Abstract
Immune evasion is a hallmark of cancer. While, there are multiple different mechanisms that cancer cells employ, myeloid deriver suppressor cells (MDSCs) are one of the key drivers of tumor mediated immune evasion. MDSCs begin as myeloid cells recruited to the tumor microenvironment where they are transformed into potent immunosuppressive cells. Our understanding of the clinical relevance of MDSCs in cancer patients, however has significantly lagged behind the preclinical literature in part due to the absence of a cognate molecule present in mice, as well as the considerable heterogeneity of MDSCs. However, if one evaluates the clinical literature through the filter of clinically robust endpoints, such as overall survival, three important phenotypes have emerged: promyelocytic, monocytic, and granulocytic. Based on these studies, MDSCs have clear prognostic importance in multiple solid tumors, and emerging data supports the utility of circulating MDSCs as a predictive marker for cancer immunotherapy, and even as an early leading marker for predicting clinical response to systemic chemotherapy in patients with advanced solid tumors. More recent preclinical data in immunosuppressed murine models suggest that MDSCs play an important role in tumor progression and the metastatic process that is independent of their immunosuppressive properties. Consequently, targeting MDSCs either in combination with cancer immunotherapy or independently as part of an approach to inhibit the metastatic process, appears to be a very clinically promising strategy. We review different approaches to target MDSCs that could potentially be tested in future clinical trials in cancer patients.
Introduction
The emergence and FDA approval in 2011 of the monoclonal antibody ipilimumab targeting cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) on the surface of T cells, as an immune based strategy in metastatic melanoma, has created a new enthusiasm for cancer immunotherapy within the oncology field.1 CTLA-4 is a negative regulator of T-cell activation, and antibody blockade is believed to foster innate immunity through blocking CTLA-4 mediated inhibition of anti-tumor immune response in metastatic melanoma.2 Further exciting clinical results with other novel monoclonal antibodies against the immune checkpoint protein programmed death-1 (PD-1) T-cell receptor and its ligand (PDL-1), as well as the data with chimeric antigen receptor adoptive T-cell therapy has brought the spotlight back on the importance of the immune system as a therapeutic target in cancer.1–3 Immune evasion by cancer cells is an important step in oncogenesis, and is considered an emerging hallmark of cancer.4
One of the challenges in the clinical development of effective immune-based therapies remains the complex interplay between the host immune system and the tumor, and different mechanisms and redundancy in pathways engaged by the tumor to evade the immune system. Multiple cell types are known to contribute to tumor mediated immune suppression, including regulatory T cells (Treg), type 2 natural killer T cells, tumor associated macrophages (TAMs), and myeloid derived suppressor cells (MDSCs).5,6 MDSCs are a heterogeneous cell population characterized by the ability to suppress T cell and natural killer (NK) cell function,5, 7–10 that arise from myeloid progenitor cells that do not differentiate into mature dendritic cells, granulocytes, or macrophages.
Myeloid cells are the predominant hematopoietic cell type in the human body, and arise from hematopoietic stem cells that differentiate into mature myeloid cells.10 The three major groups of myeloid cells are essential to the proper functioning of both our innate and adaptive immune systems: granulocytes, dendritic cells, and macrophages.10
The importance of myeloid cells in the tumor pathogenesis is not a new idea, but has its origins in the mid-1800s, when Dr. Rudolf Virchow first described a leukocytic infiltration in tumors, and hypothesized a direct connection between inflammation and cancer. At the time, he suggested that the “lymphoreticular infiltrate” reflected the origin of cancer at sites of chronic inflammation.11 Only over the last two decades have myeloid cells been recognized as playing a crucial role in the processes of tumor angiogenesis, tumor mediated immune evasion, and metastases.
Only over the last 10 years, have MDSCs been recognized as having an important role in immune evasion and progression in cancer patients. There are several well established tactics employed by MDSCs to suppress T-cells, including generation of arginase 1, nitrosylation of the T-cell receptor (TCR) though production of reactive oxygen species, down-regulation of CD62L, and cysteine sequestration.5, 7, 9, 12–17 There is an ever expanding body of clinical evidence that demonstrate elevated levels of circulating MDSCs in almost all malignancies, which appear to directly correlate with clinical cancer stage, metastatic tumor burden, and prognosis.8, 18–22 One of the challenges in the clinical data with MDSCs in cancer patients, is the absence of a clear consensus on which phenotypes are the most relevant.8 This emerging clinical data, while not definitive, provides rather compelling evidence that MDSCs represent a very important and novel therapeutic target. Targeting MDSCs, while representing a logical combination with more traditional immune based therapies, may have other non-immune related effects.10, 12 Cancer xenograft models in immune-deficient mice suggest that MDSCs have immune independent effects, and may also be important for tumor angiogenesis and pre-metastatic niches.23,24, 25 In this review, we will discuss clinical data with three distinct cellular phenotypes in cancer patients that have demonstrated a correlation between MDSCs, and overall survival (OS). We also will discuss data suggesting MDSCs to have other immune-independent effects in tumor progression. And, finally we will discuss potential strategies to target MDSCs in cancer patients, as a novel therapeutic approach.
Basic MDSCs biology
MDSCs constitute a diverse population of cells derived from bone marrow progenitor cells that are at varying stages of differentiation from early myeloid to a more granulocytic or monocytic phenotype. In murine tumor models, MDSCs have been isolated from peripheral blood, spleen, lymph nodes, and tumor sites and are known to have the ability to block both innate and adaptive immunity (Figure 1). MDSCs recruitment to the tumor microenvironment is currently thought to be an early event, and one of the key drivers by which tumor cells evade the immune system.20, 26, 27 The issue of MDSCs phenotype is much more straight forward in preclinical murine tumor models, where there are two primary MDSCs subtypes with either polymorphonuclear or monocytic characteristics, termed granulocytic and monocytic MDSCs, respectively, each of which employs slightly different mechanisms to suppress anti-tumor immunity.
Figure 1. Myeloid Derived Suppressor Cells (MDSCs) and T-cell suppression.
MDSCs are immature myeloid cells originating in the bone marrow that are recruited to the tumor microenvironment through production of various tumor derived factors (TDFs). These chemokines and cytokines stimulate the production of myeloid precursors in the marrow, and facilitate their recruitment and accumulation within the tumor microenvironment. Once in tumor sites, MDSCs are potent suppressors of T-cells via a wide array of different mechanisms. Moreover, MDSCs in tumor sites can further differentiate into tumor associated macrophages (TAMs) and also into suppressive dendritic cells.
