Abstract
BACKGROUND
Little is known about the relation between surgical care for breast cancer at for-profit hospitals and subsequent use of adjuvant radiation therapy (RT). Among Medicare beneficiaries, we examined whether hospital ownership status is associated with the use of breast brachytherapy – a newer and more expensive modality – as well as overall RT.
METHODS
We conducted a retrospective study of female Medicare beneficiaries receiving breast-conserving surgery for invasive breast cancer in 2008 and 2009. We assessed the relationship between hospital ownership and receipt of brachytherapy or overall RT using hierarchical generalized linear models.
RESULTS
The sample consisted of 35,118 women, 8.0% of whom had surgery at for-profit hospitals. Among patients who received RT, those who underwent surgery at for-profit hospitals were significantly more likely to receive brachytherapy (20.2%) than patients treated at not-for-profit hospitals (15.2%; OR for for-profit vs. not-for-profit: 1.50; 95%CI: 1.23–1.84; p<0.001). Among women aged 66–79, there was no relation between hospital ownership status and overall RT use. Among women aged 80–94 years old – the group least likely to benefit from RT due to shorter life expectancy – receipt of surgery at a for-profit hospital was significantly associated with higher overall RT use (OR: 1.22; 95%CI: 1.03–1.45, p=0.03) and brachytherapy use (OR: 1.66; 95%CI: 1.18–2.34, p=0.003).
CONCLUSIONS
Surgical care at for-profit hospitals was associated with increased use of the newer and more expensive RT modality, brachytherapy. Among the oldest women, who are least likely to benefit from RT, surgical care at a for-profit hospital was associated with higher overall RT use, with this difference largely driven by the use of brachytherapy.
INTRODUCTION
Surgical intervention plays a critical role in cancer treatment, affecting decisions made in the perioperative setting and the trajectory of cancer care beyond the initial ‘curative surgery’. Post-surgical cancer care often involves the adoption of new, expensive, and sometimes unproven technologies, which has raised concerns about inappropriate care and overuse.1–4 Many factors may affect the adoption of newer medical technologies including patient and physician preferences, reimbursement incentives, clinical evidence, and regional health system factors.5–8 Hospitals play a major role both in providing surgical care and in the adoption of new technologies, due to their ability to invest in infrastructure, their central role in the treatment of many conditions, and their being the focus of payer efforts to enhance quality and control costs. Hence, it is important to understand how hospital factors, such as ownership status, affect the adoption of new technologies after patients undergo surgery.
The effect of hospital ownership status is particularly relevant in clinical scenarios where evidence regarding treatment benefit is less definitive, and clinical decision-making is more discretionary. In this setting, hospitals owned by for-profit entities, which must return value to investors, may be more likely to encourage the adoption of highly reimbursed interventions. While both for-profit and not-for-profit hospitals have financial incentives to emphasize revenue-generating procedures, for-profit hospitals may be more responsive to these incentives given their fiduciary interests.9–11 For example, for-profit hospital ownership has been associated with increased use of cardiac revascularization interventions independent of clinical outcome.11 Similarly, receipt of care at for-profit hemodialysis centers has been associated with increased erythropoietin drug dosing in excess of recommendations from clinical guidelines.12 However, these studies focused on the use of widely used treatment strategies that had already disseminated into clinical practice with evidence-based guidelines in place. Little is known about the effect of hospital ownership status on the adoption of new medical technologies that are reimbursed at higher rates than existing technologies.13
Breast brachytherapy for older women with breast cancer is an excellent example of a newer therapy with scant comparative effectiveness data and higher reimbursements compared to the standard whole breast irradiation (WBI). Breast brachytherapy involves the implantation of the radiation source into the lumpectomy cavity and condenses the treatment course to 1 week compared to 4–6 weeks for WBI. Although breast brachytherapy has diffused into clinical practice, some recent data suggest that the harms may actually outweigh the benefits.14–20 Furthermore, brachytherapy is more highly reimbursed than the standard of care, and some authors have suggested that financial interests are driving the adoption of brachytherapy in clinical practice. 21–24 It remains unknown whether surgical care at a for-profit hospital is associated with the receipt of adjuvant brachytherapy.
It is also important to consider how the adoption of brachytherapy might affect overall use of adjuvant radiation therapy (RT). That is, after disseminating into clinical practice in either profit setting, brachytherapy may substitute for the standard of care, WBI, without any increase in the overall use of RT. Alternatively, enthusiasm for brachytherapy could expand the pool of women who are assessed to be suitable candidates for RT and instead complement the standard of care, thereby increasing overall RT use. In this context, financial incentives and increased reimbursement for brachytherapy may lead to a higher overall use of RT. This may be particularly true among older women, and especially those above age 80 years, for whom the benefit of RT diminishes and thus may be more subject to provider preferences and discretionary judgment.25–28 It remains unknown whether surgical care at a for-profit hospital is associated with brachytherapy use as a substitute for standard RT or associated with a higher likelihood of RT use overall.
To further our understanding of the relation between hospital ownership status and cancer care, we used national Medicare data to assess the relation between for-profit hospital ownership and the adoption of brachytherapy among Medicare beneficiaries with breast cancer receiving breast-conserving surgery (BCS). We hypothesized that among women receiving adjuvant RT, those who had undergone BCS at a for-profit hospital would be more likely to receive brachytherapy. We also assessed whether women undergoing BCS at for-profit hospitals would be more likely to receive RT overall. That is, we hypothesized that the use of brachytherapy in for-profit hospitals increases the proportion of women who are receiving RT, rather than simply substituting for WBI. We also hypothesized that this relation between brachytherapy and overall RT use would be stronger among older women, the group for whom RT is more discretionary.
METHODS
Data Source and Study Sample
Using the Centers for Medicare and Medicaid Services Chronic Condition Warehouse (CCW) database, we identified a sample of female Medicare beneficiaries between ages 66–94 years who received BCS and adjuvant RT for invasive breast cancer in 2008 and 2009.29,30 The CCW is a national database that contains 100% of fee-for-service Medicare claims for inpatient and outpatient institutional and non-institutional services for patients with certain chronic conditions. We identified beneficiaries with invasive breast cancer by the International Classification of Diseases, 9th revision (ICD-9) diagnosis code (174.x). Receipt of brachytherapy or WBI (traditional external beam or intensity modulated) was identified according to Healthcare Common Procedure Coding System (HCPCS) codes (Appendix 1). We only included women who received BCS between January 2008 and June 2009 and were enrolled in fee-for-service Medicare Parts A and B during the study period. Approximately 93% of all Medicare beneficiaries are enrolled in both Parts A and B.31 Women were excluded from this sample if they received an ICD-9 diagnosis code for any other cancer (including ductal carcinoma in situ) in the 9 months prior through 6 months after BCS (Appendix 2). The Yale Human Investigation Committee determined that this analysis did not directly involve human subjects.
