Abstract
Xenotropic murine leukemia virus-related virus (XMRV) was discovered in 2006 in a search for a viral etiology of human prostate cancer (PC). Substantial interest in XMRV as a potentially new pathogenic human retrovirus was driven by reports that XMRV could be detected in a significant percentage of PC samples, and also in tissues from patients with chronic fatigue syndrome (CFS). After considerable controversy, etiologic links between XMRV and these two diseases were disproven. XMRV was determined to have arisen during passage of a human PC tumor in immunocompromised nude mice, by activation and recombination between two endogenous murine leukemia viruses from cells of the mouse. The resulting XMRV had a xentropic host range, which allowed it replicate in the human tumor cells in the xenograft. This review describes the discovery of XMRV, and the molecular and virological events leading to its formation, XMRV infection in animal models and biological effects on infected cells. Lessons from XMRV for other searches of viral etiologies of cancer are discussed, as well as cautions for researchers working on human tumors or cell lines that have been passed through nude mice, includingpotential biohazards associated with XMRV or other similar xenotropic murine leukemia viruses (MLVs).
Keywords: chronic fatigue syndrome, endogenous retrovirus, murine leukemia virus, prostate cancer, retrovirus, XMRV
INTRODUCTION
Retroviruses have been associated with diseases in both animals and humans. The first retroviruses discovered caused cell-free transmission of sarcomas and acute leukemias in chickens.1,2 Although these findings were initially met with skepticism, subsequent studies led to discovery of mouse mammary tumor virus and murine leukemia virus (MLV).3,4 Studies of animal retroviruses also revealed the existence of endogenous (germline-transmitted) retroviruses,5,6,7 and of viral oncogenes and cellular proto-oncogenes.8
The existence of oncogenic animal retroviruses raised the question of whether human oncogenic retroviruses also exist. After several false starts, clinical and epidemiological studies in Japan ultimately led to isolation of the first oncogenic human retrovirus, Human T-cell leukemia virus type I (the causative agent of adult T-cell leukemia) in 1980.9,10 Subsequently, the retrovirus HIV-1 was identified as the cause of acquired immunodeficiency syndrome; together these two viruses (and the related viruses human T-cell leukemia virus type II and HIV-2) currently infect 40–50 million people worldwide. These are the only well-documented infectious human retroviruses discovered to date. However, there have been suggestions of other retroviruses in human disease, including breast cancer and primary biliary cirrhosis.11,12 Human endogenous retroviruses have also been implicated in cancers and autoimmune diseases.13,14
RETROVIRUSES
Retroviruses have been reviewed extensively,15 and a brief summary is provided here. They are enveloped RNA-containing viruses, with positive sense RNA genomes of 8–12 kb in length. All retroviruses carry three genes gag, pol and env that encode the viral core proteins, enzymes (reverse transcriptase, integrase and protease) and envelope proteins respectively. During infection virions bind to receptors on the cell surface and the viral cores are internalized and partially uncoated. In the infected cell, core-associated reverse transcriptase reverse-transcribes the viral RNA into double-stranded viral DNA that enters the nucleus where it is integrated (by way of integrase) into host DNA to generate the provirus. The provirus is then transcribed by cellular RNA polymerase II into full-length viral RNA. This RNA is transported to the cytoplasm (with and without splicing) to give viral mRNAs for synthesis of viral proteins; unspliced viral RNA is also transported to the cytoplasm as genomes for new virus particles. New virions bud from the cell surface without lysing the infected cell.
As a consequence of reverse transcription, the viral DNA is longer than the viral RNA by the presence of long terminal repeats (LTRs) at either end. In the integrated provirus, the LTRs contain the promoter and enhancer sequences necessary for initiation of viral transcription, as well as sequences for cleavage/polyadenylation. The LTR is subdivided into three regions: U3, R and U5; the promoter and enhancer sequences are contained in the U3 region.
The mechanism by which most retroviruses induce cancers is insertional activation of proto-oncogenes. Normally during infection retroviral DNAs are inserted at multiple, almost random sites in the cellular DNA. However, independent tumors induced by the same retrovirus often show integration at common insertion sites.8,16 These integration sites are largely at or near cellular proto-oncogenes—normal cell genes involved in positive stimulation of cell division and growth. The viral DNA leads to overexpression of the proto-oncogene RNA and protein, either by read-through transcription from the viral LTR (promoter insertion) or activation of the proto-oncogene's own promoter by the retroviral enhancers. A subset of retroviruses that rapidly induce cancers carry viral oncogenes—genes that directly cause oncogenic transformation.8 The viral oncogenes result from capture of cellular proto-oncogenes into the viral genome.
MURINE LEUKEMIA VIRUSES
MLVs are prototypical gammaretroviruses. They are further classified based on the species that they can infect: ecotropic viruses infect only cells of mouse or rat origin, xenotropic viruses infect only non-mouse cells; and amphotropic and polytropic viruses, infect both mouse cells and cells of other species (Table 1). MLVs such as Moloney and Friend MLV (M- and F-MLV) induce T-lymphoid and erythroid/myeloid tumors respectively. They have been extensively studied at the molecular and cellular level, and used as model systems for leukemogenesis.17
Table 1. MuLV subgroup classification by host range.
| MuLV subgroup | Host range | Receptor |
|---|---|---|
| Ecotropic MuLV | Mouse or rat cells | CAT-1a |
| Amphotropic MuLV | Mouse and non-mouse cells | PiT-2 |
| Xenotropic MuLV | Non-mouse cells | XPR-1b |
| Polytropic/MCF | Mouse and non-mouse cells | XPR-1b |
Only CAT-1 from mice and rats binds ecotropic MuLV Env protein.
Xpr-1 of mouse cells binds polytropic MuLV Env protein, but it does not bind xenotropic MuLV Env. Xpr-1 from non-murine species binds both xenotropic and polytropic Env.