Differentiating between monocytic and granulocytic MDSCs in murine cancer models was initially based on the expression of Ly6G and Ly6C.28, 29 Granulocytic MDSCs are described as Ly6G+ Ly6Clow, whereas the monocytic MDSCs are Ly6G−Ly6Chigh.29 In terms of functional differences, granulocytic MDSCs are known to express higher levels of arginase, but not inducible nitric oxide synthetase (iNOS), and have been shown to generate higher levels of reactive oxygen species (ROS).10, 29 Monocytic MDSCs are express both arginase and iNOS, but do not produce high levels of ROS. The production of ROS is of course important, as this is one mechanism by which granulocytic MDSCs are able to suppress T-cells that are in close proximity through production of high levels of ROS, such as hydrogen peroxide and peroxynitrite, that can induce T-cell apoptosis. The production of ROS also can lead to nitrosylation of the T cell receptor (TCR) during direct cell–cell contact which renders the TCR unable to bind to antigen, thus blocking activation.9, 20, 29–31
Further refinement of MDSCs in murine cancer models is also based on Gr-1 expression levels.29, 32 Monocytic MDSCs are phenotypically described as being CD11b+/Gr-1int/low, demonstrate show high expression levels of IL-4Rα compared to granulocytic MDSCs, and their activity appears to be driven by tumor-secreted GM-CSF33 and by IFN-γ released from T lymphocytes.34 Granulocytic MDSCs are phenotypically described as being CD11b+ /Gr-1high, and exert limited immune suppression in some tumor models, and only when present in relatively high numbers.35 Although granulocytic MDSCs require GM-CSF secretion for expansion, they do not appear to respond when GM-CSF is given externally, since GM-CSF is a necessary but not sufficient factor for their maturation.36
Transcriptional drivers of MDSC
The binding of select ligands (e.g.cytokines, growth factors) to their receptors expressed by hematopoietic progenitor/stem cells (HPC), dendritic cells (DC) and MDSC initiate transcriptional activity that is largely responsible for the abnormal differentiation, expansion and function of myeloid cells in the tumor microenvironment. Multiple transcription factors have been identified that play a critical role in the altered differentiation of myeloid cells in cancer, however it is beyond the scope of this review to discuss all of them but rather we will highlight some of them that have a significant impact on myeloid cell conversion to suppressive cells.
Notch signaling is thought to play a critical role in DC differentiation37 and in the accumulation of immature myeloid cells.38 More recently it was shown that the interaction between the notch receptor and the transcriptional repressor CSL which is typically required for transcription of target genes is impaired in HPC, DC and MDSC derived from tumor bearing host.39 This reduction in Notch transcription appears to be the result of serine phosphorylation of the Notch receptor mediated by the abnormal enzymatic activity of caseine kinase 2 (CK2). This impaired Notch transcriptional activity can be replicated by exposing HPC to tumor conditioned medium suggesting that the tumor microenvironment is responsible for this defect. The use of a select CK2 inhibitor, restored Notch activity in myeloid cells and when administered to tumor bearing mice promoted DC expansion and reduced tumor volume suggesting that targeting of Notch may improve normal myeloid differentiation.39
Signal transducers and activators of transcription (STATs) are known to regulate MDSC expansion and suppressive/angiogenic activity following their activation by a variety of tumor-derived products.10 STAT3 has been the most studied of the STATs and is known to enhance MDSC proliferation via the upregulation of multiple proteins involved in cell cycle progression and the promotion of cell survival (Bcl-xl, cyclin D1 and survivin).40, 41 The activation of STAT3 in myeloid precursor cells also upregulates the calcium binding proteins S1008A and S100A9, that block differentiation of dendritic cells thereby leading to the accumulation of immunosuppressive MDSC.42 Furthermore these S100 proteins are known to bind surface glycoprotein receptors on MDSC and promote their migration.43 Another function of STAT3 activation in MDSC is to upregulate the activity of NADPH oxidase (NOX2) resulting in the production of reactive oxygen species, a mechanism of T cell mediated immune suppression.13 Other STAT proteins are known to promote immune suppression in tumor bearing host. Nitric oxide induced immune suppression mediated by M-MDSC and tumor associated macrophages (TAM) was found to be dependent on STAT1 since downregulating STAT1 expression partially blocked suppression.44, 45 On the other hand STAT6 signaling via Il-4/IL-13 activation is implicated in upregulating arginase 1 and TGFb production in MDSC and macrophages leading to greater suppressive activity.10, 46
Recent evidence demonstrated that the transcription factor C/EBPb which controls emergency granulopoiesis induced by cytokines and infections is critical for the immunosuppressive program observed in tumor-induced and BM-derived MDSC.36
In vivo studies in mouse tumor models demonstrated the expression of C/EBPb in tumor infiltrating MDSC and in vitro studies indicated that GM-CSF and IL6 combination was most effective at inducing MDSC from precursors present in human and mouse bone marrow and that the loss of C/EBPb in cultured MDSC resulted in a significant reduction of arginse1 and nitric oxide synthase which are critical components of immune suppression in MDSC. The importance of C/EBPb in promoting MDSC immune suppression was shown by adoptively transferring antigen specific T cells into tumor bearing mice that were deficient in C/EBPb within the myeloid compartment. These transferred CD8+ T cells were only effective at reducing tumor growth in mice where C/EBPb was targeted in the myeloid cells but not in control harboring an intact MDSC population.
In summary, a number of transcription factors activated in MDSC by different cytokines/growth factors in the tumor microenvironment regulate their differentiation, expansion and function. Therefore a better understanding of how different transcription factors function in MDSC should provide new targets to control MDSC in the tumor and when combined with immunotherapy may improve treatment for different types of cancer.
Clinical Impact of MDSCs in Cancer Patients: Past, Present, and Future
Since the initial identification and description of MDSCs in the preclinical literature, there have been many studies in cancer patients with solid and hematologic malignancies that have evaluated the presence and clinical significance of MDSCs. In a recent review of the clinical literature of MDSCs in cancer patients, over 15 different phenotypes have been evaluated in clinical studies.8 One of the main challenges in studying MDSCs in cancer patients therefore is definitional. Thus far, no consensus has been reached on what the most important phenotypes are for advancing future clinical studies. This is due in part to the highly heterogeneous nature of MDSCs. But, the key driver in this inconsistency between the preclinical and clinical literature of MDSCs in cancer, appears to be the absence of the cognate Gr-1 molecule in humans; since in mice MDSCs are defined as CD11b+ and Gr1+.
One of the first published clinical studies that evaluated the presence of MDSCs in cancer patients was in the tumor of patients with head and neck cancer, mostly squamous histology (n = 18).47 This study reported the presence of CD34+ intra-tumoral myeloid cells that significantly correlated with secreted GM-CSF levels in tumor fragments. Moreover, CD34+ depletion via immunomagnetic separation was noted to reverse T-cell suppression, as evidenced by increased IL-2 production from intra-tumoral lymphocytes. A subsequent early pivotal study of MDSCs in cancer patients analyzed peripheral blood samples from patients (n=44) with three different cancers — head and neck squamous cell carcinoma, non-small cell lung cancer, and breast cancer—that identified a population of immature myeloid cells (ImCs).48 These cells were described as lineage negative [Lin–], defined here as CD3−, CD14−, CD19−, and CD57−. The immunosuppressive properties of those cells were confirmed by restoration of the ability of the dendritic cells to stimulate allogeneic T-cells in vitro when the ImC were depleted. From a clinical perspective, one of the shortcomings of this study was that clinical stages of patients were not reported, and there was no correlation with the presence of ImCs, or any correlation with any clinically relevant endpoints, e.g. OS or progression-free survival (PFS).
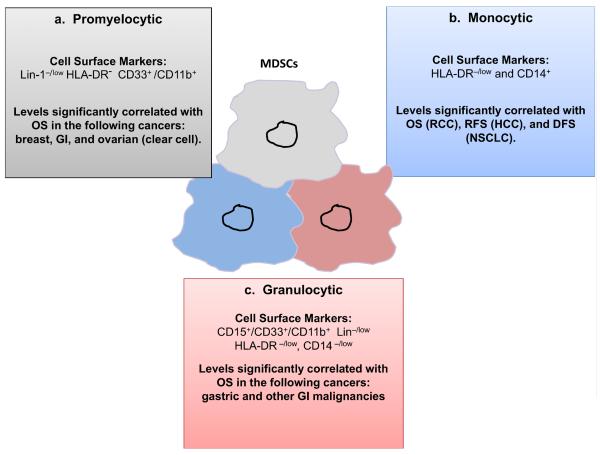
If one evaluates all published clinical data with MDSCs in cancer patients through the lens of the most clinically relevant endpoints, i.e. OS or PFS, a much clearer picture emerges. Using this restrictive filter, only three MDSCs phenotypes have been shown to correlate with true clinical efficacy measures (Figure 2). The three MDSCs phenotypes can be described as: (i) promyelocytic, (ii) monocytic, and (iii) granulocytic. We will discuss all three separately.