Radiation Therapy
Patients with any HCPCS codes indicative of brachytherapy treatment were considered to have received brachytherapy. Patients with at least four HCPCS codes indicative of the delivery of WBI were considered to have received WBI. In order to capture all patients for whom the decision was made to provide brachytherapy as a component of their therapy, patients with codes for both brachytherapy and WBI (less than 0.5% of the total sample) were assigned to the brachytherapy group.
Construction of Variables
Patient characteristics included age, race, year of surgery, residence in a metropolitan county based on Core Based Statistical Areas, and median household income at the zip code level. Clinical characteristics such as comorbid conditions, tumor laterality, lymph node dissection (including sentinel and axillary dissection), and receipt of chemotherapy were assessed using HCPCS and ICD-9 codes from the Medicare claims (Appendix 1). As proxies for access to care, we accounted for each of the following variables in the year prior to surgery: any hospital admission, receipt of a screening mammogram, receipt of a flu shot, or primary care physician visit. Comorbid conditions previously found to be associated with survival in non-cancer patients were assessed by searching claims in the 12 months through one month prior to BCS.32 We included ICD-9 diagnosis codes that were on an inpatient claim or ≥2 outpatient/physician claims billed >30 days apart.
For each patient, we identified the hospital at which BCS was performed using the Medicare provider number. Hospital ownership was determined from the Medicare Hospital General Information dataset which is a self-reported measure by hospitals during enrollment with the Centers for Medicare and Medicaid Services.33 All hospitals listed as ‘Proprietary’ under the hospital owner variable were considered for-profit. Hospitals listed as either ‘Government’ or ‘Voluntary Non-profit’ were considered not-for-profit. Patients for whom we could not identify a BCS-performing hospital or whose hospital was not included in the Hospital General Information dataset were excluded (n=6194, 15%). Hospital volume was calculated as the number of patients in our sample who received surgery at each hospital during the study period. The sample was categorized into quintiles of volume such that each quintile had approximately the same number of patients.
Patients were assigned to hospital referral regions (HRR) based on zip-code of residence using a cross-walk available from the Dartmouth Atlas of Healthcare.34 We assessed regional level factors that could be associated with the location of a for-profit hospital and use of brachytherapy including the presence of a state certificate of need (CON), two-year mammography rate, and radiation oncologist density for each HRR. The CON variable was used to assess the presence of policies that regulated the opening of new radiation facilities during the study period. We hypothesized that both two-year mammography rate, an indicator for screening practices for a given HRR, and radiation oncologist density might be associated with the use of RT because these regional characteristics may increase both the incidence rate of invasive breast cancer and access to RT.
Statistical Analysis
We used chi-square tests to determine the unadjusted association between hospital ownership and each covariate. We used hierarchical generalized linear models (HGLMs) with a logit link function to assess the unadjusted and adjusted relationship between hospital ownership and receipt of brachytherapy among patients who received RT.35 HGLMs allowed us to account for the non-independence of outcomes by clustering patients within hospitals, which were clustered within HRRs. In all HGLMs, hospital and HRR were specified as random effects, while all other covariates were specified as fixed effects. We estimated an analogous model using receipt of any RT as our outcome in the full sample. Because RT can be considered optional in many women ≥70 years of age, we hypothesized that the effect of hospital ownership on receipt of any RT might be moderated by patient age. For this reason, we repeated this model with the addition of interaction terms between hospital ownership and age category and re-estimated the model separately among age groups with and without significant interactions. Finally, in order to determine whether any association between hospital ownership and receipt of any RT was driven primarily by the differential use of brachytherapy rather than WBI among older women, we estimated two additional models in which the outcomes were receipt of brachytherapy (versus no RT) and receipt of WBI (versus no RT). All data analyses were performed using SAS version 9.2 (SAS Institute Inc., Cary, NC); HGLMs were estimated using the GLIMMIX procedure.
RESULTS
Overview of Study Sample and Hospital Characteristics
Our sample included 35,118 beneficiaries who received BCS. The mean age was 74.2 (SD: 5.9) and less than 6% of our sample was above age 85. The majority of women were white (91.1%). About 72% of the sample received adjuvant RT, of whom 22,496 (88.9%) had undergone BCS at a not-for-profit hospital and 2,816 (11.1%) at a for-profit hospital. Among women who received RT, there were significant differences between women receiving care at a for-profit compared to not-for-profit hospital with regard to race, residence in a metropolitan county, median household income, and receipt of a flu shot (Table 1). Patients from for-profit and not-for-profit hospitals were similar in all other patient characteristics.
Table 1.
Characteristics of patients who received radiation therapy according to ownership of the hospital where breast-conserving surgery was performed.
