Abstract
A major challenge of infectious disease elimination is the need to interrupt pathogen transmission across all vulnerable populations. Ethnic minorities are among the key vulnerable groups deserving special attention in disease elimination initiatives, especially because their lifestyle might be intrinsically linked to locations with high transmission risk. There has been a renewed interest in malaria elimination, which has ignited a quest to understand factors necessary for sustainable malaria elimination, highlighting the need for diverse approaches to address epidemiological heterogeneity across malaria transmission settings. An analysis of malaria incidence among the Guna Amerindians of Panamá over the last 34 years showed that this ethnic minority was highly vulnerable to changes that were assumed to not impact malaria transmission. Epidemic outbreaks were linked with El Niño Southern Oscillations and were sensitive to political instability and policy changes that did not ensure adequate attention to the malaria control needs of the Gunas. Our results illustrate how the neglect of minorities poses a threat to the sustainable control and eventual elimination of malaria in Central America and other areas where ethnic minorities do not share the benefits of malaria control strategies intended for dominant ethnic groups.
Keywords: Anopheles albimanus, climate change, earth sciences, ecology, Gunas, malaria, Plasmodium, social exclusion
INTRODUCTION
One hundred years ago, the Panamá Canal was inaugurated, and ever since, it has been a major route for global commerce.1 The Panama Canal was celebrated as a major achievement of civil and environmental engineering at the time, and its construction was also a major achievement for public sanitation.2,3 Endemic yellow fever and malaria quickly waned after intense vector control measurements were taken in ‘a hotbed of malaria'.4 Malaria control in the Canal Zone (name of the Panamá Canal under the colonial rule of the United States) emphasized the long-term benefits of environmental sanitation.1,3 This priority was derived from the need to render the Panamá Canal suitable for the colonialist exploitation of a convenient trade passage.1,3 Nevertheless, wider health benefits were observed by all residents of the Canal Zone and its surrounding neighbor, the República de Panamá.3,5,6 Malaria was rare in the Panamá Isthmus by the mid-1930s6 and had almost disappeared by the mid-1950s.7
Parallel to the ‘official' history of great achievements in malaria elimination from the Canal Zone and control in the República de Panamá, there was a silent history of malaria transmission in Amerindian populations of the Panamá Isthmus. Earlier epidemiological8,9 and entomological2,10 studies on malaria in the Canal Zone revealed that poor housing, human crowding and other consequences of social exclusion exacerbated the malaria burden among Amerindian populations. Nevertheless, following the initial assessment of the malaria situation in the Panamá Isthmus, concerns about malaria in Amerindian minorities were largely absent in studies that claimed an almost final victory against malaria in Panamá.7
Malaria transmission was considered to be negligible in the Panamá Isthmus from the 1950s onwards, including at the time of the Torrijos-Carter treaty signature in 197911—an agreement that warranted the return of the Canal Zone to Panamanian sovereignty12—and in 1999 when the Panamá Canal was returned to the República de Panamá.11 In the 1950s, the República de Panamá also began its journey toward fully recognizing the Amerindian ethnic groups as citizens, with the increased recognition of rights for the Amerindian ethnic groups through the creation of the San Blas (current Guna Yala) Comarca, the first of several Comarcas, i.e., territories designated to provide full autonomy and social integration for Amerindian minorities within the multicultural and ethnically diverse definition of the República de Panamá.13,14 In this context, Comarca Madungandí was created in 1996. This region is part of the homeland of the Guna (formerly known as Kuna), an indigenous ethnic group of Panamá (Figure 1A). The Gunas are a Chibchan group. DNA analysis showed low variability in their mitochondrial sequences, which is compatible with a population bottleneck coincident with the time of ethnogenesis for the Chibchas, approximately 10 000 years ago.15 Madungandí is a 1200 km2 area within the Chepo district of the Panamá Province, on the Atlantic basin in mid-eastern Panamá. Puente Bayano, the most accessible village in Madungandí, is 80 km from Panamá City and 120 km from Colón, the two major cities in Panamá (Figure 1B). In 2010, there were 4217 Gunas living in the 14 villages that comprise Madungandí,16 with most residing along the shores of the artificial lake Bayano (Figure 1C). Approximately 90% of the Madungandí Guna natives live in extreme poverty, which is almost five-fold higher than the percentage observed in the rest of Panamá.17 About half of the adult population, i.e., people over 15 years of age, is illiterate (45.9%), and only 17.5% completed elementary education. The major economic activities are subsistence farming and fishing, which employ 56.4% and 17.2%, respectively, of the adult population.16 The health status of the Guna lags behind that of other Panamanian citizens; the Guna have a life expectancy that is between 7 and 10 years shorter than that of other Panamanians, and a three-fold increase in the mortality rate for children under one year of age when compared with other Panamanians.17 Malaria is the most common infectious disease affecting the Guna. Since 2000, approximately 45% of the malaria cases in the entire República de Panamá have occurred in Comarca Mudangandí.11,18 The Guna are also commonly affected by respiratory and digestive infections, which are likely exacerbated by a lack of access to basic services.19 For example, 76.3% of the population has no access to potable water, and 84.3% has no access to basic sanitation, e.g., disposal of wastewater.16 Housing conditions are destitute and insect-friendly (Figure 1D), with most houses built of wood and with thatched roofs, eaves, and unscreened doors and windows that facilitate Anopheles spp. mosquito infestations.2
Figure 1.
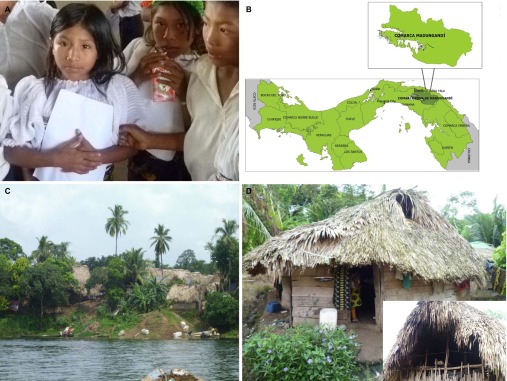
The Gunas of Comarca Madungandí, República de Panamá. (A) Guna children at Puente Bayano elementary school. (B) Comarca Madungandí, República de Panamá. The map shows the location of Comarca Madungandí in relation to Panamá City and Colón. (C) Guna Village at the shore of Lake Bayano. (D) Traditional Guna house with earthen floor, wood walls, thatched roofs and no anti-insect screening. The inset shows the typical eaves that facilitate Anopheles albimanus mosquito infestation.