Endogenous retroviruses result from infection and integration of retroviral proviruses into the germline, whereafter they are transmitted vertically as inherited genetic elements. Laboratory mice carry multiple copies of endogenous MLVs resulting from germline integrations of xenotropic MLVs (Xmv loci), polytropic MLVs (Pmv and Mpmv (modified polytropic) loci) and in some strains ecotropic MLVs (Emv loci). The proviruses in many of these loci are replication defective (e.g., from point mutations and deletions), and they are inefficiently expressed due to epigenetic modifications (e.g., DNA methylation18) or negative regulatory elements in their LTRs.19 These defects probably reflect selection against high-level expression of infectious endogenous MLVs, which could lead to development of cancers and other negative biological consequences. However, in some situations, endogenous MLVs are expressed (EMVs and/or XMVs), and even defective ones can participate in genetic recombination with exogenously infecting MLVs. When exogenous (non-endogenous) ecotropic MLVs infect mice and cause leukemias, a hallmark event is generation of recombinants containing the envelope of an endogenous polytropic or modified polytropic MLV inserted into the infecting virus genome—mink cell focus-inducing (or MCF) recombinants.20 The formation of MCF recombinants has been implicated in leukemogenesis by several MLVs.17
Another situation in which endogenous MLVs of mice have been activated is passage of human tumor xenografts in immunodeficient (e.g., nude) mice, a common procedure employed in cancer biology for establishment or passage of human tumor cell lines. During xenograft passages, XMVs can become activated; they do not replicate in the mouse since mouse cells cannot be infected by their envelopes; however, human cells are susceptible. The infection of human xenografts by xenotropic MLVs after passage through nude mice has been known for many years.21,22,23
DISCOVERY OF XMRV
Discovery in 2006 of a potential human retrovirus associated with prostate cancer (PC) originated from studies of familial PC. A familial form of PC had been genetically mapped to the gene encoding ribonuclease L (RNAse L). RNAse L is an effector molecule in the interferon antiviral response, and individuals with an inherited increased PC susceptibility were found to be homozygous for a polymorphic RNAse L allele with lower enzymatic activity (R462Q).24 The fact that RNAse L is involved in innate immunity suggested that such individuals could be more susceptible to a virus that induces PC. Based on this hypothesis investigators prepared cDNAs from familial and non-familial PC cases and hybridized them to a ‘virochip' that contained DNA sequences for genes of all known viruses.25 A virus ‘hit' was found for the familial PC tissues, and it was for xenotropic MLV; non-familial PCs appeared to have a lower frequency of hybridization with X-MLV. The hybridizing cDNAs were recovered from the virochip and cloned, and the corresponding virus was designated XMRV, and reported as a novel retrovirus potentially involved in human PC.25 In a follow-up publication the same investigators described an infectious XMRV clone (VP62) reconstituted from XMRV cDNAs from a PC patient.26 The clone produced infectious virus that could replicate in human PC cells and also in hamster cells engineered to express the X- MLV receptor XPR-1, indicating that it was xenotropic in host range. Significantly, the authors sequenced the host cell DNA adjoining integrated XMRV proviruses in primary PC tissues from two XMRV positive cases, and the cellular sequences were human. This supported the conclusion that XMRV was a retrovirus that was infecting human cells, and not likely a polymerase chain reaction (PCR) artifact (however, as it was later determined that the XMRV-infected human cells represented contamination with an infected human PC cell line,27,28 as will be discussed below).
Despite the promising initial results, follow-up studies were inconsistent in associating XMRV infection with PC. For instance an early study of a European PC cohort indicated that overall levels of XMRV detection were low (∼1%), and not associated with homozygosity for the RNAse L R462Q allele.29 On the other hand, immunohistochemistry for XMRV Gag protein in a US cohort of human PC samples detected reactivity in a significant fraction of tumors, with a positive correlation for high-grade PC tumors.30 In this study, there was also no correlation between XMRV reactivity and RNase L status, which suggested that that XMRV infection might be more widely distributed in PC patients. Also, XMRV LTR activity was reported to be significantly higher in primary stromal fibroblasts isolated from prostate tissue or the PC cell line LNCaP compared to other cell types, suggesting that the virus might replicate and express more efficiently in prostate tissue.31 An important finding in 2009 was that the cell line 22Rv1, derived from a human PC patient, harbored multiple copies of XMRV DNA and expressed it highly.32 However, other subsequent studies failed to detect XMRV in prostate tissue, serum or peripheral blood mononuclear cells (PBMC) of patients with PC from the United States and Europe.33,34,35,36,37,38
Interest in XMRV was further spurred in 2009 by Lombardi et al.39 who reported XMRV in 67% of PBMCs isolated from patients with chronic fatigue syndrome (CFS, also called myalgic encephalomyelitis or ME), a condition for which an etiology has not been found. Detection was by PCR for XMRV DNA, presence of anti-XMRV antibodies in patient sera and isolation of infectious virus from patient CD4+ T cells by coculture with susceptible cells. The following year, Lo et al.40 reported detection by PCR of polytropic MLV-related sequences, but not XMRV, in CFS patients; although this was not a finding of XMRV, it further suggested infection of gammaretroviruses in CFS patients. Despite these early reports and the excitement they generated in the CFS patient community, the association of XMRV with CFS has been disproven. Many studies have failed to detect XMRV in CFS cohorts around the globe.27,41,42,43,44,45,46,47,48,49,50,51,52 Surveys also have not identified XMRV infection in the general human population 53,54,55,56 or in humans at high risk for viral infections (e.g., HIV-1-infected individuals).57,58,59,60,61,62
When XMRV was first described, it was noteworthy that isolates from different patient sources were nearly identical.25 A hallmark of retroviruses is that they undergo significant mutation during replication, due to the error-prone nature of reverse transcriptase.15 When multiple rounds of infection take place in an infected individual (e.g., HIV-1 infection in humans), this can lead to extensive mutations in the viral genome. Moreover, an important set of host cell restriction factors, the apolipoprotein B editing complex (APOBEC) proteins, restrict retroviral replication by inducing G→A mutations during reverse transcription, and XMRV was found to be sensitive to human APOBEC3G.63,64,65 Thus, the lack of sequence variation in different XMRV isolates raised the question as to whether the virus was replicating in humans. On the other hand, human T-cell leukemia virus type -I is an established oncogenic retrovirus of humans, and it also exhibits minimal sequence variation in and between patients.66,67 This might reflect maintenance of infection by persistently infected cells, along with infrequent rounds of new infection.