| Non-profit | For-profit | ||||
|---|---|---|---|---|---|
| N | % | N | % | p-value* | |
| Total | 22,496 | 2816 | |||
| Patient Characteristics | |||||
| Age at breast-conserving surgery | 0.53 | ||||
| 66–69 | 5964 | 26.5 | 765 | 27.2 | |
| 70–74 | 6673 | 29.7 | 834 | 29.6 | |
| 75–79 | 5209 | 23.2 | 619 | 22.0 | |
| 80–84 | 3412 | 15.2 | 428 | 15.2 | |
| 85–94 | 1238 | 5.5 | 170 | 6.0 | |
| Race | <.001 | ||||
| White | 20542 | 91.3 | 2517 | 89.4 | |
| Black | 1375 | 6.1 | 183 | 6.5 | |
| Other | 579 | 2.6 | 116 | 4.1 | |
| Year of surgery | 0.83 | ||||
| 2008 | 14610 | 64.9 | 1823 | 64.7 | |
| 2009 | 7886 | 35.1 | 993 | 35.3 | |
| Residence in metro county | 0.001 | ||||
| Yes | 18038 | 80.2 | 2330 | 82.7 | |
| No | 4458 | 19.8 | 486 | 17.3 | |
| Income quintile | <.001 | ||||
| Q1 (≤$33,208) | 4265 | 19.0 | 635 | 22.6 | |
| Q2 ($33,209–$39,659) | 4297 | 19.1 | 548 | 19.5 | |
| Q3 ($39,661–$47,295) | 4360 | 19.4 | 555 | 19.7 | |
| Q4 ($47,300–$60,118) | 4401 | 19.6 | 508 | 18.0 | |
| Q5 (≥$60,124) | 4422 | 19.7 | 467 | 16.6 | |
| Unknown | 751 | 3.3 | 103 | 3.7 | |
| Clinical characteristics | |||||
| Comorbidity | 0.72 | ||||
| 0 conditions | 12761 | 56.7 | 1584 | 56.3 | |
| 1–2 conditions | 7995 | 35.5 | 1021 | 36.3 | |
| ≥3 conditions | 1740 | 7.7 | 211 | 7.5 | |
| Tumor laterality | 0.41 | ||||
| Right-sided | 9715 | 43.2 | 1227 | 43.6 | |
| Left-sided | 10056 | 44.7 | 1228 | 43.6 | |
| Unknown | 2725 | 12.1 | 361 | 12.8 | |
| Axillary node dissection | 0.06 | ||||
| No | 6285 | 27.9 | 740 | 26.3 | |
| Yes | 16211 | 72.1 | 2076 | 73.7 | |
| Chemotherapy | 0.04 | ||||
| No chemotherapy | 19721 | 87.7 | 2429 | 86.3 | |
| Chemotherapy started in month prior through month after surgery | 920 | 4.1 | 115 | 4.1 | |
| Chemotherapy started in 31–365 days after surgery | 1855 | 8.3 | 272 | 9.7 | |
| Hospital admission** | 0.96 | ||||
| No | 19460 | 86.5 | 2435 | 86.5 | |
| Yes | 3036 | 13.5 | 381 | 13.5 | |
| Screening mammogram** | 0.06 | ||||
| No | 5102 | 22.7 | 683 | 24.3 | |
| Yes | 17394 | 77.3 | 2133 | 75.8 | |
| Flu shot** | 0.02 | ||||
| No | 9390 | 41.7 | 1242 | 44.1 | |
| Yes | 13106 | 58.3 | 1574 | 55.9 | |
| Visit to primary care physician** | 0.12 | ||||
| No | 686 | 3.1 | 71 | 2.5 | |
| Yes | 21810 | 97.0 | 2745 | 97.5 | |
| Hospital characteristics | |||||
| Hospital volume*** | <0.001 | ||||
| Q1 (1–7) | 4170 | 18.5 | 855 | 30.4 | |
| Q2 (8–14) | 4284 | 19.0 | 862 | 30.6 | |
| Q3 (15–22) | 4347 | 19.3 | 445 | 15.8 | |
| Q4 (23–38) | 5013 | 22.3 | 338 | 12.0 | |
| Q5 (39–142) | 4682 | 20.8 | 316 | 11.2 | |
| Health system characteristics | |||||
| State certificate of need for radiation facility | <0.001 | ||||
| No | 11820 | 52.5 | 1773 | 63.0 | |
| Yes | 10676 | 47.5 | 1043 | 37.0 | |
| HRR-level two-year mammography rate among female Medicare enrollees 67–69, in quintiles | <0.001 | ||||
| Q1 (50.1–59.7) | 3941 | 17.5 | 793 | 28.2 | |
| Q2 (59.8–62.4) | 4400 | 19.6 | 706 | 25.1 | |
| Q3 (62.4–64.9) | 4652 | 20.7 | 468 | 16.6 | |
| Q4 (65.0–68.4) | 4762 | 21.2 | 441 | 15.7 | |
| Q5 (68.4–76.1) | 4741 | 21.1 | 408 | 14.5 | |
| Radiation oncologist density per 100,000 residents, in quintiles | <0.001 | ||||
| Q1 (0.2–1.0) | 4397 | 19.6 | 688 | 24.4 | |
| Q2 (1.0–1.1) | 4078 | 18.1 | 660 | 23.4 | |
| Q3 (1.1–1.2) | 4531 | 20.1 | 624 | 22.2 | |
| Q4 (1.2–1.4) | 4818 | 21.4 | 293 | 10.4 | |
| Q5 (1.4–2.5) | 4672 | 20.8 | 551 | 19.6 | |
P-value is for chi-square test of the association between each covariate and profit status
In year prior to breast-conserving surgery
Hospital volume is defined as the number of patients in our sample who received breast-conserving surgery at each hospital during the study period
Patients received care at 2,255 not-for-profit hospitals and 429 for-profit hospitals. Patients who received BCS at for-profit hospitals were more likely to receive surgery at lower surgical volume hospitals and reside in states without a CON for a radiation facility (63% of for-profit hospitals vs. 53% of not-for-profit hospitals, p<0.001). In addition, patients who received BCS at for-profit hospitals were more likely to reside in HRRs with a lower mammography rate and fewer radiation oncologists per capita.
Hospital Ownership and Receipt of Brachytherapy
Among beneficiaries receiving RT, 15.7% received brachytherapy. Women at for-profit hospitals who received RT were more likely to receive brachytherapy (20.2%) than women at not-for-profit hospitals (15.2%, adjusted odds ratio (OR): 1.50, 95% CI: 1.23–1.84, p<0.001, Table 2). Women who received BCS at higher surgical volume hospitals were also more likely to receive brachytherapy (OR for highest versus lowest quintile: 2.00, 95% CI: 1.57–2.53, p<0.001). In addition, patients who had left sided tumors, lymph node evaluation and screening mammograms were all more likely to have received brachytherapy (p<0.001). In contrast, patients receiving chemotherapy were less likely to receive brachytherapy.
Table 2.