The disproportionate share of the malaria burden of the República de Panamá that is held by the Guna Amerindians of Comarca Madungandi not only highlights their socially outcast condition but also their likely enhanced vulnerability to malaria, a parasitic disease with transmission that is sensitive to climatic variability.20,21,22 In this study, we analyzed the dynamics of malaria transmission among the Gunas from Comarca Madungandí, using a 34-year monthly malaria case time-series from Comarca Madungandí. We assessed whether major malaria epidemics observed in the region in the early 2000s11 represent a major dynamical regime shift, i.e., an abrupt change in the mean and/or variability of malaria incidence.23 We also assessed whether the malaria epidemics were related to temperature increases in the region or whether they were related to changes in climatic variability associated with large climatic events, such as the El Niño Southern Oscillation (ENSO). We found that inter-annual cycles in malaria epidemics were associated with ENSO during 1980–2013 and that a dynamical regime shift observed at the end of 2002 might be related to policy changes in the structure of the malaria control program, in which personnel and funding for this program were re-assigned within Panamá's Ministry of Health without adequate preparation to tackle malaria transmission. We consider that full inclusion of the Gunas into Panamanian society is the major challenge for successful malaria control, and eventual malaria elimination, in the República de Panamá.
MATERIALS AND METHODS
Malaria and demographic data
Monthly malaria cases for Comarca Madungandí, from January 1980 to April 2013, were obtained from the East Panamá Region Vector Control Department of Panamá's Ministry of Health (Departamento de Control de Vectores de la Región Panamá Este, Ministerio de Salud, República de Panamá). The time series mainly records malaria cases from a sentinel health post in Puente Bayano, the main village in Madungandí (Supplementary Figure S1A). The time series only included malaria cases with diagnoses that were confirmed by the examination of Giemsa stained blood smears that were prepared using the thick smear method8 and examined under the microscope by trained staff at the Gorgas Institute or the Panamá Este regional Hospital. All positive slides, and 10% of the negative slides, were confirmed by the Public Health Central Reference Laboratory of the Gorgas Laboratory, following the national guidelines for malaria control of the Ministry of Health of Panamá. For the study period, both the sensitivity and specificity of malaria diagnosis were consistent and were close to 100%, highlighting the quality of the data. During the study period, more than 95% of the malaria cases were due to Plasmodium vivax, but during the 2002–2006 outbreak, approximately 12% of the cases were due to Plasmodium falciparum.11
Annual population size estimates (1980–2013) for the Chepo district were obtained from the Dirección de Estadística y Censo of Contraloría General de República de Panamá. Data from the Chepo district was rescaled with the assumption that Gunas in Comarca Madungandi steadily accounted for 10% of the total population, as they did in the 2010 census (Supplementary Figure S1B).
Monthly malaria incidence per 1000 was estimated as the ratio between the number of malaria cases and population size estimates for each month, multiplied by 1000.
Study site and climatic data
The natural vegetation of Comarca Madungandí is a humid tropical rainforest, with a high and stable temperature of approximately 26–27 °C and a unimodal rainfall pattern with a dry season (mid-December to mid-April) and a wet season (mid-April to mid-December). Rainfall, maximum temperature (TMAX) and minimum temperature (TMIN) time series from the nearest weather station to Madungandí were obtained from ETESA, the Panamanian National Electrical Company. Sea surface temperature 4 (SST4, also known as, El Niño 4 index) time series, an index for ENSO, a global climatic phenomena associated with extreme weather in Central America was obtained from the United States National Oceanic and Atmospheric Administration Climate Prediction Center (http://www.cpc.ncep.noaa.gov/data/indices/ersst3b.nino.mth.81-10.ascii). The United States National Oceanic and Atmospheric Administration data were collected from the area delimited by 5°N–5°S and 160°E–150°W of the Pacific Ocean.
Statistical analysis
Trends in the climatic variables
We began our analysis by determining whether trends (increases in the average value) were present in the local climatic variables: rainfall, TMAX and TMIN. We employed locally weighted scatterplot smoothing, a non-parametric regression method,24 to fit trends in the climatic time series data. For robustness, we also employed empirical mode decomposition (EMD), a method in which a time series is decomposed by building oscillatory signals—intrinsic mode functions (IMF)—that are repeatedly subtracted from the time series and a residue, a nonlinear trend, can be obtained after the oscillatory components are removed.25 Details about the EMD decomposition algorithm and a mathematically rigorous explanation are presented by Huang et al.25
Seasonality of malaria incidence
The seasonality of the malaria incidence time series was assessed using box diagrams for each month.26
Malaria incidence time series correlation structure and association with climatic variables
We explored the correlation structure in the malaria incidence time series by inspecting its autocorrelation function (ACF), i.e., the time series correlation with itself through time, and the partial autocorrelation function (PACF), i.e., the correlation between consecutive time lags.24 Information from the ACF and PACF was used to fit an autonomous null model, i.e., a model without covariates, of the malaria incidence data. The null model was then used to pre-whiten the climatic covariates SST4, Rain, TMAX and TMIN. Pre-whitening is a process in which any common autoregressive structure is removed (often referred to as filtering) from a time series to study patterns of association with a focal time series.24 Residuals from the autonomous null model together with pre-whitened residuals of the climatic covariates were then used to estimate cross-correlation functions (CCF) of malaria incidence with each of the climatic covariates. We used the information from the CCFs to build a full non-autonomous model that considered up to two lags from each of the climatic covariates, selecting the lags with the highest correlation, based on the statistical significance of the association and the magnitude of the association. This full model was then simplified to avoid having more parameters than necessary to understand the dynamics of malaria incidence. We simplified the full model by a process of backward elimination, i.e., removing the least significant covariate in rounds.24 We used the Akaike Information Criterion (AIC) to choose the best model in each round of model simplification. AIC is a model selection criterion that can be used to choose the best model after a trade-off function between the number of parameters and likelihood is minimized.24 Finally, in all cases, assumptions about model error were verified using standard procedures for time series analysis.24
Breakpoints and dynamical regime shifts
To explore the possibility that climatic variables with trends and malaria incidence in Madungandí had dynamical regime shifts, i.e., abrupt changes in their average and/or variance during the studied period, we employed a parametric test based on an F distribution that compares the null hypothesis of no change with that of a significant change. Briefly, the method by Bai and Perron27 allows the simultaneous estimation of several breakpoints, i.e., time points when a dynamical regime shift occurs, via an iterative algorithm by which the number and temporal location of breakpoints can be estimated by minimizing the Bayes Information Criterion (BIC). BIC is a model selection criterion that guides the best model selection when a trade-off function considering the likelihood of a model, the number of parameters and data points employed in parameter estimation is minimized.24
After the breakpoints were estimated, we fitted the full non-autonomous model to the malaria incidence regimes, i.e., time series segments, which were defined by the estimated breakpoints. These models were simplified based on AIC minimization and assumptions of the error were checked using standard procedures.28 We then studied changes in the magnitude of climatic forcing in the malaria time series by comparing the time series model parameter estimates for the different segments of the split time series.28
Non-stationary patterns of association in the time–frequency domain
The time–frequency association of non-stationary time series, i.e., the association of cycles in time series over time, can be studied using continuous wavelet transforms.29 Here, we used cross-wavelet coherence analysis to determine whether peaks at a particular frequency and time in SST4 correspond to similar peaks in malaria incidence and local climatic variables (Rain, TMAX and TMIN) of Comarca Madugandi, República Panamá. We also depicted the association between malaria incidence and climatic factors by first decomposing the time series into a trend and oscillatory components via an EMD. The power spectra of the resulting trend and IMFs were estimated via the construction of a periodogram, which is a graphical representation of the distribution of power (or variance) among different frequencies, the inverse of the period of cycles per year.24 A peak in the periodogram indicates a dominant frequency, whose inverse is the period of the dominant cycles in a time series.20 Given the period of the cycles in the trend and IMFs from the EMD, we estimated their CCFs with the climatic covariates, to untangle the specific associations between the global (SST4, El Niño 4 index) and local (rainfall, TMAX and TMIN) climatic covariates with inter-annual, seasonal and noisy cycles in malaria incidence.
