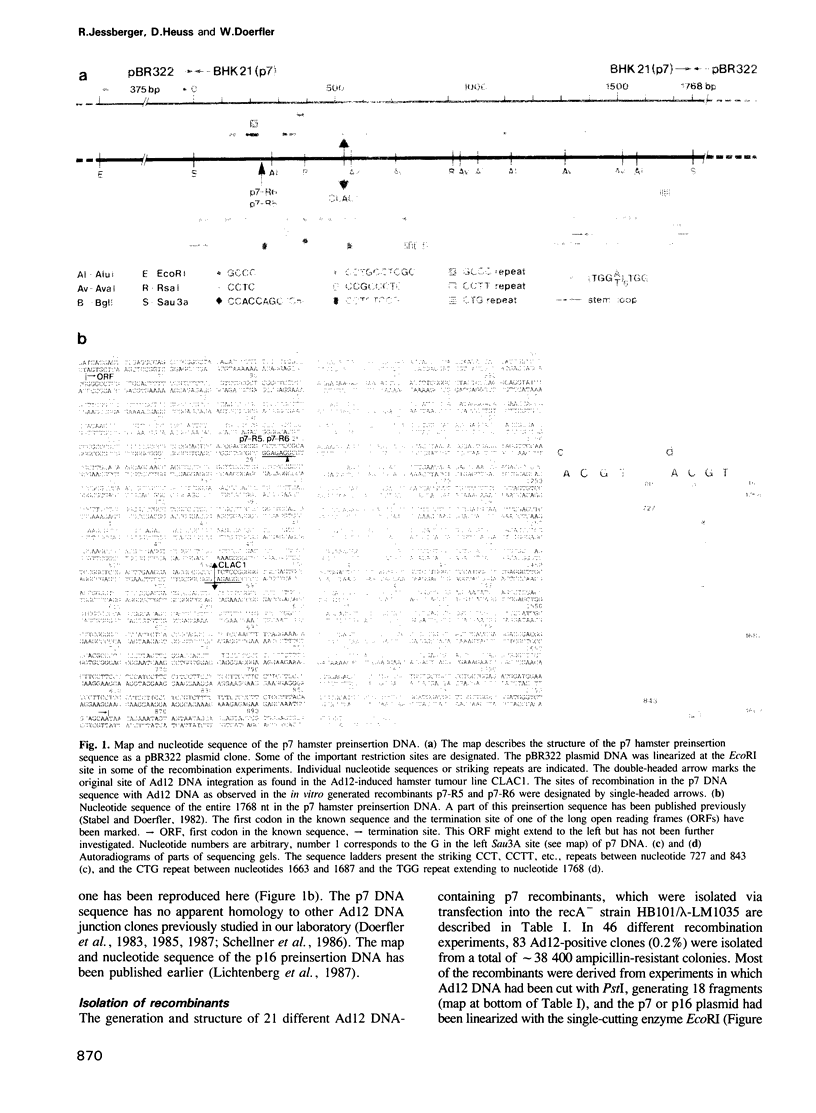
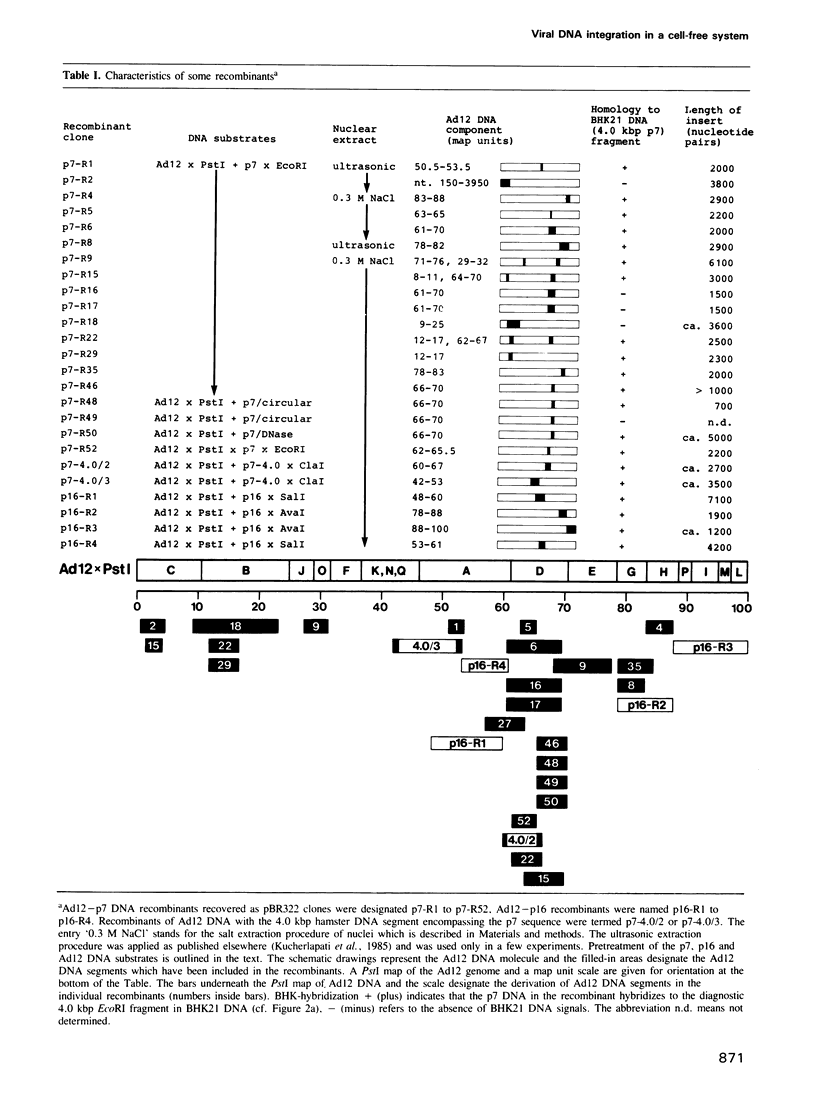
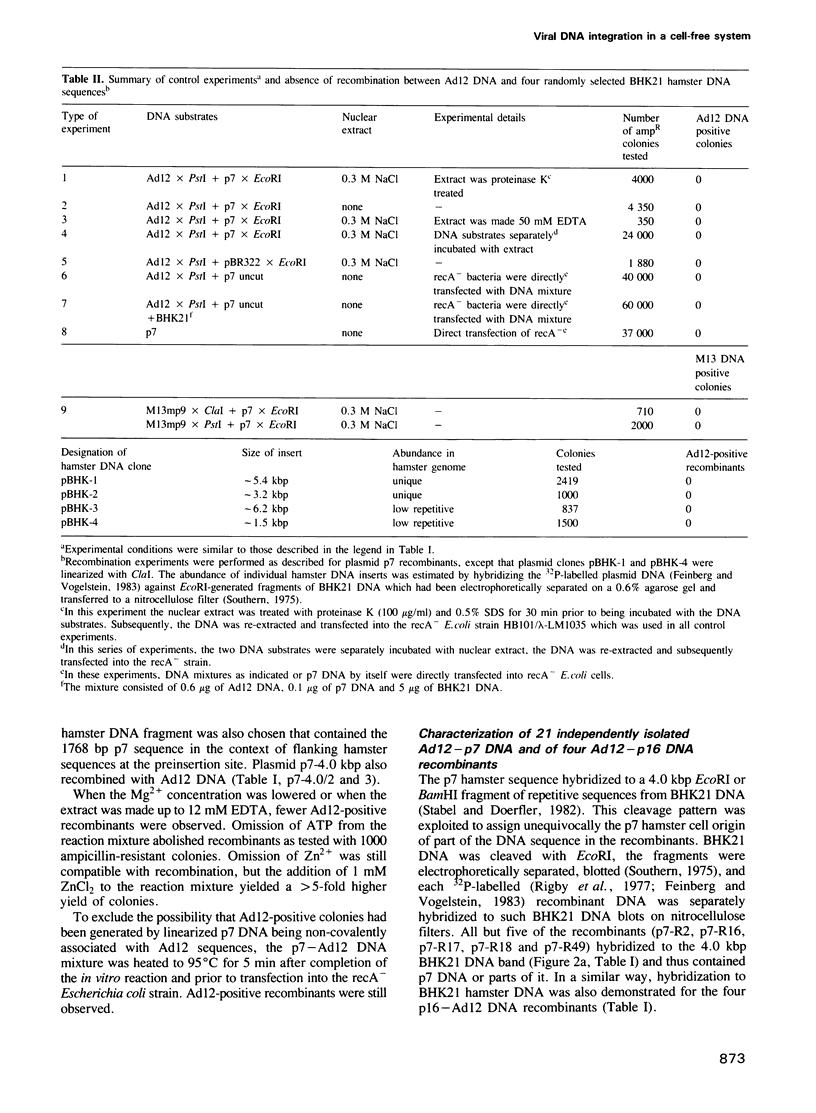
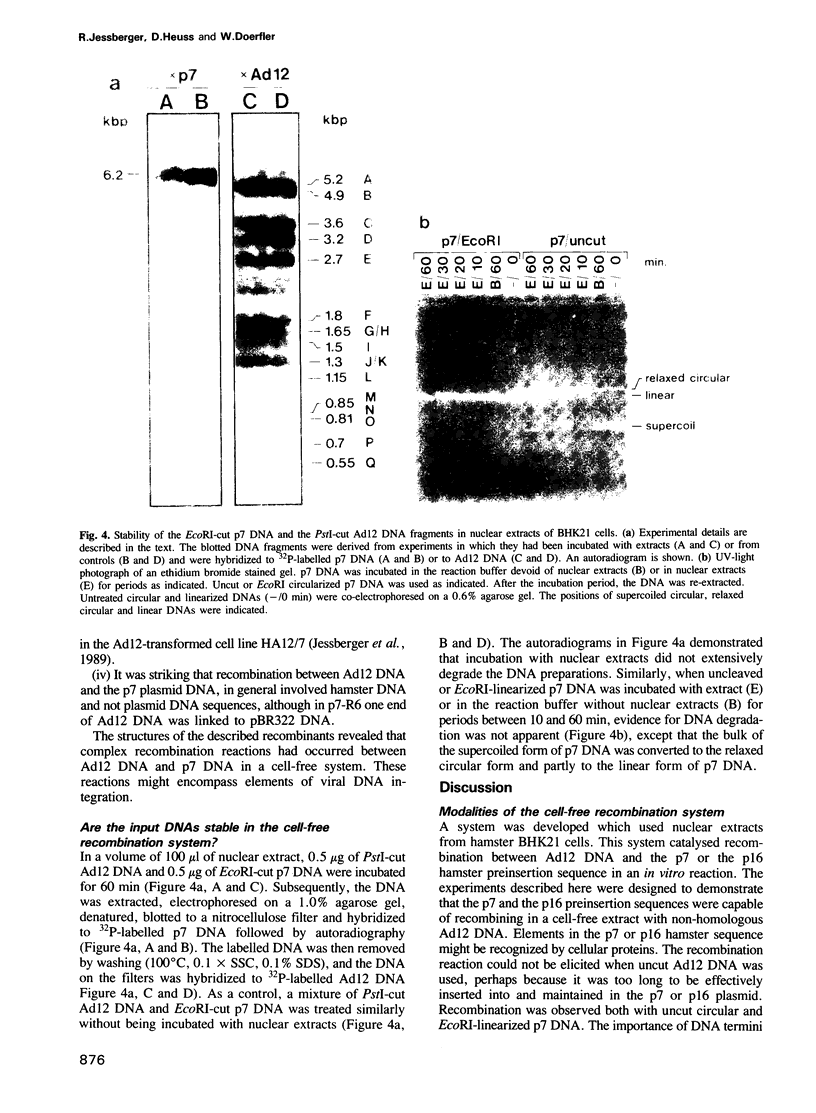
Abstract
A cell-free system of nuclear extracts from BHK21 cells has been developed to catalyse recombination in vitro between the DNA of adenovirus type 12 (Ad12) and two different hamster preinsertion sequences. The pBR322 cloned 1768 bp fragment p7 and the 3.1 kbp fragment p16 from BHK21 hamster DNA had previously been identified as the preinsertion sites corresponding to the junctions between Ad12 DNA and hamster DNA in cell line CLAC1 and in the Ad12-induced tumour T1111(2), respectively. Preinsertion sequences, which had recombined previously with foreign (Ad12) DNA, might again be recognized by the recombination system even in a cell-free system. PstI cleaved Ad12 DNA and the circular or the EcoRI linearized p7 or p16 preinsertion sequences were incubated with nuclear extracts. Recombinants were isolated by transfecting the DNA into recA- Escherichia coli strains and by screening for Ad12 DNA-positive colonies. Without a selectable eukaryotic marker, all Ad12 DNA positive recombinants were registered. Out of a total of greater than 90 p7-Ad12 DNA recombinants, 21 were studied by restriction-hybridization, and four by partial nucleotide sequence analyses. Among the p16-Ad12 DNA recombinants, four were analysed. The sites of linkage between Ad12 DNA and p7 or p16 hamster DNA were all different and distinct from the original CLAC1 or T1111(2) junction site between Ad12 and hamster DNA. The in vitro recombinants were not generated by simple end-to-end joining of the DNA fragments used in the reaction but by genetic exchange. Thirteen of the 25 recombinants were derived from the 61-71 map unit fragment of Ad12 DNA. Recombination experiments between Ad12 DNA and four randomly selected unique or repetitive hamster DNA sequences of 1.5-6.2 kbp in length did not yield recombinants. Apparently, the p7 and p16 hamster preinsertion sequences recombined with Ad12 DNA with a certain preference.