Abstract
Microfluidic devices functionalized with EpCAM antibodies were utilized for the capture of target cancer cells representing circulating tumor cells (CTCs). The fraction of cancer cells captured from homogeneous suspensions is mainly a function of flow shear rate, and can be described by an exponential function. A characteristic shear rate emerges as the most dominant parameter affecting the cell attachment ratio. Utilizing this characteristic shear rate as a scaling factor, all attachment ratio results for various combinations of receptor and ligand densities collapsed onto a single curve described by the empirical formula. The characteristic shear rate increases with both cell-receptor and surface-ligand densities, and empirical formulae featuring a product of two independent cumulative distributions described well these relationships. The minimum detection limit in isolation of target cancer cells from binary mixtures was experimentally explored utilizing microchannel arrays that allow high-throughput processing of suspensions about 0.5 ml in volume, which are clinically relevant, within a short time. Under a two-step attachment/detachment flow rate, both high sensitivity (almost 1.0) and high specificity (about 0.985) can be achieved in isolating target cancer cells from binary mixtures even for the lowest target/non-target cell concentration ratio of 1:100 000; this is a realistic ratio between CTCs and white blood cells in blood of cancer patients. Detection of CTCs from blood samples was also demonstrated using whole blood from healthy donors spiked with cancer cells. Finally, the viability of target cancer cells released after capture was confirmed by observing continuous cell growth in culture.
INTRODUCTION
Cancer progression is characterized by cells that invade locally and metastasize to nearby tissue or spread throughout the body.1 During metastatic progression, cancer cells modulate their adhesive properties to allow for invasion from the primary tumors, transit into the circulatory system and establishment of secondary colonies in distant organs.2 The clinical significance of circulating tumor cells (CTCs) in metastatic cancer has been clearly demonstrated.3, 4 The prognostic value of CTCs drug resistance profile in metastatic breast cancer patients has been confirmed,5 and the detection of CTCs before initiation of therapy in cancer patients with metastatic disease is found to be highly predictive of overall survival.6 Hence, CTCs represent a potential alternative to invasive biopsies for monitoring of non-haematologic cancers.7 CTCs, however, are rare in blood and, consequently, selectively isolating them in a timely plausible process is a formidable technical challenge.8 The main obstacle in securing viable clinical information via CTC analysis is the extremely low concentration of these cells among a high number of other cells in peripheral blood.9, 10, 11, 12, 13 Numerous reports suggest that some 10–100 CTCs are present in 1 ml whole blood of cancer patients among some 109 erythrocytes and 106 leukocytes. Sampling such rare events in a large population, three important metrics must be assessed simultaneously: sensitivity, throughput, and viability.14
Cell adhesion to a surface has long been a subject for intense research effort because of its significant physiological importance. Several studies on cell attachment and detachment have provided useful data on receptor-mediated adhesion kinetics.15, 16, 17 The adhesion force is derived from the number and strength of bonds formed between the cell and the surface. The number of active bonds, contributing to the resultant adhesion force, depends on both membrane receptor and surface ligand densities.18 Different functional properties of receptor/ligand combinations give rise to different dynamic states of adhering cells in shear flow;19 several adhesion modes have been observed: firm adhesion, transient tethering, and rolling at reduced velocities.20, 21, 22, 23
Microfluidic systems provide a unique opportunity for cell sorting and detection; they have been applied for continuous size-based separation, flow cytometry, and adhesion-based separation.24 Requiring relatively simple equipment and providing superior observation capabilities, cell capture and adhesive rolling have been extensively studied using microfluidic devices.25, 26 In particular, antibody-functionalized microchannels have been utilized for the isolation of cancer cells from either homogeneous or heterogeneous suspensions.4, 27, 28 Utilizing micro-posts coated with EpCAM antibodies, viable CTCs were selectively separated from peripheral whole blood samples.27 By combining E-selectin and anti-EpCAM molecules, efficient capture of target cells was reported in microfluidic chambers.29 Highly efficient capture of CTCs was reported by using nanostructured silicon substrates with integrated chaotic micromixers.30 Label-free cancer cell separation techniques, such as the size-based separation using deterministic lateral displacement structure31 or using dielectrophoretic techniques,32 were also reported.
In our previous work, we characterized the attachment and detachment of circulating tumor cells in antibody-functionalized microchannels,15, 16, 17 and proposed a particular flow pattern to enhance the system performance in specifically isolating target cells.33 We reported a characteristic shear rate controlling the fraction of cells captured under applied shear flow.18 In this work, an empirical formula is proposed to explicitly describe the effect of receptor and ligand densities on the number of captured cells. The detection limit of rare target cells is explored, and the viability of captured cells is tested by culturing released cells.
METHODS
Device fabrication and packaging
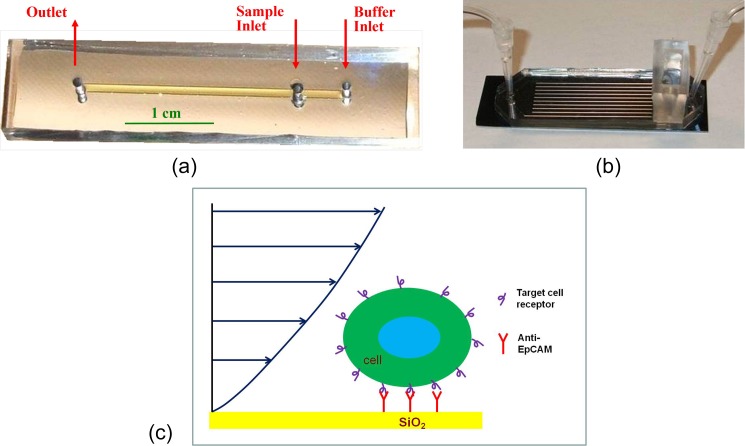
Two types of microfluidic devices were utilized for experiments in this study, single-microchannel and microchannel-array devices. The mold for the single-microchannel devices was fabricated in a silicon wafer using standard photolithography and tetramethyl-ammonium-hydroxide (TMAH) etching,33 while that for the microchannel-array devices was fabricated using polymer SU-8 (MicroChem, USA).34 Polydimethylsiloxane (PDMS) (Dow Corning, USA) microchannel replicas with inlet/outlet holes, peeled off the molds, were capped using oxidized silicon substrates to complete the fabrication process. Single-microchannel devices feature a straight channel about 100 μm in height (H), 1 mm in width (W), and 30 mm in length (L). Microchannel-array devices consist of 10 parallel channels; each is about 35 mm long, 1 mm wide, and 155 μm high, with a 500 μm-wide wall separating each pair of adjacent channels. The device length was selected such as to allow capture of all loaded target cells.17 Photographs of packaged single-microchannel and microchannel-array devices are shown in Figures 1a, 1b, respectively.