Figure 2. Three MDSC phenotypes are known to correlate with clinical outcome in cancer patients: (a) Promyelocytic, (b) Monocytic, and (c) Granulocytic.
Abbreviations: RCC (renal cell carcinoma), RFS (recurrence free survival), DFS (disease free survival), HCC (hepatocellular carcinoma), NSCLC (non-small cell lung cancer), GI (gastrointestinal).
Phenotype 1: Promyelocytic MDSCs
Promyelocytic MDSCs represent a very immature myeloid population of cells, defined phenotypically by being low/- for Lin-1[where Lin-1 is CD3, CD14, CD16, CD19, CD20, and CD56] and HLA-DR; and CD33+ and CD11b+. This phenotype is very similar to the initial phenotypic description from the early pivotal study describing ImCs. To date, four published studies have demonstrated a significant correlation with levels of promyelocytic MDSCs in the peripheral blood with clinical stage and/or metastatic tumor burden.19, 20, 22, 49 Of these, two have independently shown that in patients with advanced breast cancer and gastrointestinal malignancies, respectively that higher levels of circulating promyelocytic MDSCs were associated with poorer overall survival times. In the study by Solito et al. patients with stage IV breast cancer (n = 25) with circulating MDSCs levels >3.17 % (median) at baseline had significantly shorter median OS times than patients with circulating MDSCs less than the median at 5.5 months [95 % confidence interval (CI), 0.5–11.3] and 19.32 months (95 % CI, 8.7–NR), respectively (P < 0.048).20 Similarly, in the study by Gabitass et al., levels of circulating MDSCs greater than 2.0 % was an independent adverse prognostic factor in patients with pancreatic, esophageal, and gastric cancers on multivariate analysis.19 Patients with peripheral blood levels of promyelocytic MDSCs >2 % were found to have an overall poorer prognosis, with a median OS of only 4.6 months (95 % CI, 2.2–6.0), relative to a median OS of 9.3 months (95 % CI, 6.3–12.1) (P < 0.001), in patients with circulating MDSCs <2 %. Although these studies are retrospective in nature and involved small numbers of patients, they are the first to demonstrate the potential clinical significance of promyelocytic MDSCs. It is important to note that the method of blood collection differed, in the study by Solito et al, fresh whole blood was utilized, and in Gabitass, PBMCs were isolated using Ficoll, and later stored at −80°C for batch analysis.
Phenotyope 2: Monocytic MDSCs
Monocytic MDSCs are more differentiated that promyelocytic MDSCs, and are characterized as being HLA-DR−/lo and CD14+.50 To date, there have been three separate studies in cancer patients demonstrating a significant association between circulating levels of monocytic MDSCs and clinical outcome. The first study, by Walter et al evaluated the clinical significance of 6 different MDSCs phenotypes in patients with advanced renal cell carcinoma [RCC] who had been enrolled into a randomized phase 2 trial involving the multi-peptide therapeutic vaccine IMA901.51 In this study, pretreatment levels of 5 of the 6 different MDSCs phenotypes evaluated were found to be significantly higher in patients compared to health controls, one of these being the monocytic MDSCs phenotype. The clinical significance of pre-vaccination levels of circulating MDSCs and OS was also explored. Only the monocytic MDSCs phenotype (termed MDSCs4 in the study) was observed to be a significant discriminator of OS. Enrolled subjects having pre-vaccination monocytic MDSCs levels above the median (n = 29), had a significantly worse OS than those with levels below the median (n = 28) [HR = 0.35, P = 0.0035 by log-rank test]. Of note, a variation of the promyelocytic MDSCs phenotype was explored (called MDSCs3), but was distinct from the way it was defined in the previously discussed studies. Here, the MDSCs3 population was: Lin− HLA-DR−/lo CD33+ (whereby Lin was CD3, CD14, CD19, CD56).
The second study by Arihara and colleagues, evaluated the clinical significance of circulating monocytic MDSCs in hepatocellular carcinoma [HCC] patients (n=123).52 Circulating monocytic MDSCs ratios [HLA-DR−/low CD14+ MDSCs/ total CD14+ cells] were highest in patients with stage III/IV HCC, relative to all other examined patient groups: (i) early stage I/II HCC, (ii) patients with chronic hepatitis but no HCC, and (iii) healthy controls. In addition, and more relevant to our discussion, the clinical significance of monocytic MDSCs levels in HCC patients both before and after curative intent radiofrequency ablation [RFA] therapy in 33 patients. Blood samples were obtained on the day of treatment (baseline) and 2–4 weeks after treatment (after), and the value of monocytic MDSCs was divided by total CD14+ cells. Circulating monocytic MDSCs levels were found to significantly decreased after RFA therapy (18.0%→15.5 %, p=0.05). However, in several patients, the frequency of MDSCs remained at high levels post RFA. On multivariable analysis for recurrence, considering the variables found to be prognostic predictors on univariable analysis, only a post-treatment MDSCs ratio >22 % (HR 3.906, p = 0.014) was found to be a significant independent risk factor for recurrence.
The third study by Huang and colleagues, evaluated the clinical significance of circulating monocytic MDSCs in 89 patients with advanced non-small cell lung cancer (NSCLC).53 Levels of HLA-DR−/low CD14+ MDSCs as a percent of total CD14+ cells were present in significantly higher levels in NSCLC patients relative to healthy controls, and were proportional to clinical stage. Of the original 89 enrolled NSCLC patients, 60 had been followed until progression. Monocytic MDSCs frequency [>9.43%; 3 vs. 9 months], and absolute numbers [44.67 cells/ L; 3.5 vs. 8 months] greater than the median, significantly negatively correlated with median progression-free survival (P<0.01).
Phenotype 3: Granulocytic MDSCs
Prior clinical studies have demonstrated the presence of circulating MDSCs with more of a granulocytic morphology, negative for CD14 expression, but positive for CD15, which is expressed on circulating granulocytes and some monocytes, but not on lymphocytes.50, 54 However, these clinical studies primarily descriptive in nature, characterizing the MDSCs phenotype in the peripheral blood of cancer patients, without any correlation with either recurrence or survival. To the best of our knowledge, only two studies have correlated the presence of granulocytic MDSCs with clinical outcomes in cancer patients. The first study by Wang and colleagues evaluated the presence of MDSCs in stage I/II [n=13] or stage III/IV [n=27] gastric cancer patients.55 Blood was collected either prior to gastrectomy in operable candidates, or prior to initiating chemotherapy in patients with advanced/unresectable disease. Granulocytic MDSCs in this study were CD15+, but also rather phenotypically similar to promyelocytic MDSCs: Lin−/low (Lin being CD3/CD19/CD56), HLA-DR−/low, CD14−/low, CD11b+, and CD33+. In patients with Stage I/II gastric cancer, those with >4% circulating MDSCs (n = 23) were found to have significantly shorter median OS times when compared to those (n = 17) with <4% MDSCs (P = 0.024). A similar analysis was performed in the 27 patients with late stage gastric cancer, which demonstrated a trend towards a shorter median OS time in patients (n = 18) with >4% circulating MDSCs vs. those with <4% MDSCs (n = 7), but not surprisingly likely due to the very small numbers this difference did not reach statistical significance (P= 0.166).