THE ORIGINS OF XMRV
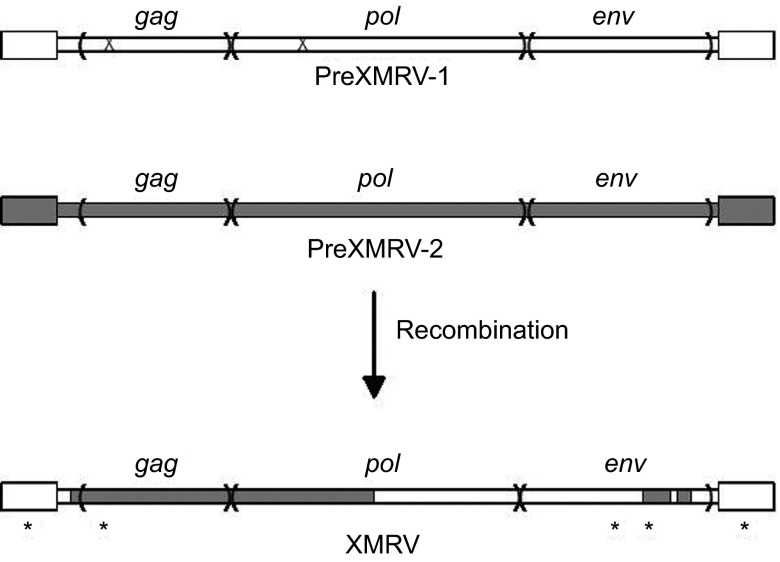
In 2011, Paprotka et et al.68 were able to deduce the origins of XMRV. They focused on the 22Rv1 cell line that harbors multiple XMRV proviruses and produces large amounts of virus. These cells resulted from multiple xenograft passages of a PC patient tumor (CWR22) in nude mice, and the investigators tested if XMRV could have arisen during the in vivo passaging. While the original patient tissue was not available, early and late xenograft passage samples were obtained, including a late passage that was an immediate precursor of 22Rv1 cells. PCR analysis using XMRV-specific primers showed that the early xenografts did not harbor XMRV, indicating that the original patient tumor was not infected. However, the late passage xenografts and the 22Rv1 cells were positive for XMRV, suggesting that XMRV arose during the in vivo passage in nude mice by infection from activated endogenous MLV proviruses. Analysis of the endogenous MLVs in nude mice identified two with stretches of high complementarity (virtual identity) to regions of XMRV: PreXMRV-1 and PreXMRV-2.68 It was possible to generate the XMRV genome by six crossovers between these two endogenous viruses, followed by three single-base substitutions and one nucleotide addition (Figure 1). Retroviral recombination requires copackaging of two heterologous viral RNAs into a virus particle, followed by template switching during reverse transcription in a subsequently infected cell.15 The PreXMRV-2 genome has open reading frames for all genes; presumably it was activated from a mouse cell during the xenografting where it also ultimately copackaged a PreXMRV-1 RNA. Thereafter, recombination and propagation of the recombinant (XMRV) took place in the human PC cells in the xenograft since human cells are susceptible to infection by xenotropic MLVs. More recent tissue culture experiments have confirmed that recombination between PreXRMV-1 and PreXMRV-2 can yield replication-competent xenotropic viruses, although the exact crossovers in the in vitro generated recombinants were different from those of XMRV.69
Figure 1.
Generation of the XMRV genome by recombination. The two endogenous MLV parental proviruses present in nude mice, PreXMRV-1 and -2 are shown at the top of the figure. The larger boxes at either end represent the LTRs. PreXMRV-1 has mutations (‘X') in the gag (a 16 nt deletion) and pol (a single-nucleotide frame-shift) that render it incapable of encoding functional proteins, but the env coding sequences are intact. PreXMRV-2 has open reading frames for all three genes. The recombinations between PreXMRV-1 and -2 that generated XMRV are shown at the bottom of the figure. There are three single nucleotide substitutions and one insertion (*) that also occurred during development of infectious XMRV (the substitution in the LTR is present at either end of the provirus).
The fact that XMRV arose from multiple recombinations between PreXMRV-1 and -2 had another implication. The high sequence identity between XMRVs detected in different studies, coupled with the multiple crossovers involved in formation of the virus, made it likely that all of the XMRVs identified in human patient samples did not arise independently, but they likely came from the 22Rv-1 cell line. Detection of XMRV in human tissues generally required multiple rounds of PCR, which could have resulted in amplification of trace amounts of XMRV-infected cells or plasmids. Subsequently, many of the tumor samples initially reported to be XMRV positive were re-analyzed and found to be contaminated with the VP62 XMRV plasmid or 22Rv1 cells,28,70 leading to an initial partial retraction of the paper by the authors.71 A large-scale collaborative blinded analysis27 tested PBMCs of CFS patients in independent laboratories and led to the conclusion that the initial reports of XMRV in the general population also resulted from laboratory contamination, or from incorrect initial identification of positive samples due in part to contaminating mouse DNA in PCR reagents.72,73 Subsequently, the key papers reporting discovery of XMRV and its association with PC or CFS25,39,40 were retracted.71,74
XMRV INFECTION IN EXPERIMENTAL ANIMALS
Despite the fact that XMRV is not a human virus circulating in humans, it is an infectious virus. Experimental infection of XMRV in three animal species has been reported: rhesus macaques,75 pigtail macaques76 and Mus pahari mice.77 Mus pahari are infectable by xenotropic MLVs due to a polymorphism in the Xpr1 receptor. In all three animals, XMRV infection could be detected shortly after infection (the first few weeks) by RT-PCR for viral RNA in plasma and/or PCR for viral DNA in tissue samples. After this phase, however, circulating virus became undetectable, although long-term persistence of proviral DNA in PBMCs or tissues could be detected by PCR in some cases. Persistence of low level viral infection was also suggested by prolonged immunological responses to XMRV in all cases. In Mus pahari mice, passage of infected cells from mother to offspring was also found.78 Similar to the tissue culture studies, the tissue tropism of XMRV in vivo seems to include a range of cell types: blood (lymphoid and other cell types) and epithelial cells. In rhesus macaques, XMRV infection in the prostate and the reproductive tract was significant early after infection, resembling the in vitro preference of XMRV replication for prostate cells;31,75 infection in the prostate was also observed in Mus pahari.77 Consistent with the in vitro sensitivity of XMRV to human and murine APOBEC3s, G→A hypermutations were observed in XMRV proviruses from PBMCs in infected pigtail macaques and Mus pahari.76,77 Thus, in all three reported animal models, XMRV establishes limited infection, although traces of the virus persist for long times. No pathological consequences of XMRV infection were reported, although the number of subjects was small and the time durations were not extensive. We have studied infection of XMRV in Sprague–Dawley rats.79 Consistent with the other animal models, there was evidence for persistent infection (up to one year) as measured by DNA PCR, and infected cells were present in multiple tissues, both hematopoietic and epithelial (including prostate). However, G→A hypermutation was not observed.