Figure 1.
Photographs of fabricated and packaged (a) single-microchannel, (b) microchannel-array devices, and (c) a schematic drawing of a captured cell under shear flow in an antibody-functionalized microchannel. Note that, in both device types, the cell sample inlet is positioned between the device inlet and outlet to allow smooth introduction of loaded cells into a pre-established stream in the functionalized microchannels.
The fabricated microdevices were then functionalized following a previously described protocol.33 The surface hydroxyl groups were silanated in 1% (vol./vol.) 3-aminopropyltriethoxysilane (APTES from Sigma)-acetone solution, and activated with 2% (vol./vol.) glutaraldehyde (Sigma) in 1 × Phosphate-buffered Solution, calcium and magnesium free (1 × CMF-PBS). Recombinant protein G from E. coli (Zymed Lab Inc.) was incubated on the activated surface; this was followed by incubation of the microchannel surface with bovine serum albumin solution (BSA from Sigma, 2 mg/ml in 1 × CMF-PBS) to block the excessive silanol sites. Finally, EpCAM antibodies (MAB9601 Clone 158 206, R&D Systems, Inc., USA) were immobilized on the protein G layer. A schematic drawing of a captured cell under shear flow in an antibody-functionalized microchannel is shown in Figure 1c. The “Indirect” enzyme-linked immunosorbent assay (ELISA) technique was used to evaluate the surface ligand density; a secondary “detection” antibody, conjugated with Cy3 fluorescent dye, was incubated on the functionalized surface at a concentration of 25 μg/ml in 1 × CMF-PBS for 1 h. After washing the microchannels in 1 × CMF-PBS, images were taken under a fluorescent microscope to record the planar light intensity distribution. The average intensity values were utilized to estimate the density of the primary capture antibody.
Cell culture and sample preparation
Two breast cancer cell lines expressing EpCAM receptors, MDA-MB-231 and BT-20, and a non-expressing EpCAM pancreatic cancer cell line, MIA PaCa-2, were utilized in this study. MDA-MB-231 cells were grown in RPMI 1640 buffer containing L-glutamine (Cellgro) with 10% fetal bovine serum (FBS, Cellgro) and 1% penicillin-streptomycin (Invitrogen). BT-20 cells were grown in Eagle's Minimum Essential Medium (EMEM from ATCC) with 10% FBS and 1% penicillin-streptomycin. MIA PaCa-2 cells were grown in Dulbecco's Modified Eagle's Medium (30–2002, ATCC) with 10% FBS and 1% penicillin-streptomycin. The cell lines were maintained in humid environment at 37 °C containing 5% CO2. Prior to each experiment, the cells were harvested using 4-mM Ethylenediaminetetraacetic acid (EDTA) and suspended in 1 × CMF-PBS. The average diameter of suspended MDA-MB-231, BT-20, and MIA PaCa-2 cells is about the same, 20 ± 2μm, as measured under a microscope. To distinguish between different cell types in mixtures, target cells (BT-20) were fluorescently labeled with CellTrackerTM Green CMFDA (5-chloromethylfluorescein diacetate, Invitrogen C7025), while non-target cells (MIA PaCa-2) were labeled with CellTrackerTM Orange CMRA (Invitrogen C34551). Labeling was accomplished, prior to EDTA harvesting, by incubating the cells for 30 min at 37 °C with the chosen CellTracker reagent (4.5 μM, diluted by 1 × DPBS, Dulbecco's PBS).
Both MIA PACA-2 and BT20 cells were derived directly from primary carcinoma and were not obtained from circulation. BT20 cells are not considered highly metastatic, while MIA PaCa-2 cells have shown strong metastatic capacity in mouse models and are therefore considered able to circulate.35 Regarding MDA-MB-231 cells, although these cells were not taken directly from a patient's blood, they were taken from metastatic sites distant from the primary tumor and they must have circulated.36 Therefore, both MDA-MB-231 and MIA PACA-2 cells can be used as models for CTC studies.
Experimental set-up
Experiments were conducted by driving cell suspensions through antibody-functionalized microfluidic devices under pre-determined flow rates. In each experiment, the microdevice was mounted on a probe station stage (Signatone S-1160), equipped with a microscope (Motic Microscope PSM-1000) and a CCD camera allowing in situ monitoring of cell motion. The number of cells entering each microfluidic device N0 was counted once the cell-sample loading had been completed. In the single channel experiment, the number of loaded cells was around N0 = 3000–4000. The total number of captured cells NA was determined after unbound cells were removed from the device under washing shear flow using 1 × CMF-PBS buffer solution. Each data point has been repeated for at least five times. To prevent cells from settling down at the inlet, the cell suspension entrance was placed downstream of the device inlet. The shear rate of the suspension flow in the channels was adjusted using two syringe pumps (PhD 2000, Harvard Apparatus) connected to the device inlet and outlet. While the inlet pump was used for driving buffer solution (1 × CMF-PBS) into the device at a desired flow rate, the outlet pump was used for withdrawing fluid out of the device at a higher flow rate. Thus, the cell suspension was forced into the device through the sample inlet due to the flow rate difference between the inlet and outlet pump. The cells were transported under a steady flow rate, and direct contact between sedimenting cells and functionalized surfaces resulted in cell adhesion to the microchannel bottom surface.
Theoretical modeling
An analytical model based on Stokesian as well as cell-adhesive dynamics is adopted to analyze cell behavior.18 The cell motion is modeled as a rigid sphere, with receptors on its surface, moving under shear flow above a solid surface immobilized with counter-receptor ligands. The motion of the sphere can be described by the following Langevin equation:37
| (1) |
where X is a state vector describing the time-dependent motion of the sphere; U∞ denotes the unperturbed velocity vector at the location of the sphere center; FS denotes the hydrodynamic loads due to viscous shear flow, while FG and FB represent the direct forces/torques acting on the sphere due to gravitation and adhesion forces, respectively; M is a mobility matrix; kB is the Boltzmann constant, and Ta is the ambient temperature. The terms kBTa∇M and wtI describe the effects of thermal fluctuations. The cell-surface adhesion force/torque due to receptor-ligand bindings, FB, depends on several parameters; among them, cell-receptor density NR and surface-ligand density NL are two parameters explicitly accounted for in the analytical model.