The second study involved patients with GI solid malignancies (n=40), where 80% of patients had pancreatic adenocarcinoma, esophageal cancer, or colon cancer.56 Three different MDSCs phenotypes were studied: (i) CD15+ granulocytic [CD33+HLADR−CD11b+CD15+]; (ii) CD15- monocytic [CD33+HLA-DR−/lowCD15−] and (iii) CD14+ monocytic [CD33+HLA-DR−/lowCD14+]. To evaluate the potential relationships between OS and these three different MDSCs subsets, an exploratory backwards elimination procedure was used to select predictors for a multivariable model. The final multivariable model found increasing CD15− MDSCs levels to be associated with an increased risk of death, while increasing CD14+ MDSCs were associated with a reduced risk of death (hazard ratio [HR] for twofold increase in CD15− = 1.42, P= 0.049; HR for twofold increase in CD14+ = 0.69, P = 0.033]. Results of these analyses should be interpreted with caution because of the overall small sample size of a very heterogeneous group of GI cancer patients, and significant co-linear relationships between each of these three different MDSCs subsets.
Intratumoral MDSCs in cancer patients
The data just discussed with the three phenotypes that have been shown to correlate with clinically relevant outcomes in cancer patients pertains only to peripheral blood. To the best of our knowledge there is only one published study that has explored the clinical impact of intra-tumoral MDSCs. The preclinical literature demonstrates a very clear relationship between MDSCs levels in the peripheral blood and tumor and/or spleen, and their recruitment occurs early on. Going back to our MDSCs model (Figure X), because MDSCs are recruited from the bone marrow to the tumor microenvironment and/or pre-metastatic niches through production of chemokines or cytokines, one would expect that there would be a rather good correlation between both blood and tumor compartments. There are indeed a handful of clinical studies that have examined the presence of intra-tumoral MDSCs. One study by Finke and colleagues in 38 patients with renal cell carcinoma (RCC) found intra-tumoral MDSCs to make up about 5% of the total tumor single cell suspension.12 At least three distinct MDSCs populations in the blood and tumor were found, with granulocytic MDSCs (CD15+CD33+HLADR−) comprising about 55% of all MDSCs, followed by promyelocytic MDSCs (40% of MDSCs) with monocytic MDSCs (CD14+CD33+HLADR−) being 5% of the total population. Another study reported the presence of intra-tumoral monocytic MDSCs in metastatic melanoma lesions.57 Another study described the presence of intra-tumoral granulocytic MDSCs (CD15+) as well as in the blood and bone marrow of pancreatic adenocarcinoma patients.58 In another study, levels of tumor-infiltrating promyelocytic MDSCs (CD11b+ CD33+) in patients with stage III colorectal cancer were found to be significantly higher than in adjacent non-cancerous tissue [6% vs. 1%; P=0.0002].49
To date, only one published study has formally evaluated the clinical impact of tumor-infiltrating MDSCs in cancer patients. The study by Cui and colleagues initially looked at the presence of promyelocytic MDSCs in fresh tumors from patients with high-grade ovarian serous cancer.59 Through reverse gating polychromatic flow cytometry analysis, it was demonstrated that CD33+ cells within ovarian tumors were confined to promyelocytic MDSCs [lin−CD45+CD33+] in fresh ovarian tumor tissue. Then utilizing ovarian tumor tissues from patients (n=140) whose clinical and pathological information was available. Specimens were scored for density of CD33+ cells by immunohistochemistry. Based on the median values of CD33+ MDSCs density, patients were classified into “low” and “high” groups. Median OS (HR = 1.99, 95% CI: 1.22, 3.25; P = 0.006) and disease-free interval (DFI) (HR = 1.75, 95% CI: 1.08, 2.87; P= 0.02) were significantly shorter in patients with high intra-tumoral MDSCs infiltration even after adjusting for relevant clinical prognostic factors. This relationship between high tumor MDSCs content and shorter OS (HR = 2.87, 95% CI: 1.11, 7.42; P=0.03) and DFI (HR = 3.25, 95% CI: 1.21, 9.63; P=0.02) remained significant even in patients with stage IV ovarian cancer.
MDSCs as a predictive marker
The important role of MDSCs in tumor immune evasion, and tumor progression would imply that circulating MDSCs levels may serve as a good predictive marker. To our knowledge, only three published studies have explored the clinical utility of peripheral blood MDSCs in predicting response to immunotherapy in cancer patients. The first study, evaluated the predictive ability of pretreatment circulating promyelocytic MDSCs (Lin−HLA-DR−CD33+) and mature dendritic cells (DC) in patients with advanced kidney cancer or melanoma (n=36) who received high dose IL-2.60 A high DC-to-MDSCs ratio, and low numbers of circulating MDSCs (pretreatment) were able to discriminate the responder subset within the cohort of patients treated with high dose IL-2.
A phase 1–2 clinical study involving a highly immunogenic MUC1 peptide vaccine in patients (n=46) with a history of colonic adenomas, but no prior colon cancer, explored predictors of long-term immune responses.61 Comparing vaccine responders to non-responders no association of response with age, family history of colorectal cancer, body mass index, or criterion for advanced adenoma was noted. However, non-responders (n=19) were observed to have significantly higher percentages of peripheral blood promyelocytic MDSCs (HLA-DR−/low CD11b+, CD33+) compared to responders (n=12; P<0.05). Circulating promyelocytic MDSCs levels in responders was found to be similar to that detected in healthy age-matched controls. A third study in breast cancer patients receiving neoadjuvant chemotherapy in combination with a glutathione disulfide mimetic, with immunomodulatory properties, found that patients with lower levels of circulating promyelocytic MDSCs at baseline and prior to the last cycle of chemotherapy, had a higher probability of achieving a pathologic complete response (P = 0.02).21
The predictive benefit of measuring changes in MDSCs over time is not limited to immune based therapies alone. Since circulating MDSCs levels in cancer patients are proportional to clinical stage and metastatic tumor burden, it stands to reason that levels should increase or decrease depending on whether a patient is responding to systemic therapy or not, provided that the systemic therapy does not have some sort of direct effect on MDSCs. Indeed, two studies provide some preliminary evidence that MDSCs may serve as a predictive marker in patients with advanced cancer receiving chemotherapy. One small study in patients with stage IV colorectal cancer receiving standard combination chemotherapy demonstrated that promyelocytic MDSCs levels over time increased in patients with radiographic evidence of progressive disease.20 Similarly, a second study showed the frequency and absolute number of monocytic MDSCs after chemotherapy to be higher in patients with progressive disease.53
MDSCs and pre-metastatic niches: Beyond immunosuppression
Immunosuppression by MDSCs is certainly an important contribution to the progression of tumors.7, 10 However, a direct role on tumorigenesis and establishment of metastatic lesions is becoming increasingly evident. Metastasis is a complex process that involves escape from the primary tumor, survival in the circulation and colonization of distant organs. It is well established that non-malignant cells from the tumor microenvironment greatly influence these processes, particularly bone marrow-derived cells of the myeloid lineage such as tumor associated macrophages (TAMs).62 MDSCs share a common progenitor with TAMs, and also are recruited to tumor in response to inflammation where they secrete factors that modulate tumor cell survival and angiogenesis such as VEGF, basic fibroblast growth factor bFGF, PDGF, and MMPs.27
A direct role of MDSCs on the dissemination of tumor cells has been recently described in RETAAD mice. RETAAD mice are transgenic for the activated RET oncogene, and spontaneously develop uveal melanoma. In this model, MDSCs preferentially infiltrate primary lesions via CXCL5, and induce epithelial-mesenchymal transition through TGF-b, EGF and HGF signaling pathways.63 During epithelial-mesenchymal transition tumor cells gain motility and become invasive, and is considered to be an important requirement for the progression of tumors.