BIOLOGICAL EFFECTS OF XMRV INFECTION
Even though XMRV is not associated causally with human PC, the virus has biological effects on infected cells in culture, some of which may have contributed to selection for the virus in 22Rv1 cells. When rat 208F cells were infected in vitro by XMRV obtained from infected hamster HT1080 cells, a small number of transformed foci formed,80 and one of the foci contained an acute transforming version of XMRV that had transduced the N-ras cellular oncogene from HT1080.81 We have found that XMRV infection of rat NRK kidney epithelial cells leads to an increase in growth rate of the infected cells and a shift to a more mesenchymal growth pattern.79
The possibility that XMRV may affect the growth properties of human prostate epithelial cells has also been examined. XMRV infection of LNCaP cells accelerates in vitro cell proliferation, transformation and invasion of cells into Matrigel; these activities were mediated by downregulation of the cyclin/cyclin-dependent kinase inhibitor p27 (Kip1).82 A role for XMRV in cell proliferation or tumorigenesis was also reported by Stieler et al.83 who found that shRNA-induced suppression of XMRV in 22Rv1 cells resulted in reduced migration and cytokine release in vitro. Furthermore, when implanted into nude mice, the shRNA-treated cells showed decreased angiogenesis, reduced tumor size and increased necrosis of the primary tumor. A positive effect of XMRV on 22Rv1 cell growth could explain why the virus was selected during establishment of these cells.
Very recently, a report by Murgai et al.84 again provided evidence for effects of XMRV or similar viruses in growth of prostate epithelial cells. LNCaP prostate epithelial cells were passaged through nude mice, and some of the resulting sublines showed enhanced metastatic potential. One such subline, B4, was found to be producing a novel xenotropic MLV (B4rv) that resulted from activation/recombination of three endogenous MLVs. Strikingly, the Env region of B4rv was largely derived from PreXMRV-1, which is also the endogenous virus that contributed most of the Env region to XMRV. In vitro infection of LNCaP cells with either B4rv or XMRV resulted in enhanced metastic potential, which was associated with disruption of tumor vasculature maturation. Chimeric viruses indicated that the growth-promoting effects were determined by the xenotropicEnv protein. It is noteworthy that passage of human PC xenografts through nude mice has twice given rise to MLVs that arise from recombination of two or more endogenous MLVs, with the envelope partner being largely derived from PreXMRV-1. On the other hand, in other human xenografts of human leukemias and solid tumors (including PCs) through nude mice, endogenous xenotropic viruses were also recovered, but they appear to be direct activations of B10 xenotropic virus 1 or other replication-competent X-MLVs without recombination.85 Thus, the nature of xenotropic MLVs recovered from human xenograft passages in nude mice may be influenced by tissue-specific and virus-specific growth-promoting properties of the activated viruses, as well as the genetic background of the nude mice, some of which do not harbor PreXMRV-1 or -2.68
LESSONS FROM XMRV
Identifying infectious agents associated with human cancer is an important topic; currently 20%–25% of human cancers have a viral etiology, and it is certainly possible that additional viruses or bacteria are involved. The experience with XMRV provides several lessons for researchers engaged in hunts for new infectious agents associated with human diseases (cancers and others). First, XMRV underlines the previously known risk of passaging human tumor tissues through immunocompromised mice. While such passages have been effective in establishing tumor cell lines, the acquisition of activated endogenous MLVs or other retroviruses (not to mention other murine viruses) should be routinely monitored in the resulting lines. It should be emphasized that the nude mice used in the tumor xenograft passaging did not themselves produce substantial replication-competent MLVs. Rather, low-frequency activation of endogenous viruses with replication potential for human cells can result in production and spread in the human xenografts. Surveys of previously derived human cell lines have revealed significant numbers that are infected with X-MLVs.86,87 Second, if a xenotropic MLV is activated or amplified in a human tumor cell line, these cells can serve as a source for viral contamination of other cell lines. If investigators are passaging several cell lines in the same laboratory without employing precautions to prevent viral contamination, spread of virus from a productively infected cell line to other cell lines can occur unnoticed. In a survey by Zhang et al.87 there was evidence for spread of X-MLVs from xenograft-derived cell lines to other cell lines passaged in the same laboratory, including apparent spread of XMRV from 22Rv1 cells to a colorectal cancer cell line.87 Indeed, the original discovery of XMRV25 likely reflected detection of contamination in PC samples of DNA or RNA from 22Rv1 cells.74
The use of high cycle PCR amplifications to detect XMRV in patient samples also likely resulted in detection of low levels of contaminating sequences in several studies. One obvious source was XMRV plasmids maintained in the same laboratory. More subtle sources of contamination result from the fact that many of the original ‘XMRV-specific' PCR primers used could also amplify endogenous PreXMRV-1 or PreXMRV-2 sequences from mouse DNA. Thus contamination of human patient samples with mouse DNA could give false positives. One potential source was the carryover of mouse DNA to patient samples if the same microtome was used for sectioning of both mouse and human tissues. Moreover mouse DNA in common laboratory reagents such as PCR buffers and RT-PCR kits gave false positive results for XMRV.88,89,90,91 Some commercial taq polymerases are prepared with use of a mouse monoclonal antibody, and trace amounts of DNA from the mouse hybridoma cells presumably resulted in positive PCR signals for XMRV.91,92
POTENTIAL BIOHAZARDS ASSOCIATED WITH XMRV
While the association of XMRV with human PC or CFS has been disproven, the fact remains that it is a replication-competent MLV with xenotropic host range, and human cell lines such as LNCaP can be infected in culture.26,31 Thus, there is potential biohazard risk from this virus for humans. Moreover, the fact that productively infected cells such as 22Rv-1 have spread infection to other cell lines, raises the possibility that laboratory workers could have been unknowingly exposed to the virus. However, several facts reduce the risk of human infection by XMRV. Gammaretroviruses require cell division for productive infection, since entry of the viral DNA into the nucleus for integration is dependent on breakdown of the nuclear envelope during mitosis.15 In human adults, the most susceptible tissues would be those where there is rapid cell division, and for gammaretroviruses, this is typically the hematopoietic system, e.g., lymphocytes and other mononuclear cells. Cells or tissues where there is little cell division would be relatively resistant to infection. Hematopoietic cells generally have high levels of APOBEC3G, which potently inhibits XMRV infection;63,64,65 thus, the main cellular targets in humans are likely to be relatively resistant to infection. Indeed XMRV is restricted for in vitro infection of human PBMCs,93 and in the animal infection experiments, hypermutation of XMRV resulting from APOBEC3 restriction was observed,76,77 which may have resulted in lack of continued productive infection. On the other hand, PC cell lines have been reported to express low or undetectable APOBEC3G,63,64,65 although this conclusion has been challenged.94 In addition, the XMRV LTR has androgen response elements in its LTR enhancers,31 which would favor transcription of the viral DNA in infected prostate cells. Thus theoretically cells of the prostate could support XMRV replication more efficiently. However, scenarios by which the relatively low number of dividing cells in the prostate could become exposed to XMRV by laboratory contamination seem rather implausible. At a practical level, a survey of laboratory workers highly exposed to mice detected no evidence for XMRV or MLV infections.95 Nevertheless, the possibility of infection by XMRV (or other xenotropic MLVs) in humans (particularly in the laboratory setting) should be considered. At a minimum, standard biosafety precautions for handling xenotropic viruses as well as screening of laboratory cell lines for infection with xenotropic MLVs is important.