RESULTS
Attachment ratio of target cancer cells in functionalized microchannels
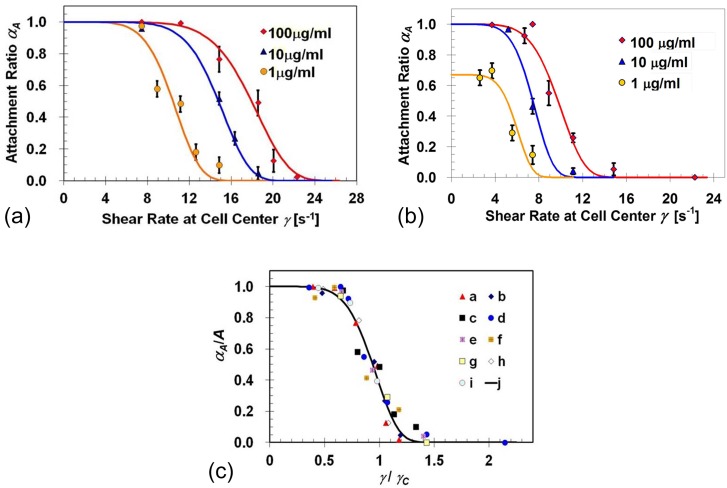
Target cell capture experiments were performed under various flow shear rates using BT-20 and MDA-MB-231 cell suspensions in microchannels functionalized with EpCAM antibody solutions at concentrations of 1, 10, or 100 μg/ml. The cell capture efficiency is characterized by determining the attachment ratio, αA, defined as the ratio between the number of captured cells, NA, and the total number of loaded cells, N0. The applied cell shear rate is taken as the shear rate expected at 10 μm above the channel bottom surface, i.e., the location of the cell-body center. Attachment-ratio results, obtained using single-channel microdevices, for BT-20 and MDA-MB-231 cells are, respectively, summarized in Figures 2a, 2b as a function of the applied shear rate. The expression level of EpCAM receptors in these two cell lines is not the same; on average, a BT-20 cell has about 139 500 while a MDA-MB-231 cell has only 1700 EpCAM receptors per cell.38 This two-orders-of-magnitude difference in the target receptor expression level is consistent with the attachment ratio results. The higher membrane receptor density allows the formation of more ligand-receptor bonds per unit area under the same shear flow rate. Consequently, the total adhesion force of BT-20 cells is higher than that of MDA-MB-231 cells resulting in a higher attachment ratio. The attachment ratio for both cell lines drops gradually from its maximum value to zero with increasing applied shear rate γ as described by the empirical formula
| (2) |
where γC is a characteristic shear rate largely depending on the type and number of receptor-ligand bonds; B is an exponent representing the non-uniformities associated with cell-surface interaction; and A is the maximum attachment ratio. Best fits of Eq. 2 to the experimental data for BT-20 cells yield values of γC = 11.13, 15.58, and 18.92 s−1 for microchannels functionalized with EpCAM antibodies at concentrations of 1, 10, and 100 μg/ml, respectively; while the corresponding values obtained for MDA-MB-231 cells were γC = 6.31, 7.94, and 10.39 s−1. Interestingly, the exponent B is about 6 ± 1 for both BT-20 and MDA-MB-231 cells captured in anti-EpCAM functionalized microchannels, at various EpCAM concentrations. Among all experiments conducted, the value of A was equal to 1 except in one single case where both the ligand and receptor densities were very low; for the low EpCAM-expressing MDA-MB-231 cells captured in microchannels functionalized with a solution of EpCAM antibodies at a low concentration of 1 μg/ml, the value of A was 0.65.
Figure 2.
Attachment-ratio measurements (symbols) as a function of the applied flow shear rate for: (a) BT-20 and (b) MDA-MB-231 cell lines in microchannels functionalized with EpCAM antibodies at three solution concentrations of 1, 10, and 100 μg/ml; the solid curves are best fits to the experimental-data sets calculated based on Eq. 2; (c) a summary of measured and computed cell-attachment ratio dependence on the applied shear rate: experimental data sets “a,” “b,” and “c” are for BT-20 cells, while “d,” “e,” and “f” are for MDA-MB-231 cells, both in microchannels functionalized with anti-EpCAM solution concentrations of 1, 10, and 100 μg/ml, respectively; computed data sets “g,” “h,” and “i” are for NR = 1.35 × 108/cm2 and NL = 6.35 × 107/cm2, NR = 1.35 × 108/cm2 and NL = 4.0 × 1010/cm2, and NR = 1.11 × 1010/cm2 and NL = 4.0 × 1010/cm2, respectively; curve “j” is calculated using Eq. 2 with exponents B = 6.
Using the theoretical model, the cell attachment ratio under various flow shear rates was calculated for cell receptor densities NR = 1.35 × 108/cm2 and 1.11 × 1010/cm2 with surface ligand densities NL = 6.35 × 107/cm2 and 4.0 × 1010/cm2. The low and high receptor densities correspond to MDA-MB-231 and BT-20 cell lines,38 respectively. The receptor density is calculated by dividing the number of cell receptors by the cell surface area. MDA-MB-231 and BT-20 cells are similar in size with a cell diameter about 20 μm and a surface area around 1.26 × 10−5 cm2. The ligand densities correspond to the low (1 μg/ml) and high (100 μg/ml) antibody-solution concentrations used in the experiments. The attachment-ratio measurements and calculations are compared in Figure 2c. All results collapse onto a single curve “j,” described by Eq. 2 with B = 6, when the attachment ratio αA is normalized by its maximum level A (obtained for γ → 0) and the applied shear rate γ by its characteristic value γC.