RESULTS
Figure 2 shows the climatic time series for Comarca Madungandí. Figure 2A shows rainfall (R) during the study period. Figure 2B and 2C shows maximum temperature (TMAX) and minimum temperature (TMIN), respectively. Minimum temperature has a clear trend of close to a 3 °C increase from 1988 to 2013, a result robustly found with locally weighted scatterplot smoothing and EMD (Figure 2C). Figure 2D shows the SST4, an index of ENSO associated with Panama's climate. Figure 2E shows the malaria incidence time series, in which the largest epidemics occurred in 1987 and 2005. During the 34 years of the time series, a total 4976 cases were reported in the Comarca Guna of Madungandí. Figure 2F shows the malaria seasonality. A higher incidence of malaria tended to occur during the dry season (December–April). Malaria incidence also shows a clear autoregressive pattern, supported by a decreasing ACF (Figure 2G) confirmed by significant PACF, coefficients at 1, 2 and 11 months of lag (Figure 2H). The ACF and PACF suggested that malaria incidence can be described by the following autonomous (i.e., without covariates) seasonal autoregressive (SAR) model:
Figure 2.
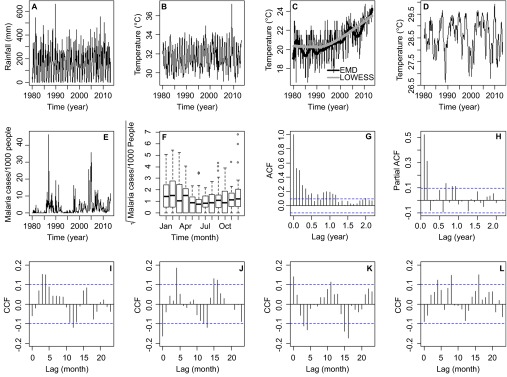
Time series data from Comarca Madungandí, República de Panamá (January 1980–April 2013) and correlation functions (A) Rainfall. (B) Maximum temperature in Comarca. (C) Minimum temperature. Lines represent the trends obtained with LOWESS and EMD. (D) El Niño 4 index, also known as, SST4. (E) Malaria cases per 1000 people. (F) Malaria transmission seasonality. Boxes delimit the 25th to 75th data quantiles, and the lines inside the boxes show the median of the distribution for each month. (G) ACF of the malaria time series shown in panel A. (H) PACF of the time series shown in A. CCF between residuals of a SAR (2, 12) model for malaria cases per 1000 people and pre-whitened: (I) SST4, (J) rainfall, (K) maximum temperature and (L) minimum temperature. In G–L, the dashed lines indicate the 95% CL for the correlations that can be expected by random. CL, confidence limits; LOWESS, locally weighted scatterplot smoothing.
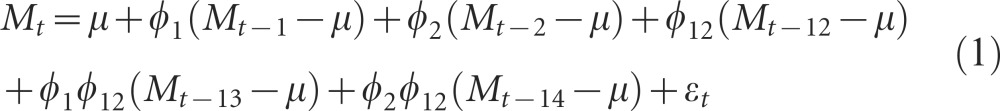 |
where M denotes malaria incidence, μ is the mean malaria incidence for different time lags and εt is an identical and independent normally distributed error. The maxima of CCFs between the residuals of the model in Equation (1) and the pre-whitened SST4 (Figure 2I), R (Figure 2J), TMAX (Figure 2K) and TMIN (Figure 2L) suggest that malaria incidence was significantly associated with SST4 (4), R (0), R (5), TMAX (0), TMAX (17), TMIN (17) and TMIN (8), with the number inside the parentheses indicating the lag of the association in months.
With the information from the CCFs, we proceeded to fit the following non-autonomous (i.e., with covariates), forced, SAR model:
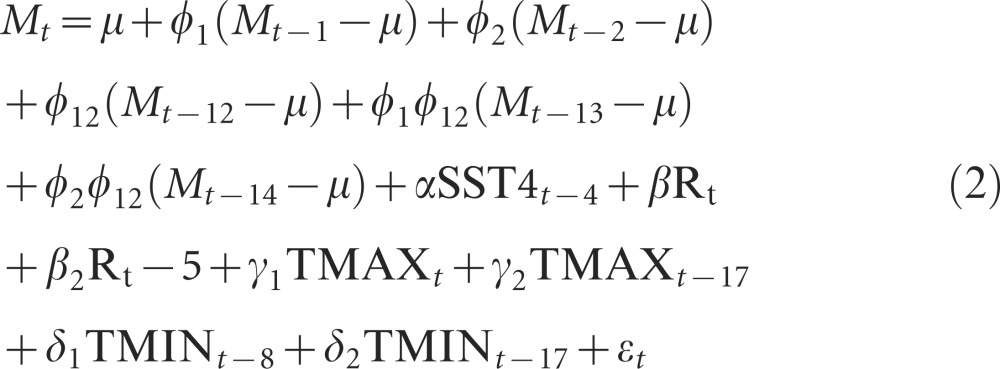 |
Hereafter, this model will be referred to as the full model. This model was simplified using the AIC (Supplementary Table S1), and several terms were dropped resulting in the following final model:
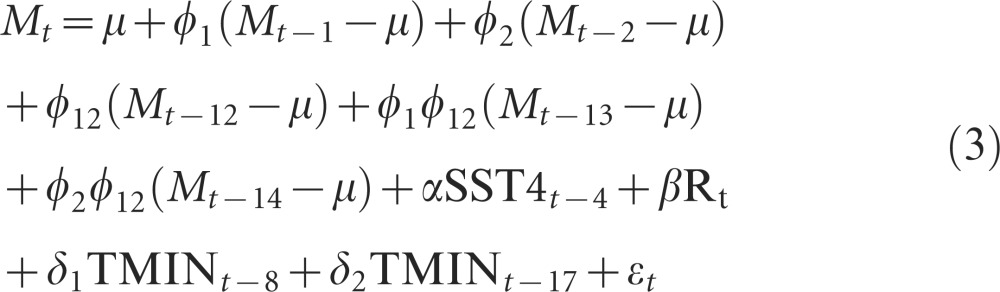 |
Parameter estimates for the model described in Equation (3) are presented in Table 1.