MDSCs are also capable of directly influencing the development of blood vessels. This was first described in MC26 cells, a murine colorectal cancer model. In this model, increased vessel formation was associated with the production of matrix metallopeptidase 9 (MMP-9) by MDSCs.64 The proteolityc activity of matrix metallopepetidases modulates the bioavailability of VGEF in tumors promoting ngiogenesis and vascular stability.65 Secretion of MMP-9 by MDSCs in a model for mammary carcinoma and the subsequent vasculogenesis has also been reported.66
Another mechanism by which MDSCs can promote tumorigensis is by differentiating into cell types that form part of the supportive network of both tumors and metastatic lesions. One example is the novel subset of MDSCs resembling fibrocytes that has been recently identified in pediatric sarcomas. Besides being able to suppress T cell responses, fibrocytes-like MDSCs are able to induce angiogenesis as measured by the standard tube formation assay.67 Another example is the differentiation of tumor infiltrating MDSCs into TAMs after exposure to hypoxia-inducible factor (HIF) 1α.68 Furthermore, MDSCs isolated from the bone marrow of tumor-bearing mice with bone metastasis are capable of differentiating into functional osteoclasts in vitro and in vivo.69 Osteoclasts are responsible for most of the destruction during osteolysis which facilitates the growth and establishment of bone metastasis. Interestingly, only MDSCs from tumors known to metastasize to the bone were able to differentiate into osteoclasts, and only when metastatic lesions were present, suggesting that coordinated signals from the bone marrow and bone metastasis are driving the differentiation of MDSCs into osteoclasts.70
Localization of MDSCs in distant sites has been associated with a favorable microenvironment for the establishment of metastasis also known as pre-metastatic niches. In a model of breast carcinoma metastasis MDSCs were found in the lungs of mice bearing primary mammary carcinomas well before metastatic lesions were established. MDSCs presence was linked with decreased levels of IFN-G and enhanced levels of MMP9 which associated with aberrant vasculature formation.71 Thus, MDSCs and other lymphoid cells can infiltrate distant sites before tumor cells, and prepare for their arrival a hospitable environment of immunosuppression and inflammation.
Therapeutic strategies targeting MDSCs as cancer therapy
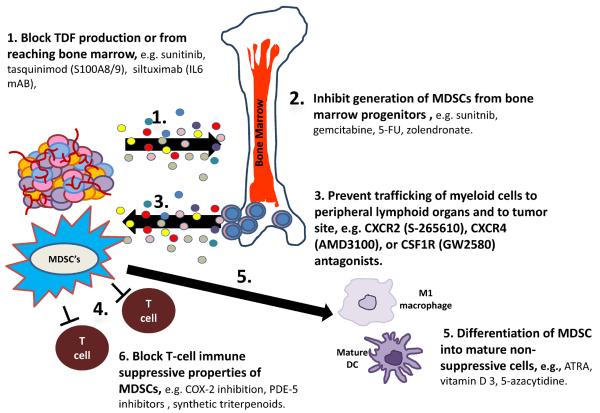
All of the clinical and preclinical data discussed provide a very strong rationale for development of therapeutic approaches targeting MDSCs in an oncologic setting either as a stand-alone approach or in combination with other immune based therapies. There are several different areas where one could envision targeting MDSCs as cancer therapy (Figure 3). Several preclinical studies suggest that abnormal myelopoiesis and recruitment of immature myeloid cells into tissues are early events in cancer progression.10 Therefore, one potential clinical strategy for targeting MDSC in an oncologic setting would be to target tumor derived factors (TDF) from being produced or from reaching the bone marrow. Key cytokines such as IL-6 or S100A8/A9 could be directly targeted. One of the challenges with this approach clinically would involve redundancy in the cytokine pathways and requirement to block several different cytokines or chemokines to curtail TDF in a meaningful enough way to have a positive impact clinically on tumor progression. A second potential therapeutic approach would be focusing on inhibiting generation of MDSCs from bone marrow progenitors or inducing apoptosis of circulating MDSCs. Various systemic therapeutic agents currently utilized in oncology are known to decrease MDSC levels, including gemcitabine, 5-FU, sunitinib, and zolendronate .5, 10 A third potential option would entail preventing trafficking of myeloid cells from the marrow to peripheral lymphoid organs or to the tumor microenvironment. Several potential drug targets would include chemokine (C-X-C motif) receptor 2 (CXCR2), chemokine (C-X-C motif) receptor 4 (CXCR4), and colony stimulating factor 1 receptor (CSF1R).10 A fourth therapeutic strategy would involve directly blocking MDSCs to suppress T-cells. Examples of drug classes that would be able to accomplish this would include phosphodiesterase type 5 inhibitors (PDE5), e.g. sildenafil and tadalafil, or cyclooxygenase 2 (COX-2) inhibitors. A fifth approach would involve drugs that would promote differentiation of MDSCs into proficient antigen presenting cells that can stimulate tumor-specific T-cells and/or into mature leukocytes. Several drugs have been shown in preclinical and/or small clinical studies to be able to promote differentiation of MDSCs including, all-trans retinoic acid (ATRA), vitamin D3, and DNA methylating agent 5-azacytidine.5, 10, 72
Figure 3.
Targeting MDSCs as an anti-cancer therapeutic strategy.