Effects of ligand and receptor density on target cancer cell attachment ratio
Among the three fitting parameters in Eq. 2, the maximum attachment ratio A is about 1 for most combinations of cell-receptor and surface-ligand densities, while the exponent B is found to be around 6 for all the data sets. Hence, for cell attachment, the characteristic shear rate γC emerges as the most dominant variable. The characteristic shear rate depends on several parameters including: (i) cell properties, diameter d and receptor density NR, (ii) microchannel properties, height h and ligand density NL, and (iii) properties intrinsic to the particular receptor-ligand interaction, such as the reaction on-rate kon, unstressed off-rate k0, and spring constant representing the bond strength κ. This relationship can be written as
| (3) |
The characteristic shear rate was reported to increase with either increasing surface-ligand or cell-receptor density,18 and error functions were empirically proposed to describe these functional relationships.39 Hence, the characteristic shear rate dependency on both cell receptor and surface ligand densities can be described explicitly by a single empirical formula consisting of a product of two error functions given by
| (4) |
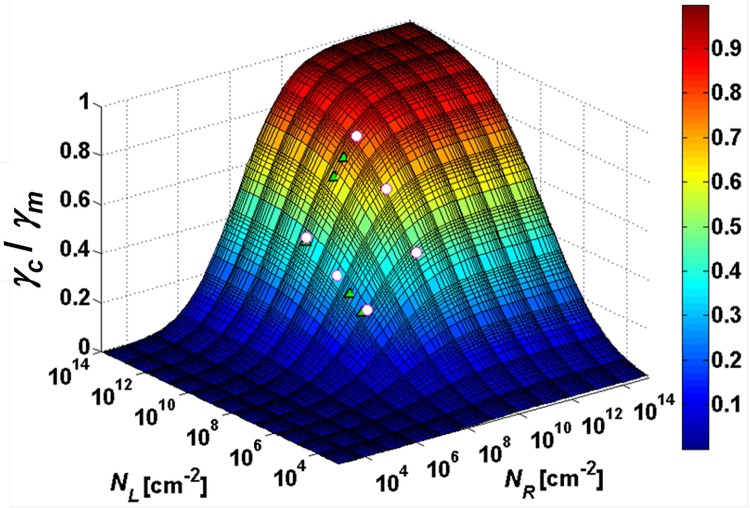
where γm is a shear rate scaling factor; NLC and NRC are characteristic ligand and receptor density, respectively, with σL and σR representing the corresponding statistical distributions. The best fit of calculations based on Eq. 4 to experimental data yields the following fitting parameters: γm = 22.71 s−1, NLC = 6.03 × 107/cm2, NRC = 1.71 × 108/cm2, σL = 4.93, and σR = 3.51. Using these parameters, a 3-D surface describing the dependence of the characteristic shear rate on both ligand and receptor densities is calculated and compared with measured values in Figure 3. The attachment ratio can now be estimated as a function of the applied shear rate for cell lines having various receptor densities passing through microchannels with different ligand densities.
Figure 3.
A calculated 3-D surface based on Eq. 4 describing the dependence of the normalized characteristic shear rate on both surface-ligand and cell-receptor densities. The white circles are the experimental data obtained for two cell lines and three antibody concentrations, whereas the green circles are simulation results based on the theoretical model presented in Eq. 1.
Detection limit of target cancer cells in binary mixtures
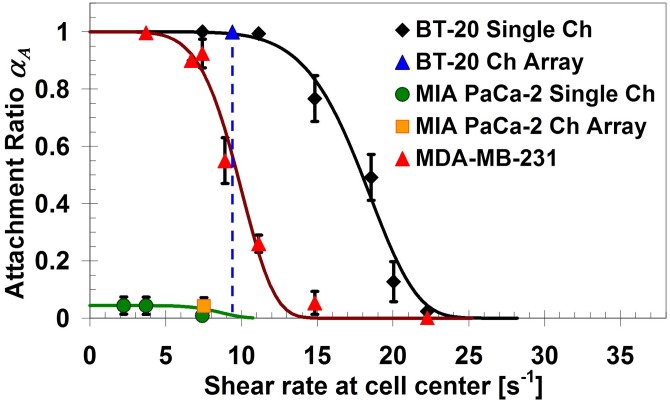
The concentration-dependent detection limit of target cancer cells in the microfluidic system was explored. Binary mixtures with various ratios between target and non-target cancer cells were prepared. Strongly expressing EpCAM BT-20 cells38 were mixed with MIA PaCa-2 cells, which do not express EpCAM,40 as target and non-target cells, respectively. The shear-rate dependent attachment ratio of the two selected cell lines, in comparison with that of MDA-MB-231 cell line, was characterized using single-microchannel devices functionalized with EpCAM antibodies. Attachment-ratio measurements for the three cell lines are plotted in Figure 4 as a function of the applied shear rate. The large difference in capture ratio between BT-20 and MIA PaCa-2 cells, in the shear-rate range of 9–11 s−1, indicates that they are, respectively, suitable for pairing as target and non-target cells in binary mixtures.
Figure 4.
Attachment ratio of BT-20 and MIA PaCa-2 cells, along with MDA-MB-231 cells, as a function of the applied shear rate in anti-EpCAM functionalized microdevices. The results in single-microchannel and microchannel-array devices are consistent. The shear rate of 9.4 s−1 is selected for isolating target BT-20 cells from binary mixtures with non-target MIA PaCa-2 cells, and the EpCAM antibody solution concentration is 100 μg/ml.
Selective capture of target cells was quantitatively characterized using binary mixtures with continuously decreasing ratio between target and non-target cells, from 1:1 to 1:100 000. Target BT-20 cells were tagged with green-fluorescent labels and non-target MIA PaCa-2 cells with red-fluorescent labels to enable visual tracking and counting of separate species. The prepared cell mixtures were driven through anti-EpCAM functionalized microchannel-array devices under a flow shear rate of γ = 9.4 s−1, as marked in Figure 4. At this shear rate, the attachment ratio of target BT-20 cells is about 1 while that of non-target MIA PaCa-2 cells is almost 0. Furthermore, the corresponding volume flow rate of 25 μl/min is sufficiently high to allow processing of a 0.5 ml sample in less than 30 min. Following the attachment stage, a gentle wash was applied under a shear rate 22.6 s−1 to remove unbound cells. Bound target and non-target cells were then counted and compared with the loaded cell population to evaluate the microsystem performance using the sensitivity and specificity criteria.33 The sensitivity was found to be close to 1.0 indicating capture of almost all target cells, while the specificity was about 0.95 suggesting that most but not all non-target cells avoided capture.