Table 1. Parameter estimates for the best models of malaria incidence (per 1000 people) in Comarca Madugandí, República de Panamá, with and without breakpoints.
| Parameter (Lag-month) | Estimates (±s.e.) | |||
|---|---|---|---|---|
| No breakpoint | Breakpoint (December 2002) | |||
| Before | After | |||
 |
Mean (–) | 3.06±0.55* | 2.45±0.53* | 4.80±0.95* |
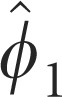 |
Autoregressive first order, AR (1) | 0.348±0.049* | 0.171±0.057* | 0.697±0.066* |
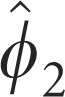 |
Autoregressive second order, AR (2) | 0.322±0.049* | 0.376±0.058* | — |
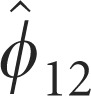 |
Seasonal Autoregressive, SAR (12) | −0.106±0.052* | — | −0.248±0.087* |
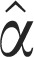 |
El Niño 4 (4) | 1.71±0.62* | 1.46±0.62* | 2.14±1.19 |
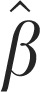 |
Rainfall (0) | −0.0043±0.0017* | — | −0.008±0.003* |
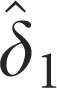 |
Minimum temperature (8) | 0.476±0.179* | — | — |
 |
Minimum temperature (17) | 0.579±0.182* | — | — |
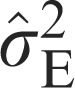 |
Variance of the error | 15.61 | 14.13 | 16.16 |
Statistically significant (P<0.05).
The dynamical regime shift analysis showed that no breakpoints (and their associated dynamical regime shifts) occurred in the TMIN time series after a model such as the one described in Equation (1) was employed to find breakpoints using the Bai and Perron27 method (Figure 3A). In contrast, a breakpoint was found when a similar procedure was applied to the malaria incidence time series, on December 2002 (95% confidence limits September 2001–February 2004), as shown by the minimization of BIC for one breakpoint in Figure 3B. There were major differences in the seasonality of malaria during the two periods separated by the dynamical regime shift. From 1980 to 2002, although epidemics were more common during December, January and February, no major differences were observed across the different months (Figure 3C). In contrast, from 2003 to 2013, a clear seasonality was observed, with the number of cases peaking during February, and the number of cases was significantly higher at the end of the wet season in November, the dry season (December–March) and the beginning of the wet season in April (Figure 3D).
Figure 3.
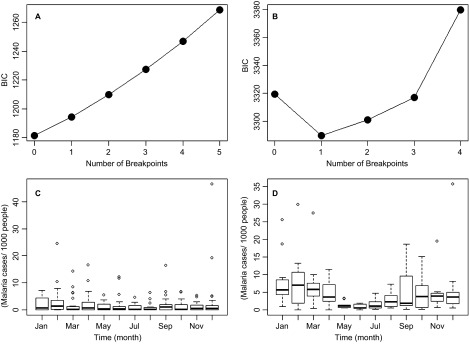
Breakpoint analysis. BIC for breakpoint selection in (A) minimum temperature and (B) malaria incidence (per 1000 people). The breakpoint in B corresponds to December 2002. Seasonal malaria incidence in (C) January 1980–December 2002 and (D) January 2003–April 2013. In C and D, the boxes delimit the 25th to 75th data quantiles, and the lines inside the boxes show the median of the distribution for each month.
To test whether there were significant changes in the association of malaria incidence with climatic variables in the regimes defined by the breakpoint, the model presented in Equation (3) was fitted to malaria incidence for the periods from 1980–2002 and 2003–2013. After a process of model selection, we found that malaria incidence for 1980–2002 (Supplementary Table S2) was a second order autoregressive process and was also significantly associated with El Niño 4 index (SST4):
Using model selection (Supplementary Table S3), we found that malaria incidence for the period from 2003–2013 was best described by a first order seasonal autoregressive process and was significantly associated with SST4 and rainfall (P<0.05):
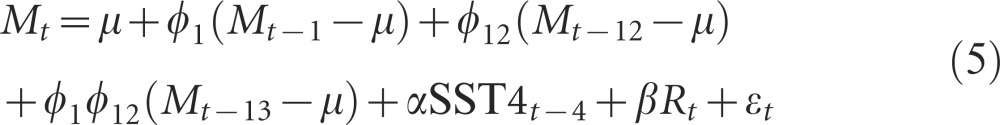 |
Parameter estimates for Equations (4) and (5) are presented in Table 1. The split model described by Equations (4) and (5) outperformed the model in Equation (3), with the former having an AIC of 2148, whereas the AIC for the other model was 2159, with both models having a total of eight parameters. The results that are displayed in Table 1 confirm the observations from Figures 2C and 2D, where a clear seasonal structure was significant only for the 2003–2013 period. Assumptions of error normality, variance homogeneity and temporal independence were confirmed for the models presented in Table 1 and Equations (1) and (2). Table 1 also shows that mean incidence was higher during the 2003–2013 period (approximately 5 cases/1000 people, for one month) than in the 1980–2002 period (approximately 2.5 cases/1000 people, for one month), regardless of the two regimes having a similar unexplained variance (approximately 15, which was also similar to the unexplained variance of the model without a breakpoint). The association between malaria incidence and El Niño 4 (SST4) was positive and became stronger after the breakpoint, yet was not statistically significant for the 2003–2013 period, when a negative and statistically significant (P<0.05) association with rainfall was observed (Table 1).
The differences between the significant covariates in the malaria incidence models with and without breakpoints (Table 1) may be related to the non-stationary association between climatic factors and malaria transmission. Non-stationary associations can be visualized through cross-wavelet coherency analysis. Therefore, we performed cross-wavelet coherency analyses between SST4 and malaria, rainfall, TMAX and TMIN. SST4 was significantly associated with inter-annual malaria cycles of four-year periods between 1980 and 1995, and eight-year periods between 1995 and 2006 (Figure 4A). Around 1995, four-year cycles in SST4 and rainfall were associated (Figure 4B), and a similar pattern was also observed for TMAX, which was also associated with SST4 near 2005 (Figure 4C). Both TMAX and TMIN where seasonally (period of one year) associated with SST4 (Figure 4C and 4D).
Figure 4.
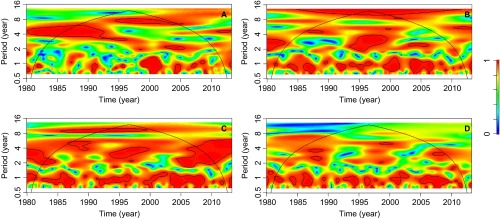
Cross-wavelet coherence analysis. Coherence between SST4 and (A) malaria cases per 1000 people from January 1980 to April 2013, (B) rainfall, (C) maximum temperature and (D) minimum temperature in Comarca Madugandí, República de Panamá. In all analyses, a smoothing window of six months was used. The cross-wavelet coherence scale is from zero (blue) to one (red). Red regions in the plots indicate frequencies and times for which the two series share variability (or power). The cone of influence (within which results are not influenced by the edges of the data) and the significant coherent time-frequency regions (P<0.05) are indicated by solid lines.