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bos J. L., Polder L. J., Bernards R., Schrier P. I., van den Elsen P. J., van der Eb A. J., van Ormondt H. The 2.2 kb E1b mRNA of human Ad12 and Ad5 codes for two tumor antigens starting at different AUG triplets. Cell. 1981 Nov;27(1 Pt 2):121–131. doi: 10.1016/0092-8674(81)90366-4. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brown P. O., Bowerman B., Varmus H. E., Bishop J. M. Correct integration of retroviral DNA in vitro. Cell. 1987 May 8;49(3):347–356. doi: 10.1016/0092-8674(87)90287-x. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Effect of growth conditions on the formation of the relaxation complex of supercoiled ColE1 deoxyribonucleic acid and protein in Escherichia coli. J Bacteriol. 1972 Jun;110(3):1135–1146. doi: 10.1128/jb.110.3.1135-1146.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darby V., Blattner F. Homologous recombination catalyzed by mammalian cell extracts in vitro. Science. 1984 Dec 7;226(4679):1213–1215. doi: 10.1126/science.6334360. [DOI] [PubMed] [Google Scholar]
- Deuring R., Winterhoff U., Tamanoi F., Stabel S., Doerfler W. Site of linkage between adenovirus type 12 and cell DNAs in hamster tumour line CLAC3. Nature. 1981 Sep 3;293(5827):81–84. doi: 10.1038/293081a0. [DOI] [PubMed] [Google Scholar]
- Doerfler W., Gahlmann R., Stabel S., Deuring R., Lichtenberg U., Schulz M., Eick D., Leisten R. On the mechanism of recombination between adenoviral and cellular DNAs: the structure of junction sites. Curr Top Microbiol Immunol. 1984;109:193–228. doi: 10.1007/978-3-642-69460-8_9. [DOI] [PubMed] [Google Scholar]