Conclusions
Immune evasion is a hallmark of cancer, and the literature provides substantial evidence that MDSCs are important in the biology of tumor progression and immune evasion. While, leukocytic infiltrates in tumors is not a new observation, MDSCs have emerged as a novel and very attractive therapeutic target in cancer, especially since they are present in the peripheral blood of patients with all major solid tumor types. However, one of the major obstacles in translating preclinical data to the clinic has been the heterogeneity of how MDSCs are defined and which phenotypes are evaluated in cancer patients. However, if one applies the filter of clinically robust endpoints, three distinct MDSC phenotypes emerge. Further appropriately prospective trials need to evaluate the importance of MDSCs as predictive and/or prognostic markers in cancer patients. More recent studies suggest that MDSCs can promote metastases with mechanisms other than immunosupression. A greater understanding of the biology of MDSCs and the role they play in tumor progression would certainly help to accelerate the clinical development of novel strategies for the prevention of metastases, and would also have the potential to greatly enhance the effectiveness of immune based therapies in cancer.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Blank CU. The perspective of immunotherapy: New molecules and new mechanisms of action in immune modulation. Curr Opin Oncol. 2014 doi: 10.1097/CCO.0000000000000054. [DOI] [PubMed] [Google Scholar]
- 2.Kyi C, Postow MA. Checkpoint blocking antibodies in cancer immunotherapy. FEBS Lett. 2014;588:368–376. doi: 10.1016/j.febslet.2013.10.015. [DOI] [PubMed] [Google Scholar]
- 3.Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, Wolchok JD, Hersey P, Joseph RW, Weber JS, Dronca R, Gangadhar TC, Patnaik A, Zarour H, Joshua AM, Gergich K, Elassaiss-Schaap J, Algazi A, Mateus C, Boasberg P, Tumeh PC, Chmielowski B, Ebbinghaus SW, Li XN, Kang SP, Ribas A. Safety and tumor responses with lambrolizumab (anti-pd-1) in melanoma. N Engl J Med. 2013;369:134–144. doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 5.Najjar YG, Finke JH. Clinical perspectives on targeting of myeloid derived suppressor cells in the treatment of cancer. Front Oncol. 2013;3:49. doi: 10.3389/fonc.2013.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gabrilovich DI, Bronte V, Chen SH, Colombo MP, Ochoa A, Ostrand-Rosenberg S, Schreiber H. The terminology issue for myeloid-derived suppressor cells. Cancer research. 2007;67:425. doi: 10.1158/0008-5472.CAN-06-3037. author reply 426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Montero AJ, Diaz-Montero CM, Kyriakopoulos CE, Bronte V, Mandruzzato S. Myeloid-derived suppressor cells in cancer patients: A clinical perspective. J Immunother. 2012;35:107–115. doi: 10.1097/CJI.0b013e318242169f. [DOI] [PubMed] [Google Scholar]
- 9.Ostrand-Rosenberg S. Myeloid-derived suppressor cells: More mechanisms for inhibiting antitumor immunity. Cancer immunology, immunotherapy : CII. 2010;59:1593–1600. doi: 10.1007/s00262-010-0855-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12:253–268. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Balkwill F, Mantovani A. Inflammation and cancer: Back to virchow? Lancet. 2001;357:539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 12.Finke J, Ko J, Rini B, Rayman P, Ireland J, Cohen P. Mdsc as a mechanism of tumor escape from sunitinib mediated anti-angiogenic therapy. Int Immunopharmacol. 2011;11:856–861. doi: 10.1016/j.intimp.2011.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Corzo CA, Cotter MJ, Cheng P, Cheng F, Kusmartsev S, Sotomayor E, Padhya T, McCaffrey TV, McCaffrey JC, Gabrilovich DI. Mechanism regulating reactive oxygen species in tumor-induced myeloid-derived suppressor cells. Journal of immunology. 2009;182:5693–5701. doi: 10.4049/jimmunol.0900092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Serafini P, Mgebroff S, Noonan K, Borrello I. Myeloid-derived suppressor cells promote cross-tolerance in b-cell lymphoma by expanding regulatory t cells. Cancer research. 2008;68:5439–5449. doi: 10.1158/0008-5472.CAN-07-6621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rodriguez PC, Quiceno DG, Ochoa AC. L-arginine availability regulates t-lymphocyte cell-cycle progression. Blood. 2007;109:1568–1573. doi: 10.1182/blood-2006-06-031856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rodriguez PC, Zea AH, Culotta KS, Zabaleta J, Ochoa JB, Ochoa AC. Regulation of t cell receptor cd3zeta chain expression by l-arginine. J Biol Chem. 2002;277:21123–21129. doi: 10.1074/jbc.M110675200. [DOI] [PubMed] [Google Scholar]
- 17.Rodriguez PC, Ochoa AC. Arginine regulation by myeloid derived suppressor cells and tolerance in cancer: Mechanisms and therapeutic perspectives. Immunol Rev. 2008;222:180–191. doi: 10.1111/j.1600-065X.2008.00608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eruslanov E, Neuberger M, Daurkin I, Perrin GQ, Algood C, Dahm P, Rosser C, Vieweg J, Gilbert SM, Kusmartsev S. Circulating and tumor-infiltrating myeloid cell subsets in patients with bladder cancer. Int J Cancer. 2012;130:1109–1119. doi: 10.1002/ijc.26123. [DOI] [PubMed] [Google Scholar]
- 19.Gabitass RF, Annels NE, Stocken DD, Pandha HA, Middleton GW. Elevated myeloid-derived suppressor cells in pancreatic, esophageal and gastric cancer are an independent prognostic factor and are associated with significant elevation of the th2 cytokine interleukin-13. Cancer immunology, immunotherapy : CII. 2011;60:1419–1430. doi: 10.1007/s00262-011-1028-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Solito S, Falisi E, Diaz-Montero CM, Doni A, Pinton L, Rosato A, Francescato S, Basso G, Zanovello P, Onicescu G, Garrett-Mayer E, Montero AJ, Bronte V, Mandruzzato S. A human promyelocytic-like population is responsible for the immune suppression mediated by myeloid-derived suppressor cells. Blood. 2011;118:2254–2265. doi: 10.1182/blood-2010-12-325753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Montero AJ, Diaz-Montero CM, Deutsch YE, Hurley J, Koniaris LG, Rumboldt T, Yasir S, Jorda M, Garret-Mayer E, Avisar E, Slingerland J, Silva O, Welsh C, Schuhwerk K, Seo P, Pegram MD, Gluck S. Phase 2 study of neoadjuvant treatment with nov-002 in combination with doxorubicin and cyclophosphamide followed by docetaxel in patients with her-2 negative clinical stage ii-iiic breast cancer. Breast Cancer Res Treat. 2012;132:215–223. doi: 10.1007/s10549-011-1889-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Diaz-Montero CM, Salem ML, Nishimura MI, Garrett-Mayer E, Cole DJ, Montero AJ. Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin-cyclophosphamide chemotherapy. Cancer immunology, immunotherapy : CII. 2009;58:49–59. doi: 10.1007/s00262-008-0523-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nature reviews. Cancer. 2009;9:239–252. doi: 10.1038/nrc2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sleeman JP. The metastatic niche and stromal progression. Cancer metastasis reviews. 2012;31:429–440. doi: 10.1007/s10555-012-9373-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Umansky V, Sevko A. Tumor microenvironment and myeloid-derived suppressor cells. Cancer microenvironment : official journal of the International Cancer Microenvironment Society. 2013;6:169–177. doi: 10.1007/s12307-012-0126-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ugel S, Delpozzo F, Desantis G, Papalini F, Simonato F, Sonda N, Zilio S, Bronte V. Therapeutic targeting of myeloid-derived suppressor cells. Curr Opin Pharmacol. 2009;9:470–481. doi: 10.1016/j.coph.2009.06.014. [DOI] [PubMed] [Google Scholar]