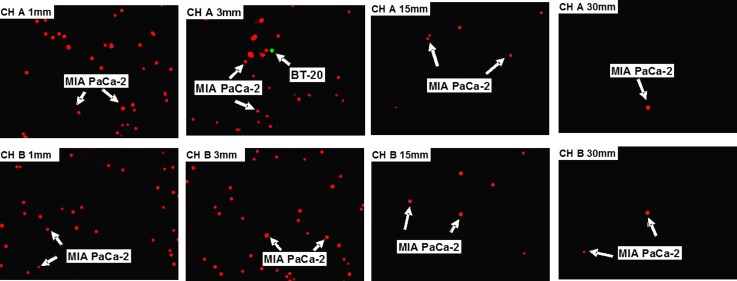
The 1:100 000 BT-20:MIA PaCa-2 cell mixture is particularly important, since this ratio is close to the ratio between CTCs and white blood cells (WBCs) frequently reported for blood samples of cancer patients.27 Therefore, a 0.5 ml suspension was prepared containing 4 BT-20 and about 400 000 MIA PaCa-2 cells. The binary cell mixture was then driven through a microchannel-array device. At the end of the attachment stage, one single target cell was found in 4 out of the 10 microchannels and about 2000 non-target cells were detected in each of the 10 microchannels; thus, the corresponding sensitivity is 1 with specificity of 0.95. To improve the microsystem specificity, a detachment flow under a shear rate of γ = 1,128 s−1 was applied. Images of the captured cell populations along two typical microchannels at four selected positions are shown in Figure 5; CH A represents 4 microchannels with a single target cell, while CH B represents the other 6 microchannels with no target cells. The average number of bound non-target cells in each microchannel was reduced from 2000 to about 600 as a direct result of the additional detachment step. Hence, the microsystem specificity was improved from 0.95 to 0.985 while the sensitivity was maintained at the same level of 1. This represents an enrichment of the relative target cell concentration from the initial 1:100 000 to a final 1:1500 ratio.
Figure 5.
Images of captured cell populations from a 0.5 ml suspension, containing four BT-20 cells (labeled green) mixed with about 400 000 MIA PaCa-2 cells (labeled red), along two representative microchannels. The experiment was performed, using an anti-EpCAM functionalized microchannel-array device, under 9.4 s−1 attachment shear rate followed by 1128 s−1 detachment shear rate. CH A represents 4 microchannels along which a single target cell was detected, while CH B represents the other 6 microchannels along which no target cell was detected. The 4 images shown for CH A and CH B were taken at distances of x = 1, 3, 15, and 30 mm downstream of the microchannel inlets.
Detection of target cancer cells suspended in whole blood
The microchannel-array device performance was examined for potential clinical applications. Samples were prepared using whole blood from a healthy donor spiked with labelled BT-20 cells (CellTrackerTM Green CMFDA). Two samples were tested, one contained 1000 and the other 100 BT-20 cells, each suspended in 2 ml whole blood. An average human blood sample of such a volume contains around 1.0 × 1010 erythrocytes (red blood cells), 1.0 × 109 thrombocytes (platelets), and 1.6 × 107 leukocytes (white blood cells). Hence, the ratio between target BT-20 cells and blood cells was in the range of 1:107–108 for the two tested samples. The samples were pretreated using a commercially available kit (Tumor Cell Enrichment Cocktail, 15167, Stemcell Technologies) according to the manufacturer's instructions. Each sample was then enriched and re-suspended in 500 μl Phosphate-buffered Saline with 2.5% fetal bovine serum (PBSF). The majority of erythrocytes and leukocytes were removed from the samples and, as counted using a hemocytometer, approximately 1.5 × 106 blood cells were left in each sample. Similar to many other CTCs enrichment methods, this pretreatment eliminated many target cancer cells as well.41 Indeed, a flow cytometry test with duplicated samples showed that about 30% of the cancer cells were lost during the pretreatment process, i.e., a recovery rate of about 70%. Hence, it is estimated that, at the end of pretreatment, one sample contained about 700 and the other about 70 BT-20 cells mixed among 1.5 × 106 blood cells.
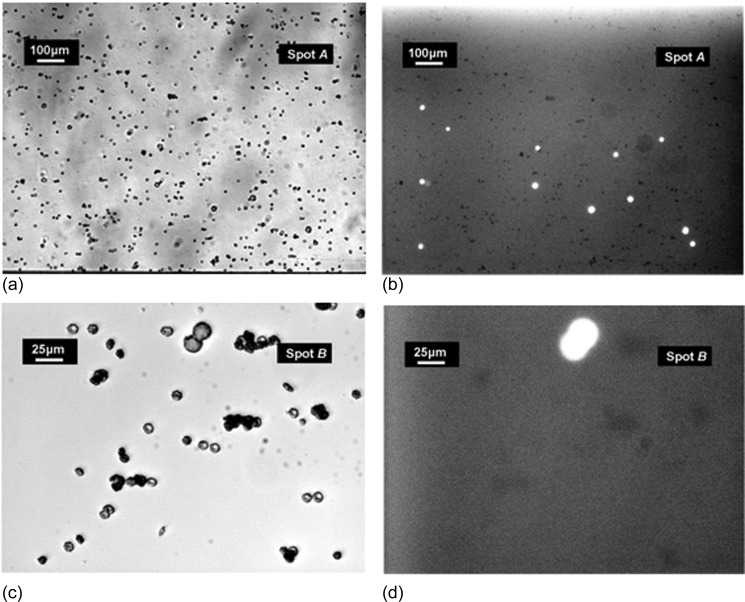
The enriched samples were driven through microchannel-array devices functionalized with EpCAM antibodies under a flow shear rate of γ = 9.4 s−1, followed by a gentle wash at a shear rate of γ = 22.6 s−1. After washing, 540 and 56 BT-20 cells were detected in the devices used for processing the samples initially spiked with 1000 and 100 cells, respectively. In each experiment, about 1.0 × 105 non-target blood cells were also captured. Thus, the tests with the two whole-blood samples in microchannel-array devices yielded system sensitivity close to 0.8 with specificity of about 0.93. Selected images of captured cells in the experiment with the 1000 BT-20 sample are shown in Figure 6. Figure 6a is the bright-field image showing the entire cell population captured inside the channel, while Figure 6b is the fluorescent image at the same spot showing only the fluorescently labeled BT-20 cells. Similarly, Figures 6c, 6d are, respectively, zoomed-in bright-field and fluorescent images of cells captured inside the channel.
Figure 6.
Images of attached cells in a microchannel array: (a) bright field and (b) fluorescent images recorded at the same location where BT-20 cells were detected. Similarly, the zoomed-in (c) bright field and (d) fluorescent images were recorded at the same location.
Viability of released cancer cell captured in microchannels
An advantage of capturing CTCs in a straight microchannel over other microfluidic devices featuring complex structures is that the captured target cells can be gently detached and collected under shear flow to minimize potential cell damage. Consequently, further analysis can be performed using released viable CTCs to gather additional biochemical information. To test the viability of target cancer cells released after capture from homogeneous suspensions, the released cells were collected at the device outlet. Cell growth in culture was then examined in comparison with both cancer cell control samples and cancer cells in a normal culture at a similar cell concentration.