Acknowledgments
This work was supported in part by the Cancer Research Coordinating Committee of the University of California, and by National Science Foundation grant DGE-0638751. Support of the University of California, Irvine Cancer Research Institute and the UCI Chao Family Comprehensive Cancer Center (grant P30CA062203 from the National Cancer Institute) is acknowledged. We thank Takayuki Nitta and Tom Hsu (University of California, Irvine) for helpful discussions.
References
- Rous P. A Sarcoma of the fowl transmissible by an agent separable from the tumor cells. J Exp Med. 1911;13:397–411. doi: 10.1084/jem.13.4.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellerman V, Bang O. Experiment elle leukamie be huhern. Zentralbl Bakteriol. 1908;46:595–609. [Google Scholar]
- Bittner JJ. Some possible effects of nursing on the mammary gland tumor incidence in mice. Science. 1936;84:162. doi: 10.1126/science.84.2172.162. [DOI] [PubMed] [Google Scholar]
- Gross L. Development and serial cellfree passage of a highly potent strain of mouse leukemia virus. Proc Soc Exp Biol Med. 1957;94:767–771. doi: 10.3181/00379727-94-23080. [DOI] [PubMed] [Google Scholar]
- Lieberman M, Kaplan HS. Leukemogenic activity of filtrates from radiation-induced lymphoid tumors of mice. Science. 1959;130:387–388. doi: 10.1126/science.130.3372.387. [DOI] [PubMed] [Google Scholar]
- Muhlbock O. Note on a new inbred mouse-strain GR-A. Eur J Cancer. 1965;1:123–124. doi: 10.1016/0014-2964(65)90003-4. [DOI] [PubMed] [Google Scholar]
- Vogt PK, Friis RR. An avian leukosis virus related to RSV(O): properties and evidence for helper activity. Virology. 1971;43:223–234. doi: 10.1016/0042-6822(71)90240-6. [DOI] [PubMed] [Google Scholar]
- DiMaio D, Fan H.Virus, cell transformation, and cancerIn: Knipke DM, Howley PM (ed.)Fields virology6th ed. Vol. 1. Philadelphia, PA; Lippincott Williams & Wilkins; 2013153–188. [Google Scholar]
- Poiesz BJ, Ruscetti FW, Gazdar AF, Bunn PA, Minna JD, Gallo RC. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc Natl Acad Sci USA. 1980;77:7415–7419. doi: 10.1073/pnas.77.12.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M, Miyoshi I, Hinuma Y. Isolation and characterization of retrovirus from cell lines of human adult T-cell leukemia and its implication in the disease. Proc Natl Acad Sci USA. 1982;79:2031–2035. doi: 10.1073/pnas.79.6.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Sakalian M, Shen Z, Loss G, Neuberger J, Mason A. Cloning the human betaretrovirus proviral genome from patients with primary biliary cirrhosis. Hepatology. 2004;39:151–156. doi: 10.1002/hep.20024. [DOI] [PubMed] [Google Scholar]
- Wang Y, Jiang JD, Xu D, et al. A mouse mammary tumor virus-like long terminal repeat superantigen in human breast cancer. Cancer Res. 2004;64:4105–4111. doi: 10.1158/0008-5472.CAN-03-3880. [DOI] [PubMed] [Google Scholar]
- Mullins CS, Linnebacher M. Human endogenous retroviruses and cancer: causality and therapeutic possibilities. World J Gastroenterol. 2012;18:6027–6035. doi: 10.3748/wjg.v18.i42.6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanish MT, Cohen CJ, Mager DL. Potential mechanisms of endogenous retroviral-mediated genomic instability in human cancer. Semin Cancer Biol. 2010;20:246–253. doi: 10.1016/j.semcancer.2010.05.005. [DOI] [PubMed] [Google Scholar]
- Goff SP.RetroviridaeIn: Knipke DM, Howley PM (ed.)Fields virology6th ed. Vol. 2. Philadelphia, PA; Lippincott Williams & Wilkins, 2013; 1424–1473. [Google Scholar]
- Hayward WS, Neel BG, Astrin SM. Activation of a cellular onc gene by promoter insertion in ALV-induced lymphoid leukosis. Nature. 1981;290:475–480. doi: 10.1038/290475a0. [DOI] [PubMed] [Google Scholar]
- Fan H. Leukemogenesis by Moloney murine leukemia virus: a multistep process. Trends Microbiol. 1997;5:74–82. doi: 10.1016/S0966-842X(96)10076-7. [DOI] [PubMed] [Google Scholar]
- Jahner D, Jaenisch R. Retrovirus-induced de novo methylation of flanking host sequences correlates with gene inactivity. Nature. 1985;315:594–597. doi: 10.1038/315594a0. [DOI] [PubMed] [Google Scholar]
- Ch'ang LY, Yang WK, Myer FE, Yang DM. Negative regulatory element associated with potentially functional promoter and enhancer elements in the long terminal repeats of endogenous murine leukemia virus-related proviral sequences. J Virol. 1989;63:2746–2757. doi: 10.1128/jvi.63.6.2746-2757.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley JW, Wolford NK, Old LJ, Rowe WP. A new class of murine leukemia virus associated with development of spontaneous lymphomas. Proc Natl Acad Sci USA. 1977;74:789–792. doi: 10.1073/pnas.74.2.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achong BG, Trumper PA, Giovanella BC.C-type virus particles in human tumours transplanted into nude mice Br J Cancer 197634203–206.183801 [Google Scholar]
- Todaro GJ, Arnstein P, Parks WP, Lennette EH, Huebner RJ. A type-C virus in human rhabdomyosarcoma cells after inoculation into NIH Swiss mice treated with antithymocyte serum. Proc Natl Acad Sci USA. 1973;70:859–862. doi: 10.1073/pnas.70.3.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tralka TS, Yee CL, Rabson AB, et al. Murine type C retroviruses and intracisternal A-particles in human tumors serially passaged in nude mice. J Natl Cancer Inst. 1983;71:591–599. [PubMed] [Google Scholar]
- Casey G, Neville PJ, Plummer SJ, et al. RNASEL Arg462Gln variant is implicated in up to 13% of prostate cancer cases. Nat Genet. 2002;32:581–583. doi: 10.1038/ng1021. [DOI] [PubMed] [Google Scholar]