- Doerfler W. Integration of the deoxyribonucleic acid of adenovirus type 12 into the deoxyribonucleic acid of baby hamster kidney cells. J Virol. 1970 Nov;6(5):652–666. doi: 10.1128/jvi.6.5.652-666.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerfler W., Lundholm U., Hirsch-Kauffmann M. Intracellular forms of adenovirus deoxyribonucleic acid. I. Evidence for a deoxyribonucleic acid-protein complex in baby hamster kidney cells infected with adenovirus type 12. J Virol. 1972 Feb;9(2):297–308. doi: 10.1128/jvi.9.2.297-308.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerfler W. The fate of the DNA of adenovirus type 12 in baby hamster kidney cells. Proc Natl Acad Sci U S A. 1968 Jun;60(2):636–643. doi: 10.1073/pnas.60.2.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Gahlmann R., Leisten R., Vardimon L., Doerfler W. Patch homologies and the integration of adenovirus DNA in mammalian cells. EMBO J. 1982;1(9):1101–1104. doi: 10.1002/j.1460-2075.1982.tb01303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gahlmann R., Schulz M., Doefler W. Low molecular weight RNAs with homologies to cellular DNA at sites of adenovirus DNA insertion in hamster or mouse cells. EMBO J. 1984 Dec 20;3(13):3263–3269. doi: 10.1002/j.1460-2075.1984.tb02288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höchtl J., Zachau H. G. A novel type of aberrant recombination in immunoglobulin genes and its implications for V-J joining mechanism. Nature. 1983 Mar 17;302(5905):260–263. doi: 10.1038/302260a0. [DOI] [PubMed] [Google Scholar]
- Kelly A., Trowsdale J. Complete nucleotide sequence of a functional HLA-DP beta gene and the region between the DP beta 1 and DP alpha 1 genes: comparison of the 5' ends of HLA class II genes. Nucleic Acids Res. 1985 Mar 11;13(5):1607–1621. doi: 10.1093/nar/13.5.