Associations between patient, clinical, and health system characteristics and the receipt of brachytherapy. Adjusted for patient, clinical, and health system characteristics
| Percent Receiving Brachytherapy | Unadjusted | Adjusted* | |||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P-value | OR | 95% CI | P-value | ||||
| Hospital characteristics | |||||||||
| Hospital profit status | 0.01 | <0.001 | |||||||
| Non-profit | 15.2 | 1.00 | -- | -- | 1.00 | -- | -- | ||
| For-profit | 20.2 | 1.28 | 1.06 | 1.55 | 1.50 | 1.23 | 1.84 | ||
| Hospital volume** | <0.001 | <0.001 | |||||||
| Q1 (1–7) | 9.1 | 1.00 | -- | -- | 1.00 | -- | -- | ||
| Q2 (8–14) | 13.2 | 1.40 | 1.15 | 1.69 | 1.38 | 1.13 | 1.67 | ||
| Q3 (15–22) | 16.6 | 1.98 | 1.61 | 2.43 | 2.02 | 1.64 | 2.50 | ||
| Q4 (23–38) | 20.0 | 2.21 | 1.79 | 2.72 | 2.24 | 1.80 | 2.77 | ||
| Q5 (39–142) | 19.6 | 1.93 | 1.53 | 2.44 | 2.00 | 1.57 | 2.53 | ||
| Patient characteristics | |||||||||
| Age at BCS | 0.52 | ||||||||
| 66–69 | 16.0 | 1.00 | -- | -- | |||||
| 70–74 | 15.6 | 0.99 | 0.89 | 1.09 | |||||
| 75–79 | 15.8 | 0.97 | 0.86 | 1.08 | |||||
| 80–84 | 15.7 | 0.90 | 0.80 | 1.03 | |||||
| 85–94 | 15.3 | 0.91 | 0.76 | 1.09 | |||||
| Race | 0.02 | 0.06 | |||||||
| White | 15.9 | 1.00 | -- | -- | 1.00 | -- | -- | ||
| Black | 13.2 | 0.77 | 0.65 | 0.92 | 0.81 | 0.67 | 0.97 | ||
| Other | 15.0 | 0.99 | 0.77 | 1.27 | 1.04 | 0.80 | 1.33 | ||
| Year of surgery | 0.53 | ||||||||
| 2008 | 15.7 | 1.00 | -- | -- | |||||
| 2009 | 15.8 | 0.97 | 0.90 | 1.06 | |||||
| Residence in metro county | 0.51 | ||||||||
| Yes | 16.4 | 1.00 | -- | -- | |||||
| No | 12.9 | 0.96 | 0.84 | 1.09 | |||||
| Income quintile | 0.29 | ||||||||
| Q1 (≤$33,208) | 15.3 | 1.00 | -- | -- | |||||
| Q2 ($33,209–$39,659) | 14.7 | 0.96 | 0.84 | 1.10 | |||||
| Q3 ($39,661–$47,295) | 16.3 | 1.05 | 0.92 | 1.20 | |||||
| Q4 ($47,300–$60,118) | 16.2 | 1.02 | 0.89 | 1.18 | |||||
| Q5 (≥$60,124) | 15.4 | 0.95 | 0.82 | 1.11 | |||||
| Unknown | 19.9 | 1.21 | 0.97 | 1.51 | |||||
| Clinical characteristics | |||||||||
| Comorbidity | 0.59 | ||||||||
| 0 conditions | 15.6 | 1.00 | -- | -- | |||||
| 1–2 conditions | 16.0 | 1.03 | 0.95 | 1.12 | |||||
| ≥3 conditions | 15.4 | 0.96 | 0.83 | 1.12 | |||||
| Tumor laterality | <0.001 | <0.001 | |||||||
| Right sided | 15.6 | 1.00 | -- | -- | 1.00 | -- | -- | ||
| Left sided | 16.7 | 1.10 | 1.02 | 1.20 | 1.11 | 1.02 | 1.21 | ||
| Unknown | 12.9 | 0.81 | 0.71 | 0.93 | 0.83 | 0.72 | 0.96 | ||
| Axillary node dissection | <0.001 | <0.001 | |||||||
| No | 11.6 | 1.00 | -- | -- | 1.00 | -- | -- | ||
| Yes | 17.3 | 1.46 | 1.32 | 1.60 | 1.52 | 1.38 | 1.68 | ||
| Chemotherapy (composite) | <0.001 | <0.001 | |||||||
| No chemotherapy | 16.3 | 1.00 | -- | -- | 1.00 | -- | -- | ||
| Chemotherapy started in month prior through month after surgery | 7.2 | 0.33 | 0.25 | 0.43 | 0.32 | 0.24 | 0.41 | ||
| Chemotherapy started in 31–365 days after surgery | 13.8 | 0.73 | 0.63 | 0.84 | 0.70 | 0.61 | 0.82 | ||
| Hospital admission*** | 0.04 | ||||||||
| No | 15.9 | 1.00 | -- | -- | |||||
| Yes | 14.3 | 0.89 | 0.79 | 1.00 | |||||
| Screening mammogram*** | <0.001 | <0.001 | |||||||
| No | 13.4 | 1.00 | -- | -- | 1.00 | -- | -- | ||
| Yes | 16.4 | 1.45 | 1.32 | 1.60 | 1.44 | 1.30 | 1.59 | ||
| Flu shot*** | 0.34 | ||||||||
| No | 15.4 | 1.00 | -- | -- | |||||
| Yes | 16.0 | 1.04 | 0.96 | 1.13 | |||||
| Visit to PCP*** | 0.03 | ||||||||
| No | 12.0 | 1.00 | -- | -- | |||||
| Yes | 15.8 | 1.32 | 1.03 | 1.71 | |||||
| Health system characteristics | |||||||||
| State CON for radiation facility | 0.09 | ||||||||
| No | 17.5 | 1.00 | -- | -- | |||||
| Yes | 13.6 | 0.85 | 0.71 | 1.02 | |||||
| HRR-level two-year mammography rate among female Medicare enrollees 67–69, in quintiles | 0.04 | 0.007 | |||||||
| Q1 (50.1–59.7) | 17.1 | 1.00 | -- | -- | 1.00 | -- | -- | ||
| Q2 (59.8–62.4) | 17.2 | 1.05 | 0.76 | 1.44 | 1.04 | 0.76 | 1.44 | ||
| Q3 (62.4–64.9) | 16.3 | 0.92 | 0.67 | 1.27 | 0.89 | 0.64 | 1.23 | ||
| Q4 (65.0–68.4) | 16.1 | 0.80 | 0.58 | 1.10 | 0.75 | 0.55 | 1.04 | ||
| Q5 (68.4–76.1) | 12.0 | 0.67 | 0.49 | 0.92 | 0.62 | 0.45 | 0.85 | ||
| Radiation oncologist density per 100,000 residents, in quintiles | 0.26 | ||||||||
| Q1 (0.2–1.0) | 16.6 | 1.00 | -- | -- | |||||
| Q2 (1.0–1.1) | 17.2 | 0.92 | 0.69 | 1.23 | |||||
| Q3 (1.1–1.2) | 17.7 | 0.85 | 0.63 | 1.16 | |||||
| Q4 (1.2–1.4) | 15.3 | 0.84 | 0.63 | 1.13 | |||||
| Q5 (1.4–2.5) | 12.0 | 0.70 | 0.51 | 0.96 | |||||
Adjusted for the following health system characteristics: state certificate of need (CON), HRR level 2-year mammography rate in quintiles, and radiation oncologist density per 100,000 enrollees
Hospital volume is defined as the number of patients in our sample who received breast-conserving surgery at each hospital during the study period
In year prior to breast-conserving surgery
Hospital Ownership and Receipt of Overall Radiation
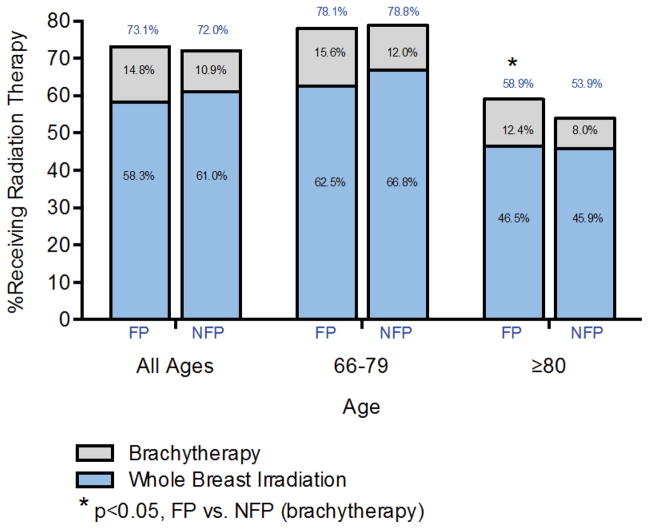
There was no association between hospital ownership and the overall use of RT. That is, 73.1% of women undergoing BCS at a for-profit hospital subsequently received adjuvant RT, compared to 72.0% of women at not-for-profit hospitals (OR: 1.08, 95% CI: 0.97–1.20, p=0.18, Figure 1). However, the relation between hospital ownership and RT use varied across age groups. Among the oldest women (aged 80–94 years), those undergoing BCS at a for-profit hospital were more likely to receive any RT compared to women receiving care at a not-for-profit hospital (58.9% vs. 53.9%, OR: 1.22, 95% CI: 1.03–1.45, p=0.03, Figure 1). There was no significant difference in receipt of RT according to hospital profit status among women age 66–79 (78.1% vs. 78.8%, p=0.74)
Figure 1.
Percent of women receiving any radiation therapy or brachytherapy based on age and hospital ownership. NFP: not-for-profit; FP: for-profit