- Urisman A, Molinaro RJ, Fischer N, et al. Identification of a novel gammaretrovirus in prostate tumors of patients homozygous for R462Q RNASEL variant. PLoS Pathog. 2006;2:e25. doi: 10.1371/journal.ppat.0020025. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Dong B, Kim S, Hong S, et al. An infectious retrovirus susceptible to an IFN antiviral pathway from human prostate tumors. Proc Natl Acad Sci USA. 2007;104:1655–1660. doi: 10.1073/pnas.0610291104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alter HJ, Mikovits JA, Switzer WM, et al. A multicenter blinded analysis indicates no association between chronic fatigue syndrome/myalgic encephalomyelitis and either xenotropic murine leukemia virus-related virus or polytropic murine leukemia virus. MBio. 2012;3:e00266–12. doi: 10.1128/mBio.00266-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusmevichientong A, das Gupta J, Elias PS, Silverman RH, Chow SA. Analysis of single-nucleotide polymorphisms in patient-derived retrovirus integration sites reveals contamination from cell lines acutely infected by xenotropic murine leukemia virus-related virus. J Virol. 2011;85:12830–12834. doi: 10.1128/JVI.05624-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer N, Hellwinkel O, Schulz C, et al. Prevalence of human gammaretrovirus XMRV in sporadic prostate cancer. J Clin Virol. 2008;43:277–283. doi: 10.1016/j.jcv.2008.04.016. [DOI] [PubMed] [Google Scholar]
- Schlaberg R, Choe DJ, Brown KR, Thaker HM, Singh IR. XMRV is present in malignant prostatic epithelium and is associated with prostate cancer, especially high-grade tumors. Proc Natl Acad Sci USA. 2009;106:16351–16356. doi: 10.1073/pnas.0906922106. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Rodriguez JJ, Goff SP. Xenotropic murine leukemia virus-related virus establishes an efficient spreading infection and exhibits enhanced transcriptional activity in prostate carcinoma cells. J Virol. 2010;84:2556–2562. doi: 10.1128/JVI.01969-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knouf EC, Metzger MJ, Mitchell PS, et al. Multiple integrated copies and high-level production of the human retrovirus XMRV (xenotropic murine leukemia virus-related virus) from 22Rv1 prostate carcinoma cells. J Virol. 2009;83:7353–7356. doi: 10.1128/JVI.00546-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akgul B, Pfister D, Knuchel R, Heidenreich A, Wieland U, Pfister H. No evidence for a role of xenotropic murine leukaemia virus-related virus and BK virus in prostate cancer of German patients. Med Microbiol Immunol. 2012;201:245–248. doi: 10.1007/s00430-011-0215-0. [DOI] [PubMed] [Google Scholar]
- Cornelissen M, Zorgdrager F, Blom P, et al. Lack of detection of XMRV in seminal plasma from HIV-1 infected men in The Netherlands. PLoS ONE. 2010;5:e12040. doi: 10.1371/journal.pone.0012040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohn O, Krause H, Barbarotto P, et al. Lack of evidence for xenotropic murine leukemia virus-related virus (XMRV) in German prostate cancer patients. Retrovirology. 2009;6:92. doi: 10.1186/1742-4690-6-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza R, Silverman RH, Klein EA, Miller AD. No biological evidence of XMRV in blood or prostatic fluid from prostate cancer patients. PLoS ONE. 2012;7:e36073. doi: 10.1371/journal.pone.0036073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakuma T, Hue S, Squillace KA, et al. No evidence of XMRV in prostate cancer cohorts in the Midwestern United States. Retrovirology. 2011;8:23. doi: 10.1186/1742-4690-8-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stieler K, Schindler S, Schlomm T, et al. No detection of XMRV in blood samples and tissue sections from prostate cancer patients in Northern Europe. PLoS ONE. 2011;6:e25592. doi: 10.1371/journal.pone.0025592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardi VC, Ruscetti FW, das Gupta J, et al. Detection of an infectious retrovirus, XMRV, in blood cells of patients with chronic fatigue syndrome. Science. 2009;326:585–589. doi: 10.1126/science.1179052. [DOI] [PubMed] [Google Scholar]
- Lo SC, Pripuzova N, Li B, et al. Detection of MLV-related virus gene sequences in blood of patients with chronic fatigue syndrome and healthy blood donors. Proc Natl Acad Sci USA. 2010;107:15874–15879. doi: 10.1073/pnas.1006901107. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Ali MA, Dale JK, Kozak CA, et al. Xenotropic murine leukemia virus-related virus is not associated with chronic fatigue syndrome in patients from different areas of the US in the 1990s. Virol J. 2011;8:450. doi: 10.1186/1743-422X-8-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cool M, Bouchard N, Masse G, et al. No detectable XMRV in subjects with chronic fatigue syndrome from Quebec. Virology. 2011;420:66–72. doi: 10.1016/j.virol.2011.08.018. [DOI] [PubMed] [Google Scholar]
- Elfaitouri A, Shao X, Mattsson Ulfstedt J, et al. Murine gammaretrovirus group G3 was not found in Swedish patients with myalgic encephalomyelitis/chronic fatigue syndrome and fibromyalgia. PLoS ONE. 2011;6:e24602. doi: 10.1371/journal.pone.0024602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlwein O, Kaye S, McClure MO, et al. Failure to detect the novel retrovirus XMRV in chronic fatigue syndrome. PLoS ONE. 2010;5:e8519. doi: 10.1371/journal.pone.0008519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta RA, Miyazawa T, Sugiyama T, et al. No association of xenotropic murine leukemia virus-related virus with prostate cancer or chronic fatigue syndrome in Japan. Retrovirology. 2011;8:20. doi: 10.1186/1742-4690-8-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groom HC, Boucherit VC, Makinson K, et al. Absence of xenotropic murine leukaemia virus-related virus in UK patients with chronic fatigue syndrome. Retrovirology. 2010;7:10. doi: 10.1186/1742-4690-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong P, Li J, Li Y. Failure to detect xenotropic murine leukaemia virus-related virus in Chinese patients with chronic fatigue syndrome. Virol J. 2010;7:224. doi: 10.1186/1743-422X-7-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakes B, Qiu X, Levine S, Hackett J, Jr, Huber BT. Failure to detect XMRV-specific antibodies in the plasma of CFS patients using highly sensitive chemiluminescence immunoassays. Adv Virol. 