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura T., Sawada Y., Shinawawa M., Shimizu Y., Shiroki K., Shimojo H., Sugisaki H., Takanami M., Uemizu Y., Fujinaga K. Nucleotide sequence of the transforming early region E1b of adenovirus type 12 DNA: structure and gene organization, and comparison with those of adenovirus type 5 DNA. Nucleic Acids Res. 1981 Dec 11;9(23):6571–6589. doi: 10.1093/nar/9.23.6571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruczek I., Doerfler W. The unmethylated state of the promoter/leader and 5'-regions of integrated adenovirus genes correlates with gene expression. EMBO J. 1982;1(4):409–414. doi: 10.1002/j.1460-2075.1982.tb01183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruijer W., van Schaik F. M., Speijer J. G., Sussenbach J. S. Structure and function of adenovirus DNA binding protein: comparison of the amino acid sequences of the Ad5 and Ad12 proteins derived from the nucleotide sequence of the corresponding genes. Virology. 1983 Jul 15;128(1):140–153. doi: 10.1016/0042-6822(83)90325-2. [DOI] [PubMed] [Google Scholar]
- Kucherlapati R. S., Spencer J., Moore P. D. Homologous recombination catalyzed by human cell extracts. Mol Cell Biol. 1985 Apr;5(4):714–720. doi: 10.1128/mcb.5.4.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larhammar D., Hammerling U., Denaro M., Lund T., Flavell R. A., Rask L., Peterson P. A. Structure of the murine immune response I-A beta locus: sequence of the I-A beta gene and an adjacent beta-chain second domain exon. Cell. 1983 Aug;34(1):179–188. doi: 10.1016/0092-8674(83)90148-4. [DOI] [PubMed] [Google Scholar]
- Lichtenberg U., Zock C., Doerfler W. Insertion of adenovirus type 12 DNA in the vicinity of an intracisternal A particle genome in Syrian hamster tumor cells. J Virol. 1987 Sep;61(9):2719–2726. doi: 10.1128/jvi.61.9.2719-2726.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison D. A. Transformation and preservation of competent bacterial cells by freezing. Methods Enzymol. 1979;68:326–331. doi: 10.1016/0076-6879(79)68023-0. [DOI] [PubMed] [Google Scholar]