- 27.Bronte V. Myeloid-derived suppressor cells in inflammation: Uncovering cell subsets with enhanced immunosuppressive functions. Eur J Immunol. 2009;39:2670–2672. doi: 10.1002/eji.200939892. [DOI] [PubMed] [Google Scholar]
- 28.Nagaraj S, Gupta K, Pisarev V, Kinarsky L, Sherman S, Kang L, Herber DL, Schneck J, Gabrilovich DI. Altered recognition of antigen is a mechanism of cd8+ t cell tolerance in cancer. Nat Med. 2007;13:828–835. doi: 10.1038/nm1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Youn JI, Nagaraj S, Collazo M, Gabrilovich DI. Subsets of myeloid-derived suppressor cells in tumor-bearing mice. Journal of immunology. 2008;181:5791–5802. doi: 10.4049/jimmunol.181.8.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kotsakis A, Harasymczuk M, Schilling B, Georgoulias V, Argiris A, Whiteside TL. Myeloid-derived suppressor cell measurements in fresh and cryopreserved blood samples. J Immunol Methods. 2012;381:14–22. doi: 10.1016/j.jim.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Whiteside TL. Disarming suppressor cells to improve immunotherapy. Cancer immunology, immunotherapy : CII. 2012;61:283–288. doi: 10.1007/s00262-011-1171-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kusmartsev S, Nefedova Y, Yoder D, Gabrilovich DI. Antigen-specific inhibition of cd8+ t cell response by immature myeloid cells in cancer is mediated by reactive oxygen species. Journal of immunology. 2004;172:989–999. doi: 10.4049/jimmunol.172.2.989. [DOI] [PubMed] [Google Scholar]
- 33.Dolcetti L, Peranzoni E, Bronte V. Measurement of myeloid cell immune suppressive activity. Curr Protoc Immunol. 2010;Chapter 14(Unit 14):17. doi: 10.1002/0471142735.im1417s91. [DOI] [PubMed] [Google Scholar]
- 34.Gallina G, Dolcetti L, Serafini P, De Santo C, Marigo I, Colombo MP, Basso G, Brombacher F, Borrello I, Zanovello P, Bicciato S, Bronte V. Tumors induce a subset of inflammatory monocytes with immunosuppressive activity on cd8+ t cells. J Clin Invest. 2006;116:2777–2790. doi: 10.1172/JCI28828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dolcetti L, Peranzoni E, Ugel S, Marigo I, Fernandez Gomez A, Mesa C, Geilich M, Winkels G, Traggiai E, Casati A, Grassi F, Bronte V. Hierarchy of immunosuppressive strength among myeloid-derived suppressor cell subsets is determined by gm-csf. Eur J Immunol. 2010;40:22–35. doi: 10.1002/eji.200939903. [DOI] [PubMed] [Google Scholar]
- 36.Marigo I, Bosio E, Solito S, Mesa C, Fernandez A, Dolcetti L, Ugel S, Sonda N, Bicciato S, Falisi E, Calabrese F, Basso G, Zanovello P, Cozzi E, Mandruzzato S, Bronte V. Tumor-induced tolerance and immune suppression depend on the c/ebpbeta transcription factor. Immunity. 2010;32:790–802. doi: 10.1016/j.immuni.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 37.Cheng P, Zlobin A, Volgina V, Gottipati S, Osborne B, Simel EJ, Miele L, Gabrilovich DI. Notch-1 regulates nf-kappab activity in hemopoietic progenitor cells. Journal of immunology. 2001;167:4458–4467. doi: 10.4049/jimmunol.167.8.4458. [DOI] [PubMed] [Google Scholar]
- 38.Gibb DR, Saleem SJ, Kang DJ, Subler MA, Conrad DH. Adam10 overexpression shifts lympho- and myelopoiesis by dysregulating site 2/site 3 cleavage products of notch. Journal of immunology. 2011;186:4244–4252. doi: 10.4049/jimmunol.1003318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cheng P, Kumar V, Liu H, Youn JI, Fishman M, Sherman S, Gabrilovich D. Effects of notch signaling on regulation of myeloid cell differentiation in cancer. Cancer research. 2014;74:141–152. doi: 10.1158/0008-5472.CAN-13-1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yu H, Pardoll D, Jove R. Stats in cancer inflammation and immunity: A leading role for stat3. Nature reviews. Cancer. 2009;9:798–809. doi: 10.1038/nrc2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Poschke I, Mougiakakos D, Hansson J, Masucci GV, Kiessling R. Immature immunosuppressive cd14+hla-dr-/low cells in melanoma patients are stat3hi and overexpress cd80, cd83, and dc-sign. Cancer research. 2010;70:4335–4345. doi: 10.1158/0008-5472.CAN-09-3767. [DOI] [PubMed] [Google Scholar]
- 42.Cheng P, Corzo CA, Luetteke N, Yu B, Nagaraj S, Bui MM, Ortiz M, Nacken W, Sorg C, Vogl T, Roth J, Gabrilovich DI. Inhibition of dendritic cell differentiation and accumulation of myeloid-derived suppressor cells in cancer is regulated by s100a9 protein. The Journal of experimental medicine. 2008;205:2235–2249. doi: 10.1084/jem.20080132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sinha P, Okoro C, Foell D, Freeze HH, Ostrand-Rosenberg S, Srikrishna G. Proinflammatory s100 proteins regulate the accumulation of myeloid-derived suppressor cells. Journal of immunology. 2008;181:4666–4675. doi: 10.4049/jimmunol.181.7.4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kusmartsev S, Gabrilovich DI. Stat1 signaling regulates tumor-associated macrophage-mediated t cell deletion. Journal of immunology. 2005;174:4880–4891. doi: 10.4049/jimmunol.174.8.4880. [DOI] [PubMed] [Google Scholar]
- 45.Movahedi K, Guilliams M, Van den Bossche J, Van den Bergh R, Gysemans C, Beschin A, De Baetselier P, Van Ginderachter JA. Identification of discrete tumor-induced myeloid-derived suppressor cell subpopulations with distinct t cell-suppressive activity. Blood. 2008;111:4233–4244. doi: 10.1182/blood-2007-07-099226. [DOI] [PubMed] [Google Scholar]
- 46.Bronte V, Serafini P, De Santo C, Marigo I, Tosello V, Mazzoni A, Segal DM, Staib C, Lowel M, Sutter G, Colombo MP, Zanovello P. Il-4-induced arginase 1 suppresses alloreactive t cells in tumor-bearing mice. Journal of immunology. 2003;170:270–278. doi: 10.4049/jimmunol.170.1.270. [DOI] [PubMed] [Google Scholar]
- 47.Pak AS, Wright MA, Matthews JP, Collins SL, Petruzzelli GJ, Young MR. Mechanisms of immune suppression in patients with head and neck cancer: Presence of cd34(+) cells which suppress immune functions within cancers that secrete granulocyte-macrophage colony-stimulating factor. Clin Cancer Res. 1995;1:95–103. [PubMed] [Google Scholar]
- 48.Almand B, Clark JI, Nikitina E, van Beynen J, English NR, Knight SC, Carbone DP, Gabrilovich DI. Increased production of immature myeloid cells in cancer patients: A mechanism of immunosuppression in cancer. Journal of immunology. 2001;166:678–689. doi: 10.4049/jimmunol.166.1.678. [DOI] [PubMed] [Google Scholar]
- 49.Zhang B, Wang Z, Wu L, Zhang M, Li W, Ding J, Zhu J, Wei H, Zhao K. Circulating and tumor-infiltrating myeloid-derived suppressor cells in patients with colorectal carcinoma. PLoS One. 2013;8:e57114. doi: 10.1371/journal.pone.0057114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Raychaudhuri B, Rayman P, Ireland J, Ko J, Rini B, Borden EC, Garcia J, Vogelbaum MA, Finke J. Myeloid-derived suppressor cell accumulation and function in patients with newly diagnosed glioblastoma. Neuro Oncol. 2011;13:591–599. doi: 10.1093/neuonc/nor042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Walter S, Weinschenk T, Stenzl A, Zdrojowy R, Pluzanska A, Szczylik C, Staehler M, Brugger W, Dietrich PY, Mendrzyk R, Hilf N, Schoor O, Fritsche J, Mahr A, Maurer D, Vass V, Trautwein C, Lewandrowski P, Flohr C, Pohla H, Stanczak JJ, Bronte V, Mandruzzato S, Biedermann T, Pawelec G, Derhovanessian E, Yamagishi H, Miki T, Hongo F, Takaha N, Hirakawa K, Tanaka H, Stevanovic S, Frisch J, Mayer-Mokler A, Kirner A, Rammensee HG, Reinhardt C, Singh-Jasuja H. Multipeptide immune response to cancer vaccine ima901 after single-dose cyclophosphamide associates with longer patient survival. Nat Med. 2012;18:1254–1261. doi: 10.1038/nm.2883. [DOI] [PubMed] [Google Scholar]