Special procedures were implemented for maintaining sterile environment throughout all experimental procedures to avoid contamination. All buffers and chemical reagents were filtered using syringe filters with a pore size of 0.2 μm, while all fluidic components were sterilized using 70% ethanol and an alcohol burner. Serum free medium (RPMI 1640 for MDA-MB-231, EMEM for BT-20) was utilized as the buffer media to suspend harvested cells and kept in ice prior to experiments. Each cell suspension was divided into an experimental sample, A, and a control sample, B. Capture and release involving attachment/detachment of cancer cells in functionalized microchannels were conducted at room temperature. While cells of the experimental sample A were processed, cells in control sample B were also kept at room temperature for the same time period (up to 20 min) to elucidate the shear flow effect.
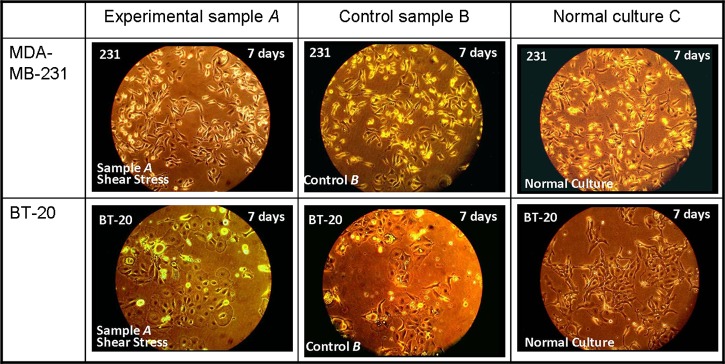
Samples A were driven through anti-EpCAM functionalized microchannels at shear rate γ = 7.4 s−1 to allow capture of most loaded cells within the microchannels. After washing unbound cells under a shear rate of γ = 37.1 s−1, the captured cancer cells were detached under much higher shear rates; γ = 7420 s−1 and 14 840 s−1 were applied for MDA-MB-231 and BT-20 cells, respectively. A high shear-rate acceleration of dγ/dt = 44 520 s−2 was selected to induce rapid detachment of captured cells.16 Subsequently, 1–2 ml suspensions of released cells were obtained at concentrations sufficiently high for immediate culturing. The collected cells of experimental samples A, released from the microdevices, and cells of control samples B were transferred at the same time into separate cell-culture dishes. The dishes were then placed in the same incubator for culture at 37 °C with 5% CO2. Typical images of cell growth, following seven-day culture, of experimental samples A and control samples B are compared in Figure 7 with samples C of normal culture for both MDA-MB-231 and BT-20 cell lines. While no sign of contamination was observed in all samples, the captured-and-released cells of samples A seem to proliferate similar to cells of control samples B and, more importantly, with no apparent differences from cells in normal cultures C. This clearly indicates that cancer cells released under shear stress, following capture in antibody-functionalized microchannels, were viable and could be cultured for further analysis.
Figure 7.
Comparisons of cell growth patterns following seven-day culture among experimental A, control B, and normal cultures C for both MDA-MB-231 and BT-20 cell lines.
DISCUSSION
Cell capture dynamics in shear flow was studied by driving BT-20 and MDA-MB-231 cells through microchannels functionalized with EpCAM antibodies. The cell attachment ratio was experimentally determined and numerically computed as a function of the applied flow shear rate with varying ligand and receptor densities. Utilizing a characteristic shear rate γC as a scaling parameter, all measured and calculated results of the normalized attachment ratio collapse onto a single curve fitted by the exponential function in Eq. 2. This empirical formula with a single exponent value of B = 6 ± 1, curve “j” in Figure 2c, is found to describe well the monotonically decreasing attachment ratio with increasing flow shear rate for all tested conditions. The exponent B is the parameter controlling the slope of the exponential function turning steeper with increasing absolute value. B is a measure of the system uniformity; the larger the value of B, the more uniform the system. Non-uniformities in cell-surface interaction arise from statistical distributions of parameters affecting cell-surface interaction including: cell size, hydrodynamic loads, receptor and ligand densities, as well as receptor-ligand bond characteristics. Therefore, the collapse of all data onto a single exponential curve, with B = 6, suggests that the combined effect of all such non-uniformities lead to a similar statistical dependence of cell capture on the applied shear stress.
The cell attachment ratio is found to depend on both cell-receptor and surface-ligand densities via the characteristic rate, γC, as explicitly expressed by Eq. 4. As shown in Figure 3, the characteristic shear rate increases gradually from zero to an asymptotic level with increasing either surface-ligand or cell-receptor density. It may seem that the attachment ratio is independent of the specific receptor-ligand pair involved in a particular target cell binding to a functionalized surface. However, a set of three parameters: γm, NLC, and NRC was needed for each tested cell-surface combination in fitting the empirical formula to experimental results. Hence, each of the three fitting parameters encompasses the influence of a particular cell-surface combination expressed symbolically as
| (5) |
The functional relationships among the various parameters in Eq. 5 are beyond the scope of this work.
The use of microchannel-array devices enabled high throughput processing of large samples, up to 0.5 ml with a concentration 106 cells/ml, under low flow rate for high-sensitivity target-cell capture. For samples of larger volumes, it is straightforward to realize devices with more microchannels to keep the processing time within an acceptable duration. Samples of about 0.5 × 106 cells allow preparation of binary mixtures at a ratio of 1:100 000 between target and non-target cells, clinically relevant for isolating CTCs from blood samples of cancer patients. Applying a two-step attachment/detachment procedure enhanced the system specificity significantly, from 0.95 to 0.985, while maintaining its sensitivity close to 1.0. The high sensitivity indicates that such affinity-based systems could be used to capture rare target cells from complex mixtures. However, the number of captured non-target cells (false positives) is still prohibitively high for clinical applications.
Isolation of target cancer cells from whole blood was demonstrated by using normal blood samples spiked with cancer cells. After a pretreatment for cancer cell enrichment, the samples were driven through microchannel arrays functionalized with antibodies. The system sensitivity in capturing target cancer cells from whole blood was substantially lower, about 0.55, in comparison with binary mixtures, close to 1. The pretreatment enrichment accounted for much of the discrepancy between the system sensitivity when processing whole-blood and binary-mixture samples. Nevertheless, blood samples are inherently more complex, and improved techniques are required in efforts to match the system performance achieved in binary mixtures.