2011;2011:854540. doi: 10.1155/2011/854540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterfield BC, Garcia RA, Jia H, Tang S, Zheng H, Switzer WM. Serologic and PCR testing of persons with chronic fatigue syndrome in the United States shows no association with xenotropic or polytropic murine leukemia virus-related viruses. Retrovirology. 2011;8:12. doi: 10.1186/1742-4690-8-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons G, Glynn SA, Komaroff AL, et al. Failure to confirm XMRV/MLVs in the blood of patients with chronic fatigue syndrome: a multi-laboratory study. Science. 2011;334:814–817. doi: 10.1126/science.1213841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffen I, Tyrrell DL, Stein E, et al. No evidence for XMRV nucleic acids, infectious virus or anti-XMRV antibodies in Canadian patients with chronic fatigue syndrome. PLoS ONE. 2011;6:e27870. doi: 10.1371/journal.pone.0027870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Switzer WM, Jia H, Hohn O, et al. Absence of evidence of xenotropic murine leukemia virus-related virus infection in persons with chronic fatigue syndrome and healthy controls in the United States. Retrovirology. 2010;7:57. doi: 10.1186/1742-4690-7-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomberg J, Blomberg F, Sjosten A, et al. No evidence for xenotropic murine leukemia-related virus infection in Sweden using internally controlled multiepitope suspension array serology. Clin Vaccine Immunol. 2012;19:1399–1410. doi: 10.1128/CVI.00391-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi Z, Lu Y, Zhang S, et al. Absence of xenotropic murine leukemia virus-related virus in blood donors in China. Transfusion. 2012;52:326–331. doi: 10.1111/j.1537-2995.2011.03267.x. [DOI] [PubMed] [Google Scholar]
- Qiu X, Swanson P, Tang N, et al. Seroprevalence of xenotropic murine leukemia virus-related virus in normal and retrovirus-infected blood donors. Transfusion. 2012;52:307–316. doi: 10.1111/j.1537-2995.2011.03395.x. [DOI] [PubMed] [Google Scholar]
- Tang S, Zhao J, Haleyur Giri Setty MK, et al. Absence of detectable XMRV and other MLV-related viruses in healthy blood donors in the United States. PLoS ONE. 2011;6:e27391. doi: 10.1371/journal.pone.0027391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes E, Flanagan P, Brown A, et al. Failure to detect xenotropic murine leukemia virus-related virus in blood of individuals at high risk of blood-borne viral infections. J Infect Dis. 2010;202:1482–1485. doi: 10.1086/657167. [DOI] [PubMed] [Google Scholar]
- Delviks-Frankenberry KA, Chaipan C, Bagni R, Wyvill K, Yarchoan R, Pathak VK. Lack of detection of xenotropic murine leukemia virus-related virus in HIV-1 lymphoma patients. Adv Virol. 2011;2011:797820. doi: 10.1155/2011/797820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingaras C, Danielson BP, Vigil KJ, Vey E, Arduino RC, Kimata JT. Absence of XMRV in peripheral blood mononuclear cells of ARV-treatment naive HIV-1 infected and HIV-1/HCV coinfected individuals and blood donors. PLoS ONE. 2012;7:e31398. doi: 10.1371/journal.pone.0031398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray ER, Garson JA, Breuer J, et al. No evidence of XMRV or related retroviruses in a London HIV-1-positive patient cohort. PLoS ONE. 2011;6:e18096. doi: 10.1371/journal.pone.0018096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X, Walker TD, Maranga IO, Oliver AW, Hampson L, Hampson IN. No biological evidence of XMRV infection in cervical smears from HIV/HPV positive and negative Kenyan women. PLoS ONE. 2012;7:e47208. doi: 10.1371/journal.pone.0047208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luczkowiak J, Martinez-Prats L, Sierra O, et al. Lack of the detection of XMRV or polytropic MLV-related sequences in blood cells from HIV-1-infected patients in Spain. J Acquir Immune Defic Syndr. 2012;59:101–104. doi: 10.1097/QAI.0b013e318238b596. [DOI] [PubMed] [Google Scholar]
- Paprotka T, Venkatachari NJ, Chaipan C, et al. Inhibition of xenotropic murine leukemia virus-related virus by APOBEC3 proteins and antiviral drugs. J Virol. 2010;84:5719–5729. doi: 10.1128/JVI.00134-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogerd HP, Zhang F, Bieniasz PD, Cullen BR. Human APOBEC3 proteins can inhibit xenotropic murine leukemia virus-related virus infectivity. Virology. 2011;410:234–239. doi: 10.1016/j.virol.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stieler K, Fischer N. Apobec 3G efficiently reduces infectivity of the human exogenous gammaretrovirus XMRV. PLoS ONE. 2010;5:e11738. doi: 10.1371/journal.pone.0011738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansky LM. In vivo analysis of human T-cell leukemia virus type 1 reverse transcription accuracy. J Virol. 2000;74:9525–9531. doi: 10.1128/jvi.74.20.9525-9531.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dooren S, Pybus OG, Salemi M, et al. The low evolutionary rate of human T-cell lymphotropic virus type-1 confirmed by analysis of vertical transmission chains. Mol Biol Evol. 2004;21:603–611. doi: 10.1093/molbev/msh053. [DOI] [PubMed] [Google Scholar]
- Paprotka T, Delviks-Frankenberry KA, Cingoz O, et al. Recombinant origin of the retrovirus XMRV. Science. 2011;333:97–101. doi: 10.1126/science.1205292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delviks-Frankenberry K, Paprotka T, Cingoz O, et al. Generation of multiple replication-competent retroviruses through recombination between PreXMRV-1 and PreXMRV-2. J Virol. 2013;87:11525–11537. doi: 10.1128/JVI.01787-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D, das Gupta J, Gaughan C, et al. In-depth investigation of archival and prospectively collected samples reveals no evidence for XMRV infection in prostate cancer. PLoS ONE. 2012;7:e44954. doi: 10.1371/journal.pone.0044954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman RH, das Gupta J, Lombardi VC, et al. Partial retraction. Detection of an infectious retrovirus, XMRV, in blood cells of patients with chronic fatigue syndrome. Science. 2011;334:176. doi: 10.1126/science.1212182. [DOI] [PubMed] [Google Scholar]