To further tease apart the non-stationary association between malaria and climatic factors, we decomposed the malaria incidence time series using EMD. The signal was decomposed into a trend and two IMFs, and the power spectra of the signal components were estimated (Figure 5). The components from EMD were the trend (Figure 5A), the first IMF (Figure 5B) and the second IMF (Figure 5C). The trend (Figure 5A) had most of its power, i.e., variability, at inter-annual time scales, with clear peaks that correspond to cycles of several periods (i.e., 1/frequency of cycles per year), primarily 16 and 4 years, as well as shorter cycles of 18 months (Figure 5D). The first IMF (Figure 5B) had a power peak that corresponds to seasonal variability, or one cycle per year (Figure 5E). The second IMF (Figure 5C) had a similar amount of variability through all of the frequencies, indicating that the variability can be considered to be white noise because no frequency dominated the power spectrum (Figure 5F). CCFs of the trend from EMD with SST4 (Figure 6A), rainfall (Figure 6B), TMAX (Figure 6C) and TMIN (Figure 6D) showed that inter-annual cycles in malaria incidence were associated with El Niño 4. By contrast, the seasonality of malaria incidence was not correlated with SST4 (Figure 6E) or rainfall (Figure 6F), but was significantly correlated with TMAX (Figure 6G) and TMIN (Figure 6H); a similar pattern was observed for the white noise component, which was also not correlated with SST4 (Figure 6I) and rainfall (Figure 6J), and was significantly correlated with TMAX (Figure 6K) and TMIN (Figure 6L). Thus, the combined outcomes of the wavelet analysis and the CCFs of the EMD decomposed malaria incidence time series indicate that inter-annual malaria cycles are associated with El Niño 4 and rainfall, whereas the seasonal dynamics are mainly associated with temperature changes.
Figure 5.
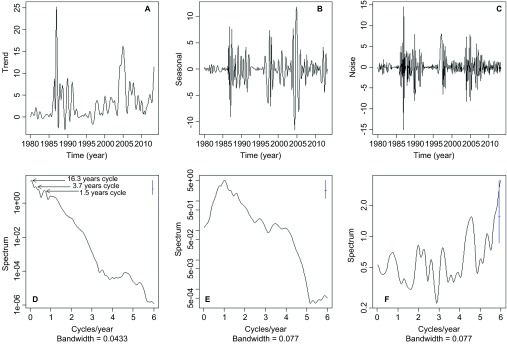
Malaria incidence (per 1000 people) EMD and spectral analysis of IMFs. (A) Residue; (B) first IMF; (C) second IMF. Periodograms for (D) EMD residue, which shows most of the power at inter-annual time scales, i.e., less than one cycle per year; (E) first IMF, which has the highest power at a seasonal time scale, i.e., one cycle per year; and (F) second IMF, which has high variability across the spectra and therefore can be considered as white noise. In D–F, the blue lines are the confidence intervals for the power spectra.
Figure 6.
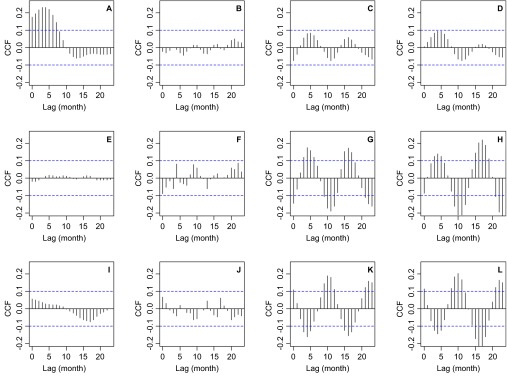
CCF between malaria incidence (per 1000 people) IMFs from an empirical mode decomposition, EMD and climatic factors. EMD residue and (A) El Niño 4 index, also known as, SST4, (B) rainfall, (C) maximum temperature and (D) minimum temperature. First IMF and (E) SST4, (F) rainfall, (G) maximum temperature and (H) minimum temperature. Second IMF and (I) SST4, (J) rainfall, (K) maximum temperature and (L) minimum temperature. In all panels, the dashed lines indicate the 95% CL for correlations that can be expected by random. CL, confidence limit.
DISCUSSION
The continued transmission of malaria among the Gunas in Panamá highlights the challenges of tropical disease elimination in multicultural and ethnically diverse nations. Although malaria was recognized as a major problem in the Amerindian populations by baseline research conducted prior to malaria control in the Canal Zone,8,9 little to no attention was directed to malaria in Amerindian populations of the Panamá Isthmus once the disease was effectively controlled in the Canal Zone and major economic centers of the República de Panamá.3,7,11,30 Lake Bayano was artificially created on the Chepo river basin in 1975. At that time, potential risks for vector-borne disease emergence among the Gunas, a vulnerable community living in the area, were ignored.31 After the lake was created, the Gunas, who used to live near the river, settled on the shores next to Lake Bayano.32 Lake shores have abundant sunlit pools, which are ideal larval habitats for Anopheles albimanus, the major malaria vector in most of Panamá and Central America.10,33,34,35 Indeed, shortly after the creation of Lake Bayano, the proliferation of ‘water lettuce' (Pistia stratiotes) and its associated mosquito fauna were linked with a major outbreak of Venezuelan equine encephalomyelitis in the area.36 Thus, the emergence of malaria in Madungandí might be partially explained by the lack of a health impact assessment prior to the creation of Lake Bayano and the lack of preventive vector management afterwards.1,37,38,39 Indeed, a similar pattern was previously observed in Panamá along the Chagres River basin.6 Following the creation of Lake Gatún in the lower Chagres River, communities in the upper Chagres River were significantly less affected by malaria than those on the shores of Lake Gatún during the 1930s.5,6 Nevertheless, the malaria burden in Madungandí was most likely unrecorded until the 1980s, when the Puente Bayano Health post was established to serve the indigenous communities around Lake Bayano.
At least three great malaria epidemics have struck the Gunas of Madungandí since 1980, including one that occurred from 1987 to 1990. An El Niño event occurred in 1987. In 1988, the use of dichloro-diphenyl-trichloroethane (DDT) as a regular tool for indoor residual spraying (IRS) in endemic areas was terminated.40 This malaria control policy change coincided with a time of major social instability associated with the end of Manuel Noriega's rule and the US invasion of Panamá,41 which greatly affected the overall functionality of public services in the República de Panamá.42 During 1997 and 1998, a new malaria epidemic emerged, synchronous with major epidemics observed in other parts of the world, especially in East Africa20,43 and South America,44,45,46 which, like Panamá, were subject to one of the strongest El Niño events on record.47 The third large epidemic began in 2002–2003, which also coincided with an El Niño event.48 This last epidemic lasted until 2005–2006, mainly because of problems associated with the decentralization of Panamá's Ministry of Health, where the malaria vector control program suffered a shortage of funding and personnel necessary to manage focalized outbreaks quickly after their identification.