Finally, the viability of cancer cells released after capture in antibody-functionalized microchannels was examined by observing cell-growth patterns in culture. The similar recovery and growth rate of released cancer cells and cancer cells in normal culture indicate that the isolated cancer cells are viable and could be used for further analysis. This confirmed that the applied shear flow for detachment of cancer cells bound to a functionalized surface did not compromise the cell viability. Further studies are needed to explore the shear flow effect on other facets of cancer cell activity such as expression level of cell-membrane receptors.
ACKNOWLEDGMENTS
This work was supported by a BCRP Grant No. BC061859, administered by the US Army Medical Research, and partially by Grant Nos. NSF 0943321 and NIH CA023074.
References
- Nguyen D. X. and Massagué J., “ Genetic determinants of cancer metastasis,” Nat. Rev. Genet. 8, 341–352 (2007). 10.1038/nrg2101 [DOI] [PubMed] [Google Scholar]
- Gupta G. P. and Massagué J., “ Cancer metastasis: Building a framework,” Cell 127, 679–695 (2006). 10.1016/j.cell.2006.11.001 [DOI] [PubMed] [Google Scholar]
- Cristofanilli M., Budd G. T., Ellis M. J., Stopeck A., Matera J., Miller M. C. et al. , “Circulating tumor cells, disease progression, and survival in metastatic breast cancer,” New Engl. J. Med. 351, 781–791 (2004). 10.1056/NEJMoa040766 [DOI] [PubMed] [Google Scholar]
- Cristofanilli M., Broglio K. R., Guarneri V., Jackson S., Fritsche H. A., Islam R. et al. , “Circulating tumor cells in metastatic breast cancer: Biologic staging beyond tumor burden,” Clin. Breast Cancer 7, 34–42 (2007). 10.3816/CBC.2007.n.004 [DOI] [PubMed] [Google Scholar]
- Gradilone A., Naso G., Raimondi C., Cortesi E., Gandini O., Vincenzi B. et al. , “Circulating tumor cells (CTCs) in metastatic breast cancer (MBC): Prognosis, drug resistance, and phenotypic characterization,” Ann. Oncol. 22, 86–92 (2011). 10.1093/annonc/mdq323 [DOI] [PubMed] [Google Scholar]
- Nakamura S., Yagata H., Ohno S., Yamaguchi H., Iwata H., Tsunoda N. et al. , “Multi-center study evaluating circulating tumor cells as a surrogate for response to treatment and overall survival in metastatic breast cancer,” Breast Cancer 17, 199–204 (2010). 10.1007/s12282-009-0139-3 [DOI] [PubMed] [Google Scholar]
- Smerage J. B. and Hayes D. F., “The measurement and therapeutic implications of circulating tumor cells in breast cancer,” Br. J. Cancer 94, 8–12 (2006). 10.1038/sj.bjc.6602871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn H. J., Presta A., Yang L.-Y., Blondal J., Trudeau M., Lickley L. et al. , “Enumeration of circulating tumor cells in the blood of breast cancer patients after filtration enrichment: Correlation with disease stage,” Breast Cancer Res. Treat. 86, 237–247 (2004). 10.1023/B:BREA.0000036897.92513.72 [DOI] [PubMed] [Google Scholar]
- Singletary S. E. and Connolly J. L., “Breast cancer staging: Working with the sixth edition of the AJCC cancer staging manual,” CA: Cancer J. Clin. 56, 37–47 (2006). 10.3322/canjclin.56.1.37 [DOI] [PubMed] [Google Scholar]
- Müller V. and Pantel K., “Bone marrow micrometastases and circulating tumor cells: Current aspects and future perspectives,” Breast Cancer Res. 6, 258–261 (2004). 10.1186/bcr942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pachmann K., Clement J. H., Schneider C. P., Willen B., Camara O., Pachmann U. et al. , “Standardized quantification of circulating peripheral tumor cells from lung and breast cancer,” Clin. Chem. Lab Med. 43, 617–627 (2005). 10.1515/CCLM.2005.107 [DOI] [PubMed] [Google Scholar]
- Pachmann K., Heiss P., Demel U., and Tilz G., “Detection and quantification of small numbers of circulating tumour cells in peripheral blood using laser scanning cytometer (LSC),” Clin. Chem. Lab Med. 39, 811–817 (2001). 10.1515/CCLM.2001.134 [DOI] [PubMed] [Google Scholar]
- Parareda A., Gallego S., Roma J., Llort A., Sábado C., Gros L. et al. , “Prognostic impact of the detection of microcirculating tumor cells by a real-time RT-PCR assay of tyrosine hydroxylase in patients with advanced neuroblastoma,” Oncol. Rep. 14, 1021–1027 (2005). 10.3892/or.14.4.1021 [DOI] [PubMed] [Google Scholar]
- Adams A. A., Okagbare P. I., Feng F., Hupert M. L., Patterson D., Göttert J. et al. , “Highly efficient circulating tumor cell isolation from whole blood and label-free enumeration using polymer-based microfluidics with an integrated conductivity sensor,” J. Am. Chem. Soc. 130, 8633–8641 (2008). 10.1021/ja8015022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung L. S. L., Zheng X., Wang L., Baygents J. C., Guzman R. Z., Schroeder J. A. et al. , “Adhesion dynamics of circulating tumor cells under shear flow in a bio-functionalized microchannel,” J. Micromech. Microeng. 21, 054033 (2011). 10.1088/0960-1317/21/5/054033 [DOI] [Google Scholar]
- Cheung L. S. L., Zheng X., Stopa A., Baygents J. C., Guzman R., Schroeder J. A. et al. , “Detachment of captured cancer cells under flow acceleration in a bio-functionalized microchannel,” Lab Chip 9, 1721–1731 (2009). 10.1039/b822172c [DOI] [PubMed] [Google Scholar]
- Cheung L. S. L., Zheng X., Wang L., Guzman R., Schroeder J. A., Heimark R. L. et al. , “Kinematics of specifically captured circulating tumor cells in bio-functionalized microchannels,” J. Microelectromech. Syst. 19, 752–763 (2010). 10.1109/JMEMS.2010.2052021 [DOI] [Google Scholar]
- Zheng X., Cheung L. S. L., Schroeder J. A., Jiang L., and Zohar Y., “Cell receptor and surface ligand density effects on dynamic states of adhering circulating tumor cells,” Lab Chip 11, 3431–3439 (2011). 10.1039/c1lc20455f [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer D. A. and Apte S. M., “Simulation of cell rolling and adhesion on surfaces in shear flow: General results and analysis of selectin-mediated neutrophil adhesion,” Biophys. J. 63, 35–57 (1992). 10.1016/S0006-3495(92)81577-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alon R., Hammer D. A., and Springer T. A., “Lifetime of the P-selectin-carbohydrate bond and its response to tensile forces in hydrodynamic flow,” Nature 374, 539–542 (1995). 10.1038/374539a0 [DOI] [PubMed] [Google Scholar]
- Lawrence M. B. and Springer T. A., “Neutrophils roll on E-selectin,” J. Immunol. 151, 6338–6346 (1993). [PubMed] [Google Scholar]
- Tempelman L. A. and Hammer D. A., “Receptor-mediated binding of IgE-sensitized rat basophilic leukemia cells to antigen-coated substrate under hydrodynamic flow,” Biophys. J. 66, 1231–1243 (1994). 10.1016/S0006-3495(94)80907-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X., Cheung L. S. L., Jiang L., Schroeder J. A., Heimark R. L., Baygents J. C.et al. , “Dynamic states of adhering cancer cells under shear flow in an antibody-functionalized microchannel,” in Proceedings of the MEMS'11 (2011), pp. 849–852.