- Knox K, Carrigan D, Simmons G, et al. No evidence of murine-like gammaretroviruses in CFS patients previously identified as XMRV-infected. Science. 2011;333:94–97. doi: 10.1126/science.1204963. [DOI] [PubMed] [Google Scholar]
- Shin CH, Bateman L, Schlaberg R, et al. Absence of XMRV retrovirus and other murine leukemia virus-related viruses in patients with chronic fatigue syndrome. J Virol. 2011;85:7195–7202. doi: 10.1128/JVI.00693-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman RH, das Gupta J, Lombardi VC, et al. Partial retraction. Detection of an infectious retrovirus, XMRV, in blood cells of patients with chronic fatigue syndrome. Science. 2011;334:176. doi: 10.1126/science.1212182. [DOI] [PubMed] [Google Scholar]
- Onlamoon N, das Gupta J, Sharma P, et al. Infection, viral dissemination, and antibody responses of rhesus macaques exposed to the human gammaretrovirus XMRV. J Virol. 2011;85:4547–4557. doi: 10.1128/JVI.02411-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Prete GQ, Kearney MF, Spindler J, et al. Restricted replication of xenotropic murine leukemia virus-related virus in pigtailed macaques. J Virol. 2012;86:3152–3166. doi: 10.1128/JVI.06886-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakuma T, Tonne JM, Squillace KA, et al. Early events in retrovirus XMRV infection of the wild-derived mouse Mus pahari. J Virol. 2011;85:1205–1213. doi: 10.1128/JVI.00886-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakuma T, Tonne JM, Malcolm JA, et al. Long-term infection and vertical transmission of a gammaretrovirus in a foreign host species. PLoS ONE. 2012;7:e29682. doi: 10.1371/journal.pone.0029682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias M. PhD thesis. University of California; Irvine, CA, USA; 2013. Molecular characterization of XMRV replication in rats. [Google Scholar]
- Metzger MJ, Holguin CJ, Mendoza R, Miller AD. The prostate cancer-associated human retrovirus XMRV lacks direct transforming activity but can induce low rates of transformation in cultured cells. J Virol. 2010;84:1874–1880. doi: 10.1128/JVI.01941-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger MJ, Miller AD. Acutely transforming retrovirus expressing Nras generated from HT-1080 fibrosarcoma cells infected with the human retrovirus XMRV. J Virol. 2010;84:7908–7910. doi: 10.1128/JVI.00389-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandhare-Dash J, Mantri CK, Gong Y, Chen Z, Dash C. XMRV accelerates cellular proliferation, transformational activity, and invasiveness of prostate cancer cells by downregulating p27(Kip1) Prostate. 2012;72:886–897. doi: 10.1002/pros.21491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stieler K, Schumacher U, Horst AK, Fischer N. XMRV induces cell migration, cytokine expression and tumor angiogenesis: are 22Rv1 cells a suitable prostate cancer model. PLoS ONE. 2012;7:e42321. doi: 10.1371/journal.pone.0042321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murgai M, Thomas J, Cherepanova O, et al. Xenotropic MLV envelope proteins induce tumor cells to secrete factors that promote the formation of immature blood vessels. Retrovirology. 2013;10:34. doi: 10.1186/1742-4690-10-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hempel HA, Burns KH, de Marzo AM, Sfanos KS. Infection of xenotransplanted human cell lines by murine retroviruses: a lesson brought back to light by XMRV. Front Oncol. 2013;3:156. doi: 10.3389/fonc.2013.00156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hue S, Gray ER, Gall A, et al. Disease-associated XMRV sequences are consistent with laboratory contamination. Retrovirology. 2010;7:111. doi: 10.1186/1742-4690-7-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YA, Maitra A, Hsieh JT, et al. Frequent detection of infectious xenotropic murine leukemia virus (XMLV) in human cultures established from mouse xenografts. Cancer Biol Ther. 2011;12:617–628. doi: 10.4161/cbt.12.7.15955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Jia H, Shankar A, Heneine W, Switzer WM. Detection of murine leukemia virus or mouse DNA in commercial RT-PCR reagents and human DNAs. PLoS ONE. 2011;6:e29050. doi: 10.1371/journal.pone.0029050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacich DJ, Sobek KM, Cummings JL, Atwood AA, O'Keefe DS. False negative results from using common PCR reagents. BMC Res Notes. 2011;4:457. doi: 10.1186/1756-0500-4-457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlwein O, Robinson MJ, Dustan S, Weber J, Kaye S, McClure MO. DNA extraction columns contaminated with murine sequences. PLoS ONE. 2011;6:e23484. doi: 10.1371/journal.pone.0023484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuke PW, Tettmar KI, Tamuri A, Stoye JP, Tedder RS. PCR master mixes harbour murine DNA sequences. Caveat emptor. PLoS ONE. 2011;6:e19953. doi: 10.1371/journal.pone.0019953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato E, Furuta RA, Miyazawa T. An endogenous murine leukemia viral genome contaminant in a commercial RT-PCR kit is amplified using standard primers for XMRV. Retrovirology. 2010;7:110. doi: 10.1186/1742-4690-7-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaipan C, Dilley KA, Paprotka T, et al. Severe restriction of xenotropic murine leukemia virus-related virus replication and spread in cultured human peripheral blood mononuclear cells. J Virol. 2011;85:4888–4897. doi: 10.1128/JVI.00046-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dey A, Mantri CK, Pandhare-Dash J, Liu B, Pratap S, Dash C. Downregulation of APOBEC3G by xenotropic murine leukemia-virus related virus (XMRV) in prostate cancer cells. Virol J. 2011;8:531. doi: 10.1186/1743-422X-8-531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks J, Lycett-Lambert K, Caminiti K, Merks H, McMillan R, Sandstrom P. No evidence of cross-species transmission of mouse retroviruses to animal workers exposed to mice. Transfusion. 2012;52:317–325. doi: 10.1111/j.1537-2995.2011.03463.x. [DOI] [PubMed] [Google Scholar]