The increased use of RT among older women at for-profit hospitals was associated primarily with receipt of brachytherapy. Specifically, women aged 80 and over receiving BCS at a for-profit hospital were more likely to receive brachytherapy (12.4% at for-profit vs. 8.0% at not-for-profit, OR for brachytherapy compared to no RT: 1.66, 95% CI: 1.18–2.34, p=0.003) while there was no relation between ownership status and the receipt of WBI (46.5% at for-profit vs. 45.9% at not-for-profit, OR for WBI vs. no RT: 1.14, 95% CI: 0.96–1.36, p=0.13).
DISCUSSION
We found that Medicare beneficiaries who underwent BCS at for-profit hospitals disproportionately received the more expensive and less proven brachytherapy over the less expensive standard of care (WBI). Furthermore, older women (≥80) receiving BCS at for-profit hospitals received more RT overall, with this difference largely driven by the use of brachytherapy. Thus, older women received more aggressive care at for-profit hospitals, despite being less likely to benefit from RT.27
Several factors may have contributed to the increased use of brachytherapy for women who had undergone BCS at for-profit hospitals. Financial incentives may be one driving factor.21,36,37 Prior studies have highlighted the high reimbursement for brachytherapy, suggesting that it is more revenue generating than the standard of care. 23,24,38 While high reimbursements do not necessarily equate to high profit margins, there has been concern that higher reimbursements have fueled the adoption of brachytherapy.24,39–41 In fact, the Centers for Medicare and Medicaid Services reduction in reimbursement for brachytherapy in 2008 and 2010 generated important debate regarding the financial incentives and feasibility of offering brachytherapy. While we do not have actual profit margin estimates for brachytherapy in individual hospitals, our findings support previous reports suggesting that higher reimbursements may be contributing to the rapid adoption of brachytherapy.39–41 In other cancer care settings, reduced reimbursement of chemotherapy has been associated with significant changes in patterns of chemotherapy use by oncologists.42–44 In addition to direct financial incentives, leaders at for-profit hospitals may prefer adopting novel therapies as a way to build market share. Indeed, hospital advertising has been shown to promote more advanced technology as a means to attract patients.45,46
It is important to note that a driving factor in the adoption of brachytherapy is the attempt to enhance convenience and tolerability of treatment. Brachytherapy has the potential of delivering RT to patients who otherwise may not seek treatment due to concerns about treatment length and toxicity, and may be a reason for some older women to choose brachytherapy over standard RT. However, it is unclear why patient preferences for radiation modality would vary with hospital ownership. Given that women older than 80 years of age are least likely to benefit from radiation overall in terms of improvements in cancer control, our analysis suggests that brachytherapy may be increasing accessibility to RT overall, but not necessarily for women who benefit from it the most. 27,47 It is also notable that we found hospital volume to be strongly associated with receipt of brachytherapy; patients treated at the highest volume hospitals were twice as likely as those treated at the lowest volume hospitals to receive brachytherapy. It is possible that larger hospitals are more likely to have the resources needed to invest in new technologies. Hospital volume has been shown in past studies to be associated with newer surgical techniques in breast cancer48–50 as well as prostate51 and rectal52 cancers.
Our study has important limitations. First, we defined hospital ownership as either not-for-profit or for-profit which does not distinguish hospital behavior that can exist in both profit settings.53 We grouped hospitals listed as ‘Government’ or ‘Voluntary Non-profit’ as not-for-profit because of our hypothesis that for-profit hospitals in particular might adopt brachytherapy to a greater extent compared to other hospital types.53 However, hospital behavior can align with financial incentives within not-for-profit organizations as well.53,54 Therefore, coarse classification of ownership into either for-profit or not-for-profit may obscure financial factors that affect brachytherapy use. Second, we examined only Medicare beneficiaries, who may not be representative of the patterns of brachytherapy utilization in younger patient populations or in patients with private insurance or no insurance. Third, we did not consider the decreases in the Centers for Medicare and Medicaid Services reimbursement for breast brachytherapy, the first of which took effect in January 2009.24 However, our study illustrates the pattern of brachytherapy use when reimbursement was higher. While our results suggest that the year when treatment occurred did not affect receipt of brachytherapy, future work exploring how these changes affect brachytherapy utilization will add to our understanding of financial incentives and adoption of new technologies of cancer care. Fourth, our analysis does not account for provider factors such as physician reimbursement structures that may differ between hospitals. Our analysis does not examine the effect of ownership status of free-standing RT facilities which also provide RT for patients and may respond differently to financial incentives. Instead, we chose to use hospital ownership where BCS was performed because patients who are referred for RT eventually seek treatment at either hospital-based facilities, freestanding facilities, or seek no RT treatment. Thus, determining the effect of hospital ownership rather than RT facility ownership captures an earlier point in the clinical decision making process. Finally, it is important to acknowledge that the long-term risks and benefits of brachytherapy are still being defined; the current work is focused on the adoption of brachytherapy during a time when there was scant comparative evidence concerning either benefits or risks.