- Chang W. C., Lee L. P., and Liepmann D., “Biomimetic technique for adhesion-based collection and separation of cells in a microfluidic channel,” Lab Chip 5, 64–73 (2005). 10.1039/b400455h [DOI] [PubMed] [Google Scholar]
- Cheng X., Irimia D., Dixon M., Sekine K., Demirci U., Zamir L. et al. , “A microfluidic device for practical label-free CD4+ T cell counting of HIV-infected subjects,” Lab Chip 7, 170–178 (2007). 10.1039/b612966h [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plouffe B. D., Radisic M., and Murthy S. K., “Microfluidic depletion of endothelial cells, smooth muscle cells, and fibroblasts from heterogeneous suspensions,” Lab Chip 8, 462–472 (2008). 10.1039/b715707j [DOI] [PubMed] [Google Scholar]
- Nagrath S., Sequist L. V., Maheswaran S., Bell D. W., Irimia D., Ulkus L. et al. , “Isolation of rare circulating tumour cells in cancer patients by microchip technology,” Nature 450, 1235–1239 (2007). 10.1038/nature06385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stott S. L., Hsu C.-L., Tsukrov D. I., Yu M., Miyamoto D. T., Waltman B. A. et al. , “Isolation of circulating tumor cells using a microvortex-generating herringbone-chip,” Proc. Natl. Acad. Sci. U. S. A. 107, 18392–18397 (2010). 10.1073/pnas.1012539107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myung J. H., Launiere C. A., Eddington D. T., and Hong S., “Enhanced tumor cell isolation by a biomimetic combination of E-selectin and anti-EpCAM: Implications for the effective separation of circulating tumor cells (CTCs),” Langmuir 26, 8589–8596 (2010). 10.1021/la904678p [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Liu K., Liu J., Yu Z. T.-F., Xu X., Zhao L. et al. , “Highly efficient capture of circulating tumor cells by using nanostructured silicon substrates with integrated chaotic micromixers,” Angew. Chem., Int. Ed. 50, 3084–3088 (2011). 10.1002/anie.201005853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z., Huang F., Du J., Shu W., Feng H., Xu X., and Chen Y., “Rapid isolation of cancer cells using microfluidic deterministic lateral displacement structure,” Biomicrofluidics 7, 011801 (2013). 10.1063/1.4774308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta V., Jafferji I., Garza M., Melnikova V. O., Hasegawa D. K., Pethig R., and Davis D. W., “ApoStream, a new dielectrophoretic device for antibody independent isolation and recovery of viable cancer cells from blood,” Biomicrofluidics 6, 024133 (2012). 10.1063/1.4731647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X., Cheung L. S. L., Schroeder J. A., Jiang L., and Zohar Y., “ A high-performance microsystem for isolating circulating tumor cells,” Lab Chip 11, 3269–3276 (2011). 10.1039/c1lc20331b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X., Jiang L., Schroeder J. A., Marron M., Iannone M., Stopeck A. T.et al. , “On the minimum detection limit of circulating tumor cells in an antibody-functionalized microchannel array,” in Proceedings of the NANOMED’12 (2012), pp. 87–91.
- Eibl G. and Reber H. A., “A xenograft nude mouse model for perineural invasion and recurrence in pancreatic cancer,” Pancreas 31, 258–262 (2005). 10.1097/01.mpa.0000175176.40045.0f [DOI] [PubMed] [Google Scholar]
- Bitler B. G., Menzl I., Huerta C. L., Sands B., Knowlton W., Chang A. et al. , “Intracellular MUC1 peptides inhibit cancer progression,” Clin. Cancer Res. 15, 100–109 (2009). 10.1158/1078-0432.CCR-08-1745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn C. B. and Schwarz U. S., “Dynamic states of cells adhering in shear flow: from slipping to rolling,” Phys. Rev. E 77, 041904 (2008). 10.1103/PhysRevE.77.041904 [DOI] [PubMed] [Google Scholar]
- Prang N., Preithner S., Brischwein K., Göster P., Wöppel A., Müller J. et al. , “Cellular and complement-dependent cytotoxicity of Ep-CAM-specific monoclonal antibody MT201 against breast cancer cell lines,” Br. J. Cancer 92, 342–349 (2005). 10.1038/sj.bjc.6602310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X., Jiang L., Schroeder J. A., Marron M., Iannone M., Stopeck A. T.et al. , “Flow-rate dependent capture of circulating tumor cells in bio-functional microchannels,” in Proceedings of the NANOMED’12 (2012), pp. 82–86.
- Ren-Heidenreich L., Davol P. A., Kouttab N. M., Elfenbein G. J., and Lum L. G., “Redirected T-cell cytotoxicity to epithelial cell adhesion molecule-overexpressing adenocarcinomas by a novel recombinant antibody, E3Bi, in vitro and in an animal model,” Cancer 100, 1095–1103 (2004). 10.1002/cncr.20060 [DOI] [PubMed] [Google Scholar]
- Gerges N., Rak J., and Jabado N., “New technologies for the detection of circulating tumor cells,” Br. Med. Bull. 94, 49–64 (2010). 10.1093/bmb/ldq011 [DOI] [PubMed] [Google Scholar]