- Norrander J., Kempe T., Messing J. Construction of improved M13 vectors using oligodeoxynucleotide-directed mutagenesis. Gene. 1983 Dec;26(1):101–106. doi: 10.1016/0378-1119(83)90040-9. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Roth D. B., Porter T. N., Wilson J. H. Mechanisms of nonhomologous recombination in mammalian cells. Mol Cell Biol. 1985 Oct;5(10):2599–2607. doi: 10.1128/mcb.5.10.2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth D. B., Wilson J. H. Nonhomologous recombination in mammalian cells: role for short sequence homologies in the joining reaction. Mol Cell Biol. 1986 Dec;6(12):4295–4304. doi: 10.1128/mcb.6.12.4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schellner J., Stüber K., Doerfler W. Computer analyses on the structure of junction sites between adenovirus DNA and cellular DNA. Biochim Biophys Acta. 1986 Jun 20;867(3):114–123. doi: 10.1016/0167-4781(86)90071-0. [DOI] [PubMed] [Google Scholar]
- Schulz M., Doerfler W. Deletion of cellular DNA at site of viral DNA insertion in the adenovirus type 12-induced mouse tumor CBA-12-1-T. Nucleic Acids Res. 1984 Jun 25;12(12):4959–4976. doi: 10.1093/nar/12.12.4959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz M., Freisem-Rabien U., Jessberger R., Doerfler W. Transcriptional activities of mammalian genomes at sites of recombination with foreign DNA. J Virol. 1987 Feb;61(2):344–353. doi: 10.1128/jvi.61.2.344-353.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stabel S., Doerfler W. Nucleotide sequence at the site of junction between adenovirus type 12 DNA and repetitive hamster cell DNA in transformed cell line CLAC1. Nucleic Acids Res. 1982 Dec 20;10(24):8007–8023. doi: 10.1093/nar/10.24.8007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringer J. R. Integrated simian virus 40 DNA: nucleotide sequences at cell-virus recombinant junctions. J Virol. 