Our analysis supports an extended role for ENSO as a potential driver of inter-annual malaria epidemics in Madungandí, given the robust results derived from our different methods of analysis, which showed a close association between the inter-annual cycles of ENSO, measured by SST4, and malaria epidemics in Madungandí. One of the dominant cycle periods of the epidemics is approximately 4 (exactly 3.7) years, and its harmonics, i.e., cycles whose periods are a multiple of the dominant period,20 such as 16 years, are oscillation periods observed in ENSO.49 A remarkable 10-year study of malaria in eastern Panamá revealed the occurrence of epidemics every 4 years in the Chagres river basin, with the largest epidemic observed in 1939,5,6 an El Niño year.49
Multidisciplinary studies in the Chagres river basin in the 1930s5,6 showed that dry El Niño years were associated with the dynamic generation of larval habitats in Lake Gatún shores and riverbeds along the Chagres River basin, ideal habitats for Anopheles albimanus, the main malaria vector around lakes in Panamá, including Lake Bayano.2,33,50 The negative association between rainfall and malaria incidence in our models suggest that a similar biological mechanism might be driving the recent malaria epidemics in Madungandí. Furthermore, our wavelet analysis indicates that rainfall patterns were associated with SST4 in Panamá during the study period, supporting the hypothesis that ENSO may be linked to changes in malaria risk levels, via the dynamic generation of An. albimanus larval habitats by abnormal rainfall patterns. Mathematical models have suggested that dynamic larval habitat generation can lead to increased entomological vector-borne disease risk via the increase in mosquito density.51 In addition, recent epidemics could have also been enhanced by the rising minimum temperatures in eastern Panamá, which might be reducing the variance in overall temperature that mosquitoes experience during their development, a condition known to maximize the fitness of exothermic organisms.52 This enhanced fitness pattern in stable environments has been observed in non-anopheline mosquitoes,53 and in malaria parasite development inside anopheline mosquitoes,54 thus providing a biological explanation for the associations we found between malaria incidence, temperature and SST4.
Nevertheless, the increase in temperature likely played no role in the dynamical regime shift that was observed for 2002. First, temperature was not an important variable in the split model. Additionally, P. falciparum invaded Panamá in 2002 after being absent from the Panamá Isthmus since the 1970s.11 Moreover, the circulating P. falciparum parasites carried mutations that conferred resistance to chloroquine, the main antimalarial drug used to treat the disease at that time.11,18 This invasion was likely mediated by the constant movement of the Gunas across their homeland, which comprises certain malaria hotspots in northern Colombia where human mobility has been shown to play a major role in malaria persistence,55 a possibility supported by mathematical modeling.56 An interesting characteristic of the shift was that mean incidence increased, but there was no change in the incidence variance. In contrast, dynamical regime shifts observed in East Africa in the late 1990s had breakpoints, which indicated changes in both the variance and mean incidence,28 which would be expected in the presence of a biological amplification of temperature trends by vectors.57 The control of the epidemic that began in 2002 also illustrates the boldness necessary to contain malaria on the edge of elimination. In 2005, a Vector Control Task Group was created and funded by the Panamá Ministry of Health to tackle the malaria epidemic in Madungandí.19 This administratively autonomous group devised an intensive operational plan to guarantee the following activities in the remote areas affected by malaria: active surveillance, rapid outbreak containment and a high IRS coverage, aiming to cover at least 80% of the domiciles in Madungandí.19 Thus, by means of administrative modifications, without changes in the attention guidelines or malaria control procedures, a significant drop in the incidence rate was observed 4 months after the installation of the program. At the end of 2005, the annual parasite index (API) was 1.4, which represents a 29% decrease compared with the API observed in the previous year (API=1.7%). This decreasing trend continued during 2006 with a 70% reduction (API=0.5%) and during 2007 with a 76% reduction (API=0.4%). Additionally, the autochthonous transmission of P. falciparum was eliminated and malaria ceased to be a cause of mortality in Madungandí.
Some of the current challenges for malaria control and elimination among the Gunas are biological. Insecticide resistance has become common among An. albimanus in Madungandí, where this mosquito species is resistant to pyrethroids, but susceptible to organophosphates and carbamates,58 which has led to a change in the selection of insecticides, similar to when An. albimanus was among the first malaria vector species in which DDT resistance was observed,59 with vector control now being based on the application of organophosphates and carbamates.58 In addition to An. albimanus, other vectors of concern in the area are An. punctimacula,33 An. triannulatus and An. apicimacula, whose larval ecology is not related to lake shore habitats58 and whose control will not benefit from environmental modifications to the shores of Lake Bayano. However, the major obstacles to malaria control and elimination among the Gunas are likely not biological, but socioeconomic.60 A lack of: (i) intercultural understanding, (ii) economic inclusion of the Gunas and (iii) multinational political commitment, heavily undermines any sustainable malaria control or elimination among this ethnic minority and, consequently, from the República de Panamá. Control measures such as IRS tend to be unwelcome among the Gunas and are probably not the most effective malaria control tool for their traditional housing style.19 Due to lack of consent, the average IRS coverage per village at best reaches 50%, with coverage ranging between 12% and 70%.19 Efforts for housing improvement require a better understanding of ‘western housing' benefits by the Gunas, who are, in general, cautious of any policy that is supposed to improve their livelihoods,13 due to a long history of neglect and abuse by the dominant groups of the Panamá Isthmus. Moreover, the deplorable socioeconomic exclusion status of the Gunas renders impossible housing improvement as an endogenous initiative, led and funded by an ‘autonomous' Comarca. Adapting traditional Guna housing to be malaria proof requires multidisciplinary research, and excellent communication between researchers and the Guna communities to ensure a successful change, as shown for vector-borne diseases.61 Poverty and wariness of the government can also interact and lead to the misuse of other effective malaria control tools, as observed with insecticide treated nets in Lake Victoria Basin.62 Similarly, the nature of the Gunas as a multinational ethnic group highlights the need for setting a common agenda for malaria elimination across borders, and focusing on the ethnic group beyond the nationality of its members. Similar policies have been suggested and implemented for mobile populations, including the Ngäbe, another major Amerindian group in Panamá, many of whom seasonally migrate across the Panamá/Costa Rica border for economic reasons.34 For the Gunas, the commitment requires the development of an integrated policy between Colombia and Panamá, which is hindered by the ongoing civil conflict in the former.63 Thus, a major challenge for malaria control and elimination among the Gunas, and from the República de Panamá, is the full inclusion of native Amerindian minorities into a multiethnic and pluricultural Panamanian society.
In summary, our data showed a clear association between ENSO and the incidence of malaria in Comarca Madungandí, which put into context, is likely exacerbated by the precarious status of the Gunas within Panamanian society. Our analysis also supports that a dynamical regime shift in transmission that was observed at the end of 2002 was likely related to malaria control policy changes, wherein the re-assignment of personnel and funding for malaria control within the Ministry of Health undermined the ability to combat malaria. This policy change also coincided with the re-introduction of P. falciparum in Panamá, including drug-resistant parasites, and these elements led to an increase in malaria incidence among the Madungandí Gunas, which was controlled only after the malaria control unit was re-assembled.