- 52.Arihara F, Mizukoshi E, Kitahara M, Takata Y, Arai K, Yamashita T, Nakamoto Y, Kaneko S. Increase in cd14+hla-dr −/low myeloid-derived suppressor cells in hepatocellular carcinoma patients and its impact on prognosis. Cancer immunology, immunotherapy : CII. 2013;62:1421–1430. doi: 10.1007/s00262-013-1447-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Huang A, Zhang B, Wang B, Zhang F, Fan KX, Guo YJ. Increased cd14(+)hla-dr (−/low) myeloid-derived suppressor cells correlate with extrathoracic metastasis and poor response to chemotherapy in non-small cell lung cancer patients. Cancer immunology, immunotherapy : CII. 2013;62:1439–1451. doi: 10.1007/s00262-013-1450-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zea AH, Rodriguez PC, Atkins MB, Hernandez C, Signoretti S, Zabaleta J, McDermott D, Quiceno D, Youmans A, O'Neill A, Mier J, Ochoa AC. Arginase-producing myeloid suppressor cells in renal cell carcinoma patients: A mechanism of tumor evasion. Cancer research. 2005;65:3044–3048. doi: 10.1158/0008-5472.CAN-04-4505. [DOI] [PubMed] [Google Scholar]
- 55.Wang L, Chang EW, Wong SC, Ong SM, Chong DQ, Ling KL. Increased myeloid-derived suppressor cells in gastric cancer correlate with cancer stage and plasma s100a8/a9 proinflammatory proteins. Journal of immunology. 2013;190:794–804. doi: 10.4049/jimmunol.1202088. [DOI] [PubMed] [Google Scholar]
- 56.Mundy-Bosse BL, Young GS, Bauer T, Binkley E, Bloomston M, Bill MA, Bekaii-Saab T, Carson WE, 3rd, Lesinski GB. Distinct myeloid suppressor cell subsets correlate with plasma il-6 and il-10 and reduced interferon-alpha signaling in cd4(+) t cells from patients with gi malignancy. Cancer immunology, immunotherapy : CII. 2011;60:1269–1279. doi: 10.1007/s00262-011-1029-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kaufman HL, Kim DW, DeRaffele G, Mitcham J, Coffin RS, Kim-Schulze S. Local and distant immunity induced by intralesional vaccination with an oncolytic herpes virus encoding gm-csf in patients with stage iiic and iv melanoma. Ann Surg Oncol. 2010;17:718–730. doi: 10.1245/s10434-009-0809-6. [DOI] [PubMed] [Google Scholar]
- 58.Porembka MR, Mitchem JB, Belt BA, Hsieh CS, Lee HM, Herndon J, Gillanders WE, Linehan DC, Goedegebuure P. Pancreatic adenocarcinoma induces bone marrow mobilization of myeloid-derived suppressor cells which promote primary tumor growth. Cancer immunology, immunotherapy : CII. 2012;61:1373–1385. doi: 10.1007/s00262-011-1178-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cui TX, Kryczek I, Zhao L, Zhao E, Kuick R, Roh MH, Vatan L, Szeliga W, Mao Y, Thomas DG, Kotarski J, Tarkowski R, Wicha M, Cho K, Giordano T, Liu R, Zou W. Myeloid-derived suppressor cells enhance stemness of cancer cells by inducing microrna101 and suppressing the corepressor ctbp2. Immunity. 2013;39:611–621. doi: 10.1016/j.immuni.2013.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Finkelstein SE, Carey T, Fricke I, Yu D, Goetz D, Gratz M, Dunn M, Urbas P, Daud A, DeConti R, Antonia S, Gabrilovich D, Fishman M. Changes in dendritic cell phenotype after a new high-dose weekly schedule of interleukin-2 therapy for kidney cancer and melanoma. J Immunother. 2010;33:817–827. doi: 10.1097/CJI.0b013e3181ecccad. [DOI] [PubMed] [Google Scholar]
- 61.Kimura T, McKolanis JR, Dzubinski LA, Islam K, Potter DM, Salazar AM, Schoen RE, Finn OJ. Muc1 vaccine for individuals with advanced adenoma of the colon: A cancer immunoprevention feasibility study. Cancer Prev Res (Phila) 2013;6:18–26. doi: 10.1158/1940-6207.CAPR-12-0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Murdoch C, Muthana M, Coffelt SB, Lewis CE. The role of myeloid cells in the promotion of tumour angiogenesis. Nature reviews. Cancer. 2008;8:618–631. doi: 10.1038/nrc2444. [DOI] [PubMed] [Google Scholar]
- 63.Toh B, Wang X, Keeble J, Sim WJ, Khoo K, Wong WC, Kato M, Prevost-Blondel A, Thiery JP, Abastado JP. Mesenchymal transition and dissemination of cancer cells is driven by myeloid-derived suppressor cells infiltrating the primary tumor. PLoS biology. 2011;9:e1001162. doi: 10.1371/journal.pbio.1001162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yang L, DeBusk LM, Fukuda K, Fingleton B, Green-Jarvis B, Shyr Y, Matrisian LM, Carbone DP, Lin PC. Expansion of myeloid immune suppressor gr+cd11b+ cells in tumor-bearing host directly promotes tumor angiogenesis. Cancer cell. 2004;6:409–421. doi: 10.1016/j.ccr.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 65.Yang L, Huang J, Ren X, Gorska AE, Chytil A, Aakre M, Carbone DP, Matrisian LM, Richmond A, Lin PC, Moses HL. Abrogation of tgf beta signaling in mammary carcinomas recruits gr-1+cd11b+ myeloid cells that promote metastasis. Cancer cell. 2008;13:23–35. doi: 10.1016/j.ccr.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ahn GO, Brown JM. Matrix metalloproteinase-9 is required for tumor vasculogenesis but not for angiogenesis: Role of bone marrow-derived myelomonocytic cells. Cancer cell. 2008;13:193–205. doi: 10.1016/j.ccr.2007.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang H, Maric I, DiPrima MJ, Khan J, Orentas RJ, Kaplan RN, Mackall CL. Fibrocytes represent a novel mdsc subset circulating in patients with metastatic cancer. Blood. 2013;122:1105–1113. doi: 10.1182/blood-2012-08-449413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Corzo CA, Condamine T, Lu L, Cotter MJ, Youn JI, Cheng P, Cho HI, Celis E, Quiceno DG, Padhya T, McCaffrey TV, McCaffrey JC, Gabrilovich DI. Hif-1alpha regulates function and differentiation of myeloid-derived suppressor cells in the tumor microenvironment. The Journal of experimental medicine. 2010;207:2439–2453. doi: 10.1084/jem.20100587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sawant A, Deshane J, Jules J, Lee CM, Harris BA, Feng X, Ponnazhagan S. Myeloid-derived suppressor cells function as novel osteoclast progenitors enhancing bone loss in breast cancer. Cancer research. 2013;73:672–682. doi: 10.1158/0008-5472.CAN-12-2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sawant A, Ponnazhagan S. Myeloid-derived suppressor cells as osteoclast progenitors: A novel target for controlling osteolytic bone metastasis. Cancer research. 2013;73:4606–4610. doi: 10.1158/0008-5472.CAN-13-0305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yan HH, Pickup M, Pang Y, Gorska AE, Li Z, Chytil A, Geng Y, Gray JW, Moses HL, Yang L. Gr-1+cd11b+ myeloid cells tip the balance of immune protection to tumor promotion in the premetastatic lung. Cancer research. 2010;70:6139–6149. doi: 10.1158/0008-5472.CAN-10-0706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Daurkin I, Eruslanov E, Vieweg J, Kusmartsev S. Generation of antigen-presenting cells from tumor-infiltrated cd11b myeloid cells with DNA demethylating agent 5-aza-2'-deoxycytidine. Cancer immunology, immunotherapy : CII. 2010;59:697–706. doi: 10.1007/s00262-009-0786-4. [DOI] [PMC free article] [PubMed] [Google Scholar]