1981 May;38(2):671–679. doi: 10.1128/jvi.38.2.671-679.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugisaki H., Sugimoto K., Takanami M., Shiroki K., Saito I., Shimojo H., Sawada Y., Uemizu Y., Uesugi S., Fujinaga K. Structure and gene organization in the transformed Hind III-G fragment of Ad12. Cell. 1980 Jul;20(3):777–786. doi: 10.1016/0092-8674(80)90324-4. [DOI] [PubMed] [Google Scholar]
- Sutcliffe J. G. Complete nucleotide sequence of the Escherichia coli plasmid pBR322. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):77–90. doi: 10.1101/sqb.1979.043.01.013. [DOI] [PubMed] [Google Scholar]
- Sutter D., Westphal M., Doerfler W. Patterns of integration of viral DNA sequences in the genomes of adenovirus type 12-transformed hamster cells. Cell. 1978 Jul;14(3):569–585. doi: 10.1016/0092-8674(78)90243-x. [DOI] [PubMed] [Google Scholar]
- Wallace R. B., Johnson M. J., Suggs S. V., Miyoshi K., Bhatt R., Itakura K. A set of synthetic oligodeoxyribonucleotide primers for DNA sequencing in the plasmid vector pBR322. Gene. 1981 Dec;16(1-3):21–26. doi: 10.1016/0378-1119(81)90057-3. [DOI] [PubMed] [Google Scholar]
- Wilson J. H., Berget P. B., Pipas J. M. Somatic cells efficiently join unrelated DNA segments end-to-end. Mol Cell Biol. 1982 Oct;2(10):1258–1269. doi: 10.1128/mcb.2.10.1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel R., Hendriks W., Quax W., Bloemendal H. Complete structure of the hamster alpha A crystallin gene. Reflection of an evolutionary history by means of exon shuffling. J Mol Biol. 1985 Sep 20;185(2):273–284. doi: 10.1016/0022-2836(85)90403-6. [DOI] [PubMed] [Google Scholar]