Acknowledgments
We dedicate this work to the Gunas of Comarca Madungandí, with whom we are working to find suitable methods for malaria elimination from their communities, and the República de Panamá. We thank the Departamento de Control de Vectores del Ministerio de Salud, República de Panamá for their sustained support and cooperation with the malaria research performed by Instituto Gorgas in Comarca Madungandí. We also thank ETESA for sharing meteorological data. We thank Ms Milixa Perea from the Graduate Program in Epidemiology and Tropical Health at Universidad Latina de Panamá for Figure 1A. Ms Chystrie Rigg from the ICGES helped with the elaboration of Figure 1B. Luis Fernando Chaves is thankful to Ms. Junko Sakemoto for her administrative support at Nagasaki University. This study was supported by Instituto Conmemorativo Gorgas de Estudios de La Salud (José E Calzada, Lisbeth Amarilis Hurtado and Lorenzo Cáceres) and Nagasaki University-Program for Nurturing Global Leaders in Tropical and Emerging Communicable Diseases (Luis Fernando Chaves).
Footnotes
Note: Supplementary Information for this article can be found on Emerging Microbes and Infections' website (http://www.nature.com/EMI/).
Supplementary Information
References
- Sutter PS. Nature's agents or agents of empire? Entomological workers and environmental change during the construction of the Panama Canal. Isis. 2007;98:724–754. doi: 10.1086/529265. [DOI] [PubMed] [Google Scholar]
- Zetek J. Behavior of Anopheles albimanus Wiede. and tarsimaculata Goeldi. Ann Entomol Soc Am. 1915;8:221–271. [Google Scholar]
- Gorgas WC. The conquest of the tropics for the white race. J Am Med Assoc. 1909;52:1967–1969. [Google Scholar]
- Leigh JG. America's triumph in Panama: three years' medical and sanitary record in the Canal Zone. Lancet. 1908;171:1646–1649. [Google Scholar]
- Clark HC, Komp WH. A seventh year's observations on malaria in Panama. Am J Trop Med Hyg. 1938;s1–18:271–288. [Google Scholar]
- Clark HC, Komp WH, Jobbins DM. A tenth year's observations on malaria in Panama, with reference to the occurrence of variations in the parasite index, during continued treatment with atabrine and plasmochine. Am J Trop Med Hyg. 1941;s1–21:191–216. [Google Scholar]
- Dehné EJ. Fifty years of malaria control in the Panama area. Am J Trop Med Hyg. 1955;4:800–811. [PubMed] [Google Scholar]
- Kendall AI. Malarial infection in certain native villages of the canal zone. J Am Med Assoc. 1906;46:1266–1273. [Google Scholar]
- Kendall AI. Malarial infection in certain native villages of the canal zone. J Am Med Assoc. 1906;46:1151–1154. [Google Scholar]
- Jennings AH. Some problems of mosquito control in the tropics. J Econ Entomol. 1912;5:131–142. [Google Scholar]
- Calzada JE, Samudio F, Bayard V, et al. Revising antimalarial drug policy in Central America: experience in Panama. Trans R Soc Trop Med Hyg. 2008;102:694–698. doi: 10.1016/j.trstmh.2008.03.012. [DOI] [PubMed] [Google Scholar]
- Strong RA. Jimmy Carter and the Panama Canal Treaties. President Stud Q. 1991;21:269–286. [Google Scholar]
- Horton L. Contesting state multiculturalisms: indigenous land struggles in eastern Panama. J Latin Am Stud. 2006;38:829–858. [Google Scholar]
- Runk JV. Indigenous land and environmental conflicts in panama: neoliberal multiculturalism, changing legislation, and human rights. J Latin Am Geogr. 2012;11:21–47. [Google Scholar]
- Batista O, Kolman CJ, Bermingham E. Mitochondrial DNA diversity in the Kuna Amerinds of Panamá. Hum Mol Genet. 1995;4:921–929. doi: 10.1093/hmg/4.5.921. [DOI] [PubMed] [Google Scholar]
- Dirección de Estadística y Censo . Panamá: Contraloría General de República de Panamá. Spanish; 2011. [National Census for Population XI and Housing VII.] [Google Scholar]
- Dirección de Política Social . Panamá: Ministerio de Economia y Finanzas de República de Panamá. Spanish; 2005. [Poverty in Panamá: 2003 life level survey.] [Google Scholar]
- Samudio F, Santamaría AM, Obaldía N, et al. Prevalence of Plasmodium falciparum mutations associated with antimalrial drug resistance during an epidemic in Kuna Yala, Panama, Central America. Am J Trop Med Hyg. 2005;73:839–841. [PubMed] [Google Scholar]
- Cáceres L, Griffith M, Calzada JE, Rovira JE, Torres R. Panamá: Ministerio de Salud, República de Panamá. Spanish; 2013. [Guidelines for the intercultural study of malaria in Comarca Kuna of Madugandi.] [Google Scholar]
- Chaves LF, Koenraadt CJ. Climate change and highland malaria: fresh air for a hot debate. Q Rev Biol. 2010;85:27–55. doi: 10.1086/650284. [DOI] [PubMed] [Google Scholar]
- Lindsay SW, Birley MH. Climate change and malaria transmission. Ann Trop Med Parasitol. 1996;90:573–588. doi: 10.1080/00034983.1996.11813087. [DOI] [PubMed] [Google Scholar]
- Béguin A, Hales S, Rocklov J, et al. The opposing effects of climate change and socio-economic development on the global distribution of malaria. Global Environ Change. 2011;21:1209–1214. [Google Scholar]
- Scheffer M. Critical transitions in nature and society. Princeton, NJ; Princeton University Press; 2009. [Google Scholar]
- Shumway TH, Stoffer DS.Time series analysis and its applications3rd ed. New York; Springer; 2011 [Google Scholar]
- Huang NE, Shen Z, Long SR, et al. The empirical mode decomposition and the Hilbert spectrum for nonlinear and non-stationary time series analysis. Proc R Soc Lond Ser A Math Phys Eng Sci. 1998;454:903–995. [Google Scholar]
- Venables WN, Ripley BD. Modern applied statistics with S. New York; Springer; 2002. [Google Scholar]
- Bai J, Perron P. Computation and analysis of multiple structural change models. J Appl Econometr. 2003;18:1–22. [Google Scholar]
- Chaves LF, Hashizume M, Satake A, Minakawa N. Regime shifts and heterogeneous trends in malaria time series from Western Kenya Highlands. Parasitology. 2012;139:14–25. doi: 10.1017/S0031182011001685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazelles B, Chavez M, de Magny GC, Guégan JF, Hales S. Time-dependent spectral analysis of epidemiological time-series with wavelets. J R Soc Interface. 2007;4:625–636. doi: 10.1098/rsif.2007.0212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons JS. Observations on the Importance of Anopheles punctimacula as a malaria vector in Panama, and report of experimental infections in A. neomaculipalpus, A. apicimacula, and A. eiseni. Am J Trop Med Hyg. 1937;s1–17:191–212. [Google Scholar]
- Finley-Brook M, Thomas C. Treatment of displaced indigenous populations in two large hydro projects in Panama. Water Altern. 2010;3:269–290. [Google Scholar]