Our study extends the quality of surgical care literature by examining how hospital ownership affects the adoption of newer, more expensive cancer technologies. In addition, our study highlights the important role surgical providers may have in affecting post-surgical care decisions especially for older women for whom there exists considerable debate regarding the benefit of adjuvant RT.
Acknowledgments
The authors acknowledge the efforts of General Dynamics Information Technology (Buccaneer Computer Systems & Service, Inc.) Chronic Condition Data Warehouse, under contract with CMS.
Funding/support and role of the sponsor: Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under Award Number R01CA149045. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health..
APPENDIX 1 Procedure and diagnosis codes used in analysis
| HCPCS | ICD-9 PROCEDURE | ICD-9 DIAGNOSIS | |
|---|---|---|---|
| Breast-Conserving Surgery | 19110, 19120, 19125, 19126, 19160, 19162, 19301, 19302 | 85.20, 85.21, 85.22, 85.23, 85.25, | |
| Whole Breast Irradiation | 77402, 77403, 77404, 77406, 77407, 77408, 77409, 77411, 77412, 77413, 77414, 77416, 77418, 0073T, G0174 | ||
| Brachytherapy | 77761, 77762, 77763, 77776, 77777, 77778, 77781, 77782, 77783, 77784, 77785, 77786, 77787, 77799, 0182T | ||
| Tumor laterality | Breast-conserving surgery code with a HCPCS modifier indicating a left or right sided procedure, which is optionally included for procedures | ||
| Axillary node dissection | 19302, 38740, 38745, 38525, 38500 | 40.23, 40.51 | |
| Chemotherapy | 96400-96549, Q0083-Q0085, J9000-J9999, G0355-G0362, J8510, J8520, J8521, J8530, J8560, J8565, J8600, J8610, J8700 | 99.25 | V58.1 |
| Screening mammogram | 76092, 77057, G0202, G0203 | V76.1, V76.11, V76.12 | |
| Flu shot | 90656, 90658, 90659, 90660, 90661, 90662, 90724 | V04.81 | |
| Visit to primary care physician | 99202, 99203, 99204, 99205, 99212, 99213, 99214, 99215, 99387, 99397 |
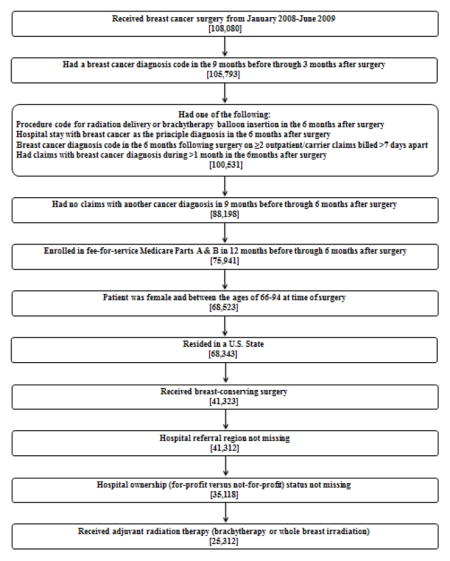
APPENDIX 2 Sample Selection Algorithm
Footnotes
Data access and responsibility: All authors had full access to all the data in the study and Dr. Gross takes responsibility for the integrity of the data and the accuracy of the data analysis.
Disclosures and Conflicts of interest: Drs. Ross and Gross are members of a scientific advisory board for FAIR Health, Inc. Drs. Ross and Gross receive support from Medtronic, Inc. to develop and implement methods of clinical trial data sharing and patient-level meta-analyses. Dr. Ross is supported by the National Institute on Aging (K08 AG032886) and by the American Federation for Aging Research through the Paul B. Beeson Career Development Award Program, from the Centers of Medicare and Medicaid Services (CMS) to develop and maintain performance measures that are used for public reporting, and from the Pew Charitable Trusts to examine regulatory issues at the U.S. Food and Drug Administration. Dr. Krumholz is supported, in part, by grant U01 HL105270-03 (Center for Cardiovascular Outcomes Research at Yale University) from the National Heart, Lung, and Blood Institute in Bethesda, Maryland. Dr. Krumholz is the recipient of a research grant from Medtronic, Inc. through Yale University and is chair of a cardiac scientific advisory board for UnitedHealth.
