier JM. Consumer perception of pork eating quality as affected by pork quality attributes and end point cooked temperature. Meat Sci. 2010;84:14–22. doi: 10.1016/j.meatsci.2009.06.023. [DOI] [PubMed] [Google Scholar]
- Onibi GE, Osho IB. Oxidative stability and bacteriological assessment of meat from broiler chicken fed diets containing Hibiscus sabdariffa calyces. African J Biotech. 2007;6:2721–2726. [Google Scholar]
- Osborn HM, Brown H, Adams JB, Ledward DA. High temperature reduction in metmyoglobin in aqueous muscle extracts. Meat Sci. 2003;65:631–637. doi: 10.1016/S0309-1740(02)00258-9. [DOI] [PubMed] [Google Scholar]
- Patil DA, Gunjal BB, Pawar VD, Surve VD, Machewad GM. Effect of aging on quality of chevon. J Food Sci Tech. 2003;40:528–530. [Google Scholar]
- Sen AR, Muthukumar M, Naveena BM, Babji Y. Colour changes in broiler and sheep muscles during frozen storage. J Food Sci Tech. 2004;41:678–680. [Google Scholar]
- Su Y, Ang CYW, Lillard DA. Precooking method affects warmed-over-flavors broiler breast patties. J Food Sci. 1991;56:881–898. doi: 10.1111/j.1365-2621.1991.tb14597.x. [DOI] [Google Scholar]
- Trout GR. Variation in myoglobin denaturation and color of cooked beef, pork and turkey meat as influenced by pH, sodium chloride, sodium tripolyphosphate and cooking temperature. J Food Sci. 1989;54:536–540. doi: 10.1111/j.1365-2621.1989.tb04644.x. [DOI] [Google Scholar]
- Wardlaw FB, Mc Caskill LH, Acton JC. Effect of postmortem muscle changes in poultry meat loaf properties. J Food Sci. 1973;38:421–424. doi: 10.1111/j.1365-2621.1973.tb01444.x. [DOI] [Google Scholar]
- Warriss PD. The extraction of haeme pigments from fresh meat. J Food Tech. 1979;14:75–80. doi: 10.1111/j.1365-2621.1979.tb00849.x. [DOI] [Google Scholar]
- Yancey JWS, Wharton MD, Apple JK. Cooking method and end point temperature can affect the Warner-Bratzler shear force, cooking loss and internal cooked colour of beef longissimus steaks. Meat Sci. 2011;88:1–7. doi: 10.1016/j.meatsci.2010.11.020. [DOI] [PubMed] [Google Scholar]