- Wali A. The transformation of a frontier: State and regional relationships in Panama, 1972–1990. Hum Org. 1993;52:115–129. [Google Scholar]
- Loaiza JR, Bermingham E, Scott ME, Rovira JR, Conn JE. Species composition and distribution of adult Anopheles (Diptera: Culicidae) in Panama. J Med Entomol. 2008;45:841–851. doi: 10.1603/0022-2585(2008)45[841:scadoa]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cáceres L, Rovira J, Torres R, et al. [Characterization of Plasmodium vivax malaria transmission at the border of Panamá and Costa Rica.] Biomedica 201232557–569.Spanish. [DOI] [PubMed] [Google Scholar]
- Rejmankova E, Roberts DR, Pawley A, Manguin S, Polanco J. Predictions of adult Anopheles albimanus densities in villages based on distances to remotely sensed larval habitats. Am J Trop Med Hyg. 1995;53:482–488. doi: 10.4269/ajtmh.1995.53.482. [DOI] [PubMed] [Google Scholar]
- Adames A, Peralta P, Saenz R, Johnson C, Galindo P.[Venezuelan Equine Encephalomyelitis (VEE) during the creation of Lake Bayano in Panamá, 1977.] Rev Méd Panamá 19794246–257.Spanish. [Google Scholar]
- Mindell J, Boaz A, Joffe M, Curtis S, Birley M. Enhancing the evidence base for health impact assessment. J Epidemiol Community Health. 2004;58:546–551. doi: 10.1136/jech.2003.012401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird JK. Resurgent malaria at the millennium: control strategies in crisis. Drugs. 2000;58:719–743. doi: 10.2165/00003495-200059040-00001. [DOI] [PubMed] [Google Scholar]
- Yasuoka J, Levins R. Impact of deforestation and agricultural development on anopheline ecology and malaria epidemiology. Am J Trop Med Hyg. 2007;76:450–460. [PubMed] [Google Scholar]
- Cohen JM, Smith DL, Cotter C, et al. Malaria resurgence: a systematic review and assessment of its causes. Malar J. 2012;11:122. doi: 10.1186/1475-2875-11-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilboa E. The Panama invasion revisited: lessons for the use of force in the Post Cold War Era. Polit Sci Q. 1995;110:539–562. [Google Scholar]
- Nasi C.[Panama: Crisis, invasion and the New American Hegemony.] Colomb Int 1990113–24.Spanish. [Google Scholar]
- Lindsay SW, Bødker R, Malima R, Msangeni HA, Kisinza W. Effect of 1997–98 EI Niño on highland malaria in Tanzania. Lancet. 2000;355:989–990. doi: 10.1016/s0140-6736(00)90022-9. [DOI] [PubMed] [Google Scholar]
- Gagnon AS, Smoyer-Tomic KE, Bush AB. The El Niño southern oscillation and malaria epidemics in South America. Int J Biometeorol. 2002;46:81–89. doi: 10.1007/s00484-001-0119-6. [DOI] [PubMed] [Google Scholar]
- Poveda G, Estrada-Restrepo OA, Morales JE, et al. Integrating knowledge and management regarding the climate–malaria linkages in Colombia. Curr Opin Environ Sustain. 2011;3:448–460. [Google Scholar]
- Poveda G, Rojas W, Quiñones ML, et al. Coupling between annual and ENSO timescales in the malaria–climate association in Colombia. Environ Health Perspect. 2001;109:489. doi: 10.1289/ehp.01109489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPhaden MJ. Genesis and evolution of the 1997–98 El Niño. Science. 1999;283:950–954. doi: 10.1126/science.283.5404.950. [DOI] [PubMed] [Google Scholar]
- Chaves LF, Satake A, Hashizume M, Minakawa N. Indian Ocean dipole and rainfall drive a moran effect in East Africa malaria transmission. J Infect Dis. 2012;205:1885–1891. doi: 10.1093/infdis/jis289. [DOI] [PubMed] [Google Scholar]
- Allan R, Lindesay J, Parker D. El Niño: Southern oscillation and climatic variability. Collingwood; CSIRO; 1996. [Google Scholar]
- Rozeboom LE. The life cycle of laboratory-bred Anopheles albimanus Widemann. Ann Entomol Soc Am. 1936;29:480–489. [Google Scholar]
- Predescu M, Levins R, Awerbuch-Friedlander T. Analysis of a nonlinear system for community intervention in mosquito control. Discrete Contin Dyn Syst Ser B. 2006;6:605–622. [Google Scholar]
- Huey RB, Berrigan D. Temperature, demography, and ectotherm fitness. Am Nat. 2001;158:204–210. doi: 10.1086/321314. [DOI] [PubMed] [Google Scholar]
- Carrington LB, Seifert SN, Willits NH, Lambrechts L, Scott TW. Large diurnal temperature fluctuations negatively influence Aedes aegypti (Diptera: Culicidae) life-history traits. J Med Entomol. 2013;50:43–51. doi: 10.1603/me11242. [DOI] [PubMed] [Google Scholar]
- Paaijmans KP, et al. Influence of climate on malaria transmission depends on daily temperature variation. Proc Natl Acad Sci USA. 2010;107:15135–15139. doi: 10.1073/pnas.1006422107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevilla-Casas E. Human mobility and malaria risk in the Naya river basin of Colombia. Soc Sci Med. 1993;37:1155–1167. doi: 10.1016/0277-9536(93)90255-3. [DOI] [PubMed] [Google Scholar]
- Reiner RC, et al. A systematic review of mathematical models of mosquito-borne pathogen transmission: 1970–2010. J R Soc Interface. 2013;10:20120921. doi: 10.1098/rsif.2012.0921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual M, Ahumada JA, Chaves LF, Rodo X, Bouma M. Malaria resurgence in the East African highlands: temperature trends revisited. Proc Natl Acad Sci USA. 2006;103:5829–5834. doi: 10.1073/pnas.0508929103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cáceres L, Rovira J, García A, Torres R.[Determination of the resistance to organophosphate, carbamate, and pyrethroid insecticides in Panamanian Anopheles albimanus (Diptera: Culicidae) mosquitoes.] Biomedica 201131419–427.Spanish. [DOI] [PubMed] [Google Scholar]
- Trapido H. Modified response of Anopheles albimanus to DDT residual house spraying in Panama. Am J Trop Med Hyg. 1952;1:853–861. doi: 10.4269/ajtmh.1952.1.853. [DOI] [PubMed] [Google Scholar]
- Greenwood B. Can malaria be eliminated. Trans R Soc Trop Med Hyg. 2009;103:S2–S5. doi: 10.1016/j.trstmh.2008.10.027. [DOI] [PubMed] [Google Scholar]
- Lucero DE, Morrissey LA, Rizzo DM, et al. Ecohealth interventions limit triatomine reinfestation following insecticide spraying in La Brea, Guatemala. Am J Trop Med Hyg. 2013;88:630–637. doi: 10.4269/ajtmh.12-0448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honjo K, Chaves LF, Satake A, Kaneko A, Minakawa N. When they don't bite, we smell money: understanding malaria bednet misuse. Parasitology. 2013;140:580–586. doi: 10.1017/S0031182012002077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis RE. The impact of instability in Latin and South America. IEEE Eng Med Biol Mag. 2004;23:187–193. doi: 10.1109/memb.2004.1297191. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