Author contributions: Drs. Gross, Lesnikoski, and Sen and Ms. Soulos were responsible for the conception and design of this work. Drs. Gross and Sen and Ms. Soulos drafted the manuscript. Ms. Soulos conducted the statistical analysis. Dr. Gross was responsible for acquisition of data and obtained funding. Dr. Gross provided supervision. All authors participated in the analysis and interpretation of the data and critically revised the manuscript for important intellectual content.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dentzer S. ‘Swinging for the fences’ versus striking out on cancer. Health Aff (Millwood) 2012;31:662. doi: 10.1377/hlthaff.2012.0301. [DOI] [PubMed] [Google Scholar]
- 2.Gross CP, LJBRJS, et al. The cost of breast cancer screening in the medicare population. JAMA Internal Medicine. 2013:1–7. doi: 10.1001/jamainternmed.2013.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lakdawalla DN, Romley JA, Sanchez Y, Maclean JR, Penrod JR, Philipson T. How cancer patients value hope and the implications for cost-effectiveness assessments of high-cost cancer therapies. Health Aff (Millwood) 2012;31:676–82. doi: 10.1377/hlthaff.2011.1300. [DOI] [PubMed] [Google Scholar]
- 4.Philipson T, Eber M, Lakdawalla DN, Corral M, Conti R, Goldman DP. An analysis of whether higher health care spending in the United States versus europe is ‘worth it’ in the case of cancer. Health Aff (Millwood) 2012;31:667–75. doi: 10.1377/hlthaff.2011.1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilson CB. Adoption of new surgical technology. BMJ. 2006;332:112–4. doi: 10.1136/bmj.332.7533.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bodenheimer T. High and rising health care costs. Part 2: technologic innovation. Annals of internal medicine. 2005;142:932–7. doi: 10.7326/0003-4819-142-11-200506070-00012. [DOI] [PubMed] [Google Scholar]
- 7.Gelijns A, Rosenberg N. The dynamics of technological change in medicine. Health Aff (Millwood) 1994;13:28–46. doi: 10.1377/hlthaff.13.3.28. [DOI] [PubMed] [Google Scholar]
- 8.Weissman JS, Blumenthal D, Silk AJ, Zapert K, Newman M, Leitman R. Consumers’ reports on the health effects of direct-to-consumer drug advertising. Health Aff (Millwood) 2003:W3–82–95. doi: 10.1377/hlthaff.w3.82. Suppl Web Exclusives. [DOI] [PubMed] [Google Scholar]
- 9.Culyer AJ, Newhouse JP. Handbook of health economics. 1. Amsterdam ; New York: Elsevier; 2000. [Google Scholar]
- 10.Lee DK, Chertow GM, Zenios SA. Reexploring differences among for-profit and nonprofit dialysis providers. Health services research. 2010;45:633–46. doi: 10.1111/j.1475-6773.2010.01103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sloan FA, Trogdon JG, Curtis LH, Schulman KA. Does the ownership of the admitting hospital make a difference? Outcomes and process of care of Medicare beneficiaries admitted with acute myocardial infarction. Medical care. 2003;41:1193–205. doi: 10.1097/01.MLR.0000088569.50763.15. [DOI] [PubMed] [Google Scholar]
- 12.Thamer M, Zhang Y, Kaufman J, Cotter D, Dong F, Hernan MA. Dialysis facility ownership and epoetin dosing in patients receiving hemodialysis. JAMA : the journal of the American Medical Association. 2007;297:1667–74. doi: 10.1001/jama.297.15.1667. [DOI] [PubMed] [Google Scholar]
- 13.Pauly MV, McGuire TG, Barros PP. Handbook of health economics. 1 [Google Scholar]
- 14.Presley CJ, Soulos PR, Herrin J, et al. Patterns of Use and Short-Term Complications of Breast Brachytherapy in the National Medicare Population From 2008–2009. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012 doi: 10.1200/JCO.2012.43.5297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hill-Kayser CE, Chacko D, Hwang WT, Vapiwala N, Solin LJ. Long-term clinical and cosmetic outcomes after breast conservation treatment for women with early-stage breast carcinoma according to the type of breast boost. International journal of radiation oncology, biology, physics. 2011;79:1048–54. doi: 10.1016/j.ijrobp.2009.12.026. [DOI] [PubMed] [Google Scholar]
- 16.Vass S, Bairati I. A cosmetic evaluation of breast cancer treatment: a randomized study of radiotherapy boost technique. International journal of radiation oncology, biology, physics. 2005;62:1274–82. doi: 10.1016/j.ijrobp.2004.12.039. [DOI] [PubMed] [Google Scholar]
- 17.Aristei C, Palumbo I, Cucciarelli F, et al. Partial breast irradiation with interstitial high-dose-rate brachytherapy in early breast cancer: results of a phase II prospective study. Eur J Surg Oncol. 2009;35:144–50. doi: 10.1016/j.ejso.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 18.Smith GL, Xu Y, Buchholz TA, et al. Brachytherapy for Accelerated Partial-Breast Irradiation: A Rapidly Emerging Technology in Breast Cancer Care. Journal of Clinical Oncology. 2011;29:157–65. doi: 10.1200/JCO.2009.27.0942. [DOI] [PubMed] [Google Scholar]
- 19.Suh WW, Pierce LJ, Vicini FA, Hayman JA. A cost comparison analysis of partial versus whole-breast irradiation after breast-conserving surgery for early-stage breast cancer. International journal of radiation oncology, biology, physics. 2005;62:790–6. doi: 10.1016/j.ijrobp.2004.10.039. [DOI] [PubMed] [Google Scholar]
- 20.Smith GL, Xu Y, Buchholz TA, et al. Association between treatment with brachytherapy vs whole-breast irradiation and subsequent mastectomy, complications, and survival among older women with invasive breast cancer. JAMA. 2012;307:1827–37. doi: 10.1001/jama.2012.3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suh WW, Pierce LJ, Vicini FA, Hayman JA. A cost comparison analysis of partial versus whole-breast irradiation after breast-conserving surgery for early-stage breast cancer. Int J Radiat Oncol. 2005;62:790–6. doi: 10.1016/j.ijrobp.2004.10.039. [DOI] [PubMed] [Google Scholar]
- 22.Hattangadi JA, Taback N, Neville BA, Harris JR, Punglia RS. Accelerated partial breast irradiation using brachytherapy for breast cancer: patterns in utilization and guideline concordance. J Natl Cancer Inst. 2012;104:29–41. doi: 10.1093/jnci/djr495. [DOI] [PubMed] [Google Scholar]
- 23.Peres J. Intraoperative radiotherapy makes uncertain headway in the U.S. Journal of the National Cancer Institute. 2012;104:895–7. doi: 10.1093/jnci/djs294. [DOI] [PubMed] [Google Scholar]
- 24.Shaitelman SF. Sounding a warning bell? Documentation of the increased utilization of accelerated partial breast irradiation. Journal of the National Cancer Institute. 2012;104:5–7. doi: 10.1093/jnci/djr501. [DOI] [PubMed] [Google Scholar]
- 25.Hughes KS, Schnaper LA, Berry D, et al. Lumpectomy plus tamoxifen with or without irradiation in women 70 years of age or older with early breast cancer. N Engl J Med. 2004;351:971–7. doi: 10.1056/NEJMoa040587. [DOI] [PubMed] [Google Scholar]
- 26.Hughes KS, Schnaper LA, Cirrincione C, et al. Lumpectomy plus tamoxifen with or without irradiation in women age 70 or older with early breast cancer. 2010 ASCO Annual Meeting; 2010; 2010. [Google Scholar]
- 27.Smith BD, Gross CP, Smith GL, Galusha DH, Bekelman JE, Haffty BG. Effectiveness of radiation therapy for older women with early breast cancer. J Natl Cancer Inst. 2006;98:681–90. doi: 10.1093/jnci/djj186. [DOI] [PubMed] [Google Scholar]
- 28.Schonberg MA, Marcantonio ER, Ngo L, Silliman RA, McCarthy EP. Does Life Expectancy Affect Treatment of Women Aged 80 and Older with Early Stage Breast Cancers? Journal of geriatric oncology. 2012;3:8–16. doi: 10.1016/j.jgo.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nattinger AB, Laud PW, Bajorunaite R, Sparapani RA, Freeman JL. An algorithm for the use of Medicare claims data to identify women with incident breast cancer. Health Serv Res. 2004;39:1733–49. doi: 10.1111/j.1475-6773.2004.00315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith GL, Shih YC, Giordano SH, Smith BD, Buchholz TA. A method to predict breast cancer stage using Medicare claims. Epidemiol Perspect Innov. 2010;7:1. doi: 10.1186/1742-5573-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. [Accessed 12/1/2012, 2012];Medicare Enrollment Reports. 2012 at http://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/MedicareEnrpts.)
- 32.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 33.Medicare Hospital General Information Dataset.
- 34. [Accessed 5/21/2011];The Dartmouth Atlas of Health Care: Zip-Code Crosswalk. at http://www.dartmouthatlas.org/tools/downloads.aspx?tab=35#crosswalks.)
- 35.Snijders TAB, Bosker RJ. Multilevel analysis : an introduction to basic and advanced multilevel modeling. London ; Thousand Oaks, Calif: Sage Publications; 1999. [Google Scholar]
- 36.Sher DJ, Wittenberg E, Suh WW, Taghian AG, Punglia RS. Partial-Breast Irradiation Versus Whole-Breast Irradiation for Early-Stage Breast Cancer: A Cost-Effectiveness Analysis. Int J Radiat Oncol. 2009;74:440–6. doi: 10.1016/j.ijrobp.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roberts KB, Soulos PR, Herrin J, et al. The Adoption of New Adjuvant Radiation Therapy Modalities Among Medicare Beneficiaries With Breast Cancer: Clinical Correlates and Cost Implications. International journal of radiation oncology, biology, physics. 2012 doi: 10.1016/j.ijrobp.2012.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hattangadi JA, Taback N, Neville BA, Harris JR, Punglia RS. Accelerated Partial Breast Irradiation using Brachytherapy (APBIb) for Breast Cancer (BCA): Predictors of Use and Guideline Concordance. Int J Radiat Oncol. 2011;81:S141-S. doi: 10.1093/jnci/djr495. [DOI] [PubMed] [Google Scholar]
- 39.Smith GL, Xu Y, Buchholz TA, et al. Brachytherapy for accelerated partial-breast irradiation: a rapidly emerging technology in breast cancer care. J Clin Oncol. 2011;29:157–65. doi: 10.1200/JCO.2009.27.0942. [DOI] [PubMed] [Google Scholar]
- 40.DiFronzo LA, Tsai PI, Hwang JM, et al. Breast conserving surgery and accelerated partial breast irradiation using the MammoSite system: initial clinical experience. Arch Surg. 2005;140:787–94. doi: 10.1001/archsurg.140.8.787. [DOI] [PubMed] [Google Scholar]
- 41.Abelson R. Quickly Vetted, Treatment is Offered to Patients. New York Times; 2008. [Google Scholar]
- 42.Shahinian VB, Kuo YF, Gilbert SM. Reimbursement policy and androgen-deprivation therapy for prostate cancer. N Engl J Med. 2010;363:1822–32. doi: 10.1056/NEJMsa0910784. [DOI] [PubMed] [Google Scholar]
- 43.Jacobson M, Earle CC, Price M, Newhouse JP. How medicare’s payment cuts for cancer chemotherapy drugs changed patterns of treatment. Health Aff (Millwood) 2010;29:1391–9. doi: 10.1377/hlthaff.2009.0563. [DOI] [PubMed] [Google Scholar]
- 44.Jacobson M, Earle CC, Newhouse JP. Geographic variation in physicians’ responses to a reimbursement change. The New England journal of medicine. 2011;365:2049–52. doi: 10.1056/NEJMp1110117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Larson RJ, Schwartz LM, Woloshin S, Welch HG. Advertising by academic medical centers. Archives of internal medicine. 2005;165:645–51. doi: 10.1001/archinte.165.6.645. [DOI] [PubMed] [Google Scholar]
- 46.Blumenthal D, Weissman JS, Griner PF. Academic health centers on the front lines: survival strategies in highly competitive markets. Academic medicine : journal of the Association of American Medical Colleges. 1999;74:1038–49. doi: 10.1097/00001888-199909000-00021. [DOI] [PubMed] [Google Scholar]
- 47.Carlson RW. Industry “rewrites” of NCCN Guidelines. Journal of the National Comprehensive Cancer Network : JNCCN. 2011;9:257. doi: 10.6004/jnccn.2011.0023. [DOI] [PubMed] [Google Scholar]
- 48.Fedeli U, Alba N, Schievano E, et al. Diffusion of good practices of care and decline of the association with case volume: the example of breast conserving surgery. BMC Health Serv Res. 2007;7:167. doi: 10.1186/1472-6963-7-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hiotis K, Ye W, Sposto R, Skinner KA. Predictors of breast conservation therapy: size is not all that matters. Cancer. 2005;103:892–9. doi: 10.1002/cncr.20853. [DOI] [PubMed] [Google Scholar]
- 50.Kotwall CA, Covington DL, Rutledge R, Churchill MP, Meyer AA. Patient, hospital, and surgeon factors associated with breast conservation surgery. A statewide analysis in North Carolina Ann Surg. 1996;224:419–26. doi: 10.1097/00000658-199610000-00001. discussion 26–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ulmer WD, Prasad SM, Kowalczyk KJ, et al. Factors associated with the adoption of minimally invasive radical prostatectomy in the United States. J Urol. 2012;188:775–80. doi: 10.1016/j.juro.2012.05.014. [DOI] [PubMed] [Google Scholar]
- 52.Paquette IM, Kemp JA, Finlayson SR. Patient and hospital factors associated with use of sphincter-sparing surgery for rectal cancer. Dis Colon Rectum. 2010;53:115–20. doi: 10.1007/DCR.0b013e3181bc98a1. [DOI] [PubMed] [Google Scholar]
- 53.Folland S, Goodman AC, Stano M. The economics of health and health care. 5. Upper Saddle River, NJ: Pearson Prentice Hall; 2007. [Google Scholar]
- 54.Pauly M, Redisch M. Not-for-Profit Hospital as a Physicians Cooperative. American Economic Review. 1973;63:87–99. [